 Found 31536 hits with Last Name = 'kim' and Initial = 's'
Found 31536 hits with Last Name = 'kim' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50434903
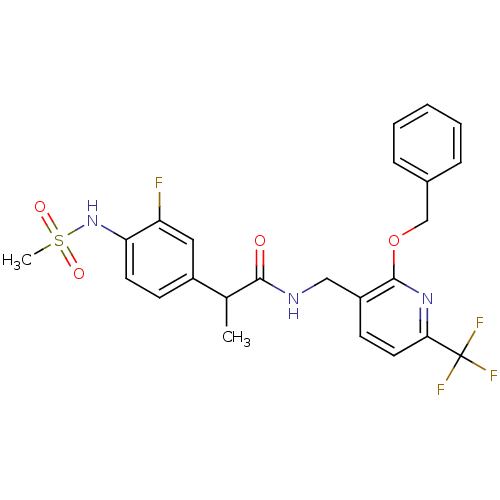
(CHEMBL2385223)Show SMILES CC(C(=O)NCc1ccc(nc1OCc1ccccc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H23F4N3O4S/c1-15(17-8-10-20(19(25)12-17)31-36(2,33)34)22(32)29-13-18-9-11-21(24(26,27)28)30-23(18)35-14-16-6-4-3-5-7-16/h3-12,15,31H,13-14H2,1-2H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-arachidonoyldopamine-induced activity after 5 mins by FLIPR a... |
Eur J Med Chem 64: 589-602 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.003
BindingDB Entry DOI: 10.7270/Q2BZ67FC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50434903

(CHEMBL2385223)Show SMILES CC(C(=O)NCc1ccc(nc1OCc1ccccc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H23F4N3O4S/c1-15(17-8-10-20(19(25)12-17)31-36(2,33)34)22(32)29-13-18-9-11-21(24(26,27)28)30-23(18)35-14-16-6-4-3-5-7-16/h3-12,15,31H,13-14H2,1-2H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-arachidonoyldopamine-induced activity after 5 mins by FLIPR a... |
Eur J Med Chem 64: 589-602 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.003
BindingDB Entry DOI: 10.7270/Q2BZ67FC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50434903

(CHEMBL2385223)Show SMILES CC(C(=O)NCc1ccc(nc1OCc1ccccc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H23F4N3O4S/c1-15(17-8-10-20(19(25)12-17)31-36(2,33)34)22(32)29-13-18-9-11-21(24(26,27)28)30-23(18)35-14-16-6-4-3-5-7-16/h3-12,15,31H,13-14H2,1-2H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-arachidonoyldopamine-induced activity after 5 mins by FLIPR a... |
Eur J Med Chem 64: 589-602 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.003
BindingDB Entry DOI: 10.7270/Q2BZ67FC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50455135
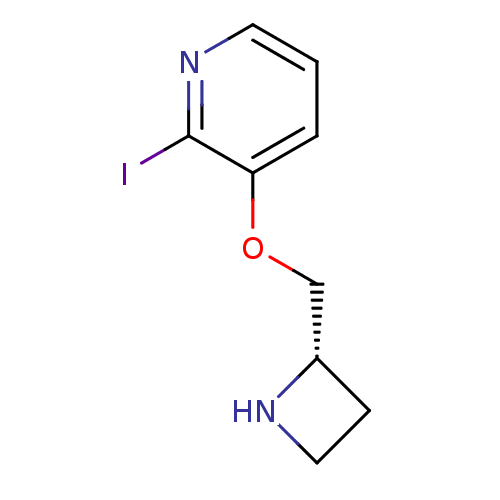
(CHEMBL123225)Show InChI InChI=1S/C9H11IN2O/c10-9-8(2-1-4-12-9)13-6-7-3-5-11-7/h1-2,4,7,11H,3,5-6H2/t7-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Binding affinity for alpha4 beta-2 nAChR using [3H]epibatidine in rat brain |
J Med Chem 41: 3690-8 (1998)
Article DOI: 10.1021/jm980170a
BindingDB Entry DOI: 10.7270/Q2HM59ZN |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474596

