

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50358321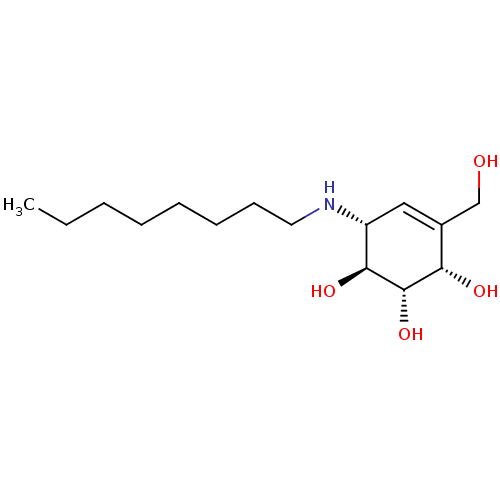 (CHEMBL1922579 | CHEMBL1922581) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amicus Therapeutics Curated by ChEMBL | Assay Description Inhibition of human beta galactosidase | J Med Chem 56: 2705-25 (2013) Article DOI: 10.1021/jm301557k BindingDB Entry DOI: 10.7270/Q26111N4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182801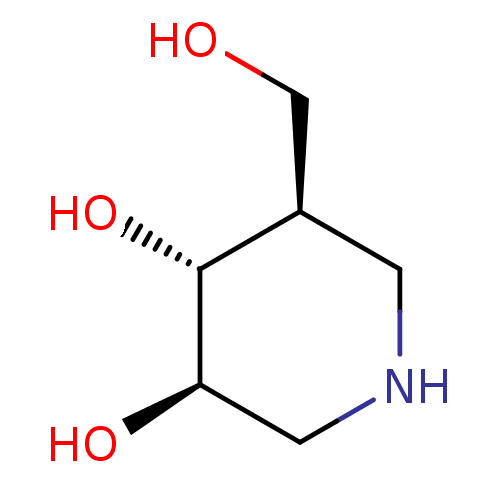 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of GCase assessed as 4-methylumbelliferone release assay after 30 mins by fluorimetry | Nat Chem Biol 3: 101-7 (2007) Article DOI: 10.1038/nchembio850 BindingDB Entry DOI: 10.7270/Q2639PX9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Palmitoyl-protein thioesterase 1 (Homo sapiens (Human)) | BDBM50429511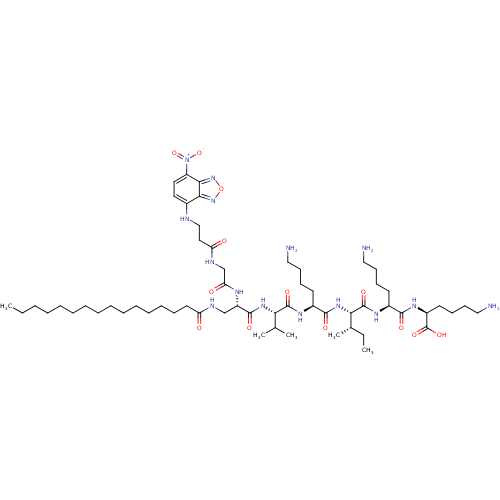 (CHEMBL2332876) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amicus Therapeutics Curated by ChEMBL | Assay Description Inhibition of PPT1 in human fibroblast/lymphoblast cell lysate using fluorescent-based MUGSP as substrate after 1 to 3 hrs | J Med Chem 56: 2705-25 (2013) Article DOI: 10.1021/jm301557k BindingDB Entry DOI: 10.7270/Q26111N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoyl-protein thioesterase 1 (Homo sapiens (Human)) | BDBM50429510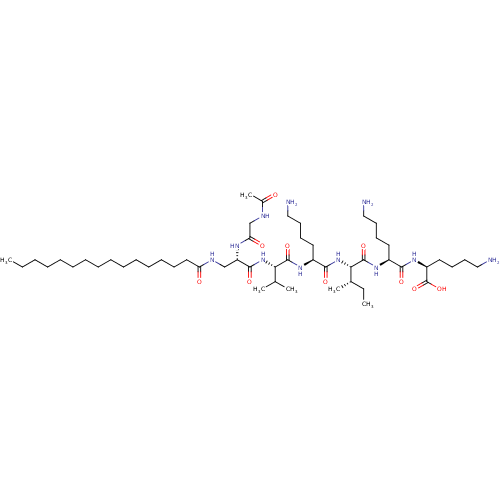 (CHEMBL2332875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amicus Therapeutics Curated by ChEMBL | Assay Description Inhibition of PPT1 (unknown origin) in cell lysates using fluorescent-based MUGSP as substrate after 1 to 3 hrs | J Med Chem 56: 2705-25 (2013) Article DOI: 10.1021/jm301557k BindingDB Entry DOI: 10.7270/Q26111N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||