(CHEMBL79332)Show InChI InChI=1S/C16H15ClFN3O/c17-16-12(2-1-11-3-5-20-15(18)7-11)8-14(9-21-16)22-10-13-4-6-19-13/h1-3,5,7-9,13,19H,4,6,10H2/b2-1+/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474584

(CHEMBL79387)Show InChI InChI=1S/C14H13BrClN3O/c15-13-5-9(1-3-18-13)12-6-11(7-19-14(12)16)20-8-10-2-4-17-10/h1,3,5-7,10,17H,2,4,8H2/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474594

(CHEMBL86050)Show InChI InChI=1S/C14H12BrClFN3O/c15-12-3-8(5-20-14(12)17)11-4-10(6-19-13(11)16)21-7-9-1-2-18-9/h3-6,9,18H,1-2,7H2/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474583

(CHEMBL86051)Show InChI InChI=1S/C14H13BrClN3O/c15-13-2-1-9(6-18-13)12-5-11(7-19-14(12)16)20-8-10-3-4-17-10/h1-2,5-7,10,17H,3-4,8H2/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474587
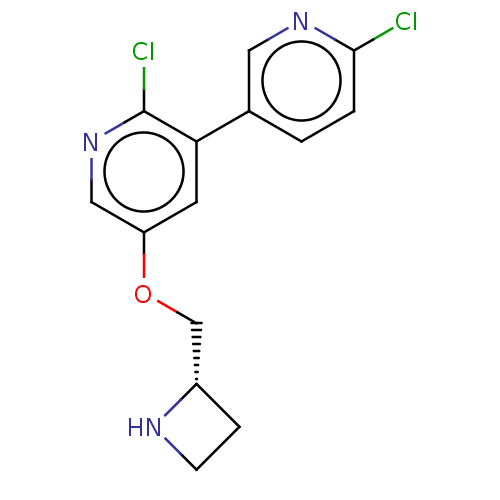
(CHEMBL314718)Show InChI InChI=1S/C14H13Cl2N3O/c15-13-2-1-9(6-18-13)12-5-11(7-19-14(12)16)20-8-10-3-4-17-10/h1-2,5-7,10,17H,3-4,8H2/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474595

(CHEMBL79594)Show InChI InChI=1S/C14H13ClFN3O/c15-14-12(9-1-3-18-13(16)5-9)6-11(7-19-14)20-8-10-2-4-17-10/h1,3,5-7,10,17H,2,4,8H2/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474582
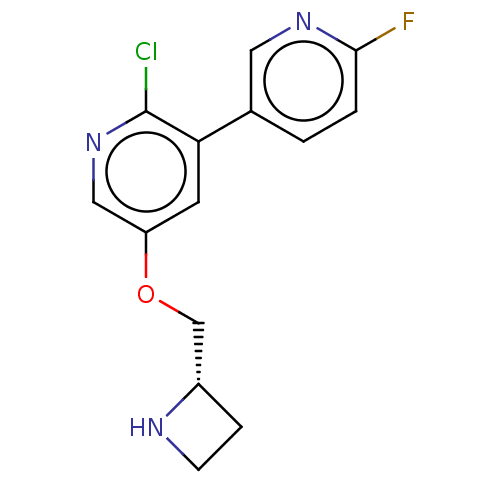
(CHEMBL83444)Show InChI InChI=1S/C14H13ClFN3O/c15-14-12(9-1-2-13(16)18-6-9)5-11(7-19-14)20-8-10-3-4-17-10/h1-2,5-7,10,17H,3-4,8H2/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474593

(CHEMBL313877)Show InChI InChI=1S/C16H15BrClN3O/c17-15-7-11(3-5-20-15)1-2-12-8-14(9-21-16(12)18)22-10-13-4-6-19-13/h1-3,5,7-9,13,19H,4,6,10H2/b2-1+/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474591

(CHEMBL79515)Show SMILES Clc1ncc(OC[C@@H]2CCCN2)cc1\C=C\c1ccnc(Br)c1 Show InChI InChI=1S/C17H17BrClN3O/c18-16-8-12(5-7-21-16)3-4-13-9-15(10-22-17(13)19)23-11-14-2-1-6-20-14/h3-5,7-10,14,20H,1-2,6,11H2/b4-3+/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500526
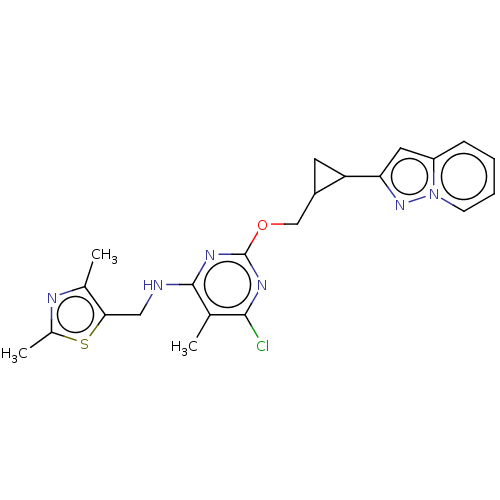
(CHEMBL3747517)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc4ccccn4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C22H23ClN6OS/c1-12-20(23)26-22(27-21(12)24-10-19-13(2)25-14(3)31-19)30-11-15-8-17(15)18-9-16-6-4-5-7-29(16)28-18/h4-7,9,15,17H,8,10-11H2,1-3H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474598

(CHEMBL79702)Show InChI InChI=1S/C14H14BrN3O/c15-14-13(10-2-1-4-16-7-10)6-12(8-18-14)19-9-11-3-5-17-11/h1-2,4,6-8,11,17H,3,5,9H2/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474592
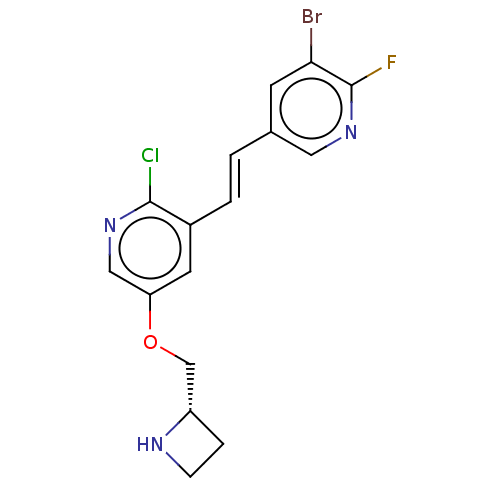
(CHEMBL83738)Show SMILES Fc1ncc(\C=C\c2cc(OC[C@@H]3CCN3)cnc2Cl)cc1Br Show InChI InChI=1S/C16H14BrClFN3O/c17-14-5-10(7-22-16(14)19)1-2-11-6-13(8-21-15(11)18)23-9-12-3-4-20-12/h1-2,5-8,12,20H,3-4,9H2/b2-1+/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474585

(CHEMBL312132)Show InChI InChI=1S/C14H14ClN3O/c15-14-13(10-2-1-4-16-7-10)6-12(8-18-14)19-9-11-3-5-17-11/h1-2,4,6-8,11,17H,3,5,9H2/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50143320
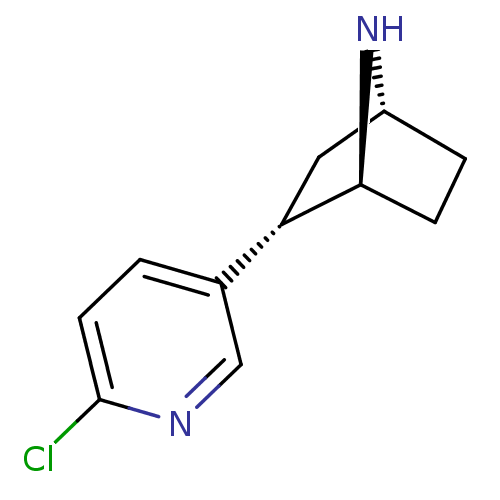
((+)-epibatidine | (-)-1-epidatidine | (1S,2S,4R)-2...)Show SMILES Clc1ccc(cn1)[C@@H]1C[C@H]2CC[C@@H]1N2 |THB:4:7:13:11.10| Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8-,9+,10+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Binding affinity for alpha4 beta-2 nAChR using [3H]epibatidine in rat brain |
J Med Chem 41: 3690-8 (1998)
Article DOI: 10.1021/jm980170a
BindingDB Entry DOI: 10.7270/Q2HM59ZN |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50450722
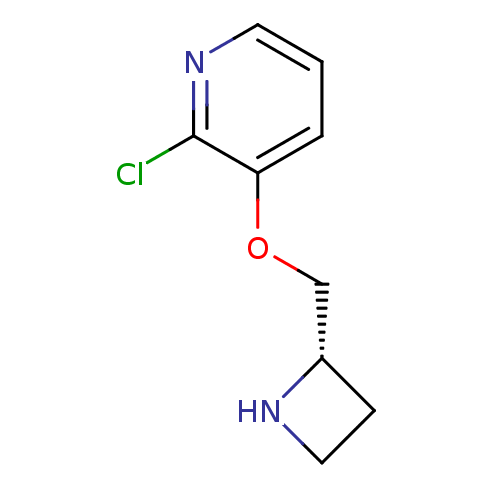
(CHEMBL94843)Show InChI InChI=1S/C9H11ClN2O/c10-9-8(2-1-4-12-9)13-6-7-3-5-11-7/h1-2,4,7,11H,3,5-6H2/t7-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Binding affinity for alpha4 beta-2 nAChR using [3H]epibatidine in rat brain |
J Med Chem 41: 3690-8 (1998)
Article DOI: 10.1021/jm980170a
BindingDB Entry DOI: 10.7270/Q2HM59ZN |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474590

(CHEMBL309292)Show InChI InChI=1S/C13H13ClFN4O/c14-13-11(19-8-16-3-2-12(19)15)5-10(6-18-13)20-7-9-1-4-17-9/h2-3,5-6,8-9,17H,1,4,7H2/q+1/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474586

(CHEMBL80040)Show InChI InChI=1S/C16H16ClN3O/c17-16-13(2-1-12-3-6-18-7-4-12)9-15(10-20-16)21-11-14-5-8-19-14/h1-4,6-7,9-10,14,19H,5,8,11H2/b2-1+/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474588
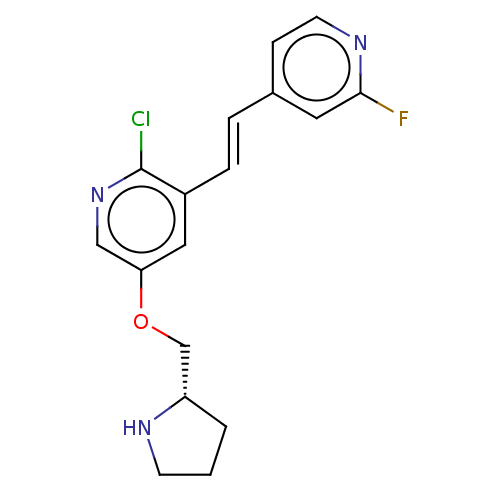
(CHEMBL310197)Show InChI InChI=1S/C17H17ClFN3O/c18-17-13(4-3-12-5-7-21-16(19)8-12)9-15(10-22-17)23-11-14-2-1-6-20-14/h3-5,7-10,14,20H,1-2,6,11H2/b4-3+/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50066789
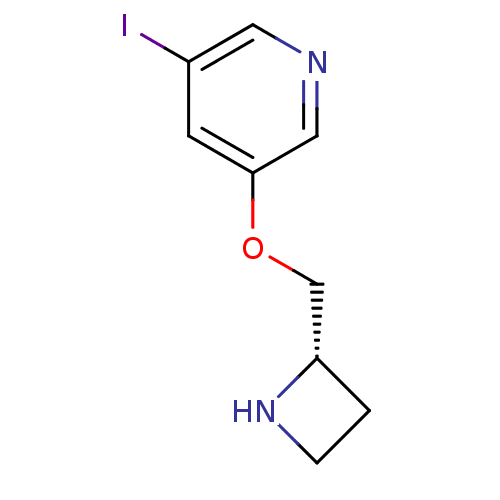
(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500521

(CHEMBL3746277)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc(C)ccn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H24ClN5OS/c1-11-5-6-23-17(7-11)16-8-15(16)10-28-21-26-19(22)12(2)20(27-21)24-9-18-13(3)25-14(4)29-18/h5-7,15-16H,8-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500520

(CHEMBL3746993)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C24H24ClN5OS/c1-13-22(25)29-24(30-23(13)26-11-21-14(2)27-15(3)32-21)31-12-17-10-18(17)20-9-8-16-6-4-5-7-19(16)28-20/h4-9,17-18H,10-12H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500538

(CHEMBL3745790)Show SMILES COc1ccnc(c1)C1CC1COc1nc(Cl)c(C)c(NCc2sc(C)nc2C)n1 Show InChI InChI=1S/C21H24ClN5O2S/c1-11-19(22)26-21(27-20(11)24-9-18-12(2)25-13(3)30-18)29-10-14-7-16(14)17-8-15(28-4)5-6-23-17/h5-6,8,14,16H,7,9-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50066789

(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay |
Bioorg Med Chem 21: 6657-64 (2013)
Article DOI: 10.1016/j.bmc.2013.08.015
BindingDB Entry DOI: 10.7270/Q26Q1ZPN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay |
Bioorg Med Chem 21: 6657-64 (2013)
Article DOI: 10.1016/j.bmc.2013.08.015
BindingDB Entry DOI: 10.7270/Q26Q1ZPN |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251]
(Staphylococcus aureus) | BDBM97445
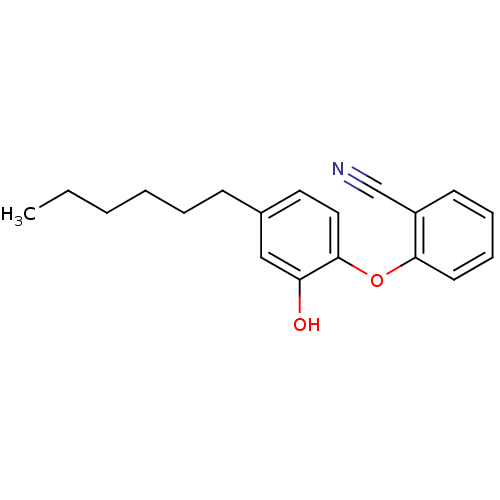
(PT119)Show InChI InChI=1S/C19H21NO2/c1-2-3-4-5-8-15-11-12-19(17(21)13-15)22-18-10-7-6-9-16(18)14-20/h6-7,9-13,21H,2-5,8H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus enoyl ACP reductase |
Eur J Med Chem 88: 66-73 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.008
BindingDB Entry DOI: 10.7270/Q25T3N3S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50066789

(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Binding affinity for alpha4 beta-2 nAChR using [3H]epibatidine in rat brain |
J Med Chem 41: 3690-8 (1998)
Article DOI: 10.1021/jm980170a
BindingDB Entry DOI: 10.7270/Q2HM59ZN |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474597

(CHEMBL83608)Show InChI InChI=1S/C14H14IN3O/c15-14-13(10-2-1-4-16-7-10)6-12(8-18-14)19-9-11-3-5-17-11/h1-2,4,6-8,11,17H,3,5,9H2/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Binding affinity for alpha4 beta-2 nAChR using [3H]epibatidine in rat brain |
J Med Chem 41: 3690-8 (1998)
Article DOI: 10.1021/jm980170a
BindingDB Entry DOI: 10.7270/Q2HM59ZN |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474581
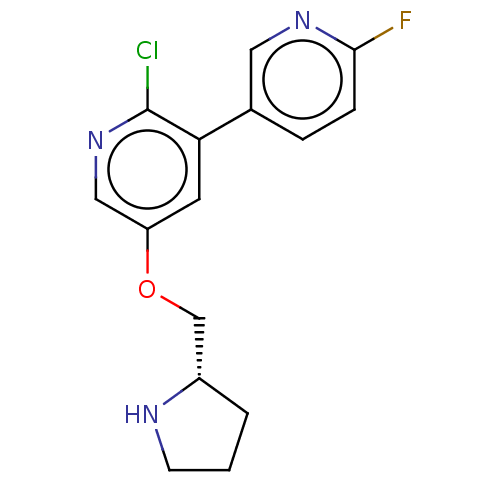
(CHEMBL83986)Show InChI InChI=1S/C15H15ClFN3O/c16-15-13(10-3-4-14(17)19-7-10)6-12(8-20-15)21-9-11-2-1-5-18-11/h3-4,6-8,11,18H,1-2,5,9H2/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50100712
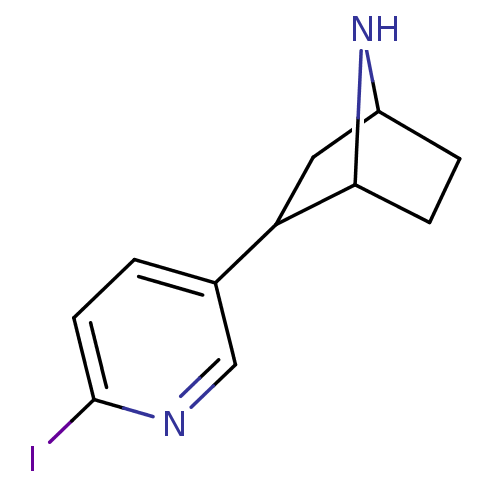
(2-(6-Iodo-pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptan...)Show InChI InChI=1S/C11H13IN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049553
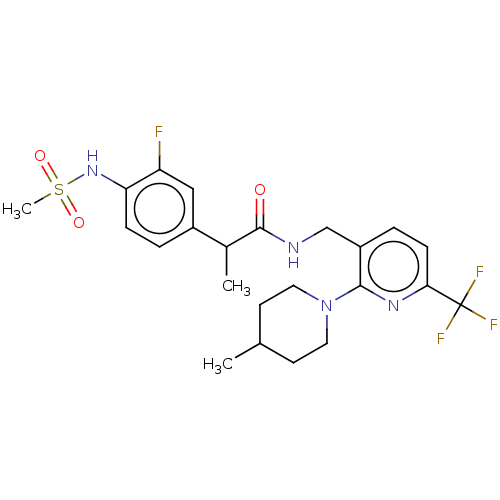
(CHEMBL2177428)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474580

(CHEMBL84154)Show InChI InChI=1S/C15H15BrClN3O/c16-14-4-3-10(7-19-14)13-6-12(8-20-15(13)17)21-9-11-2-1-5-18-11/h3-4,6-8,11,18H,1-2,5,9H2/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature |
J Med Chem 47: 2453-65 (2004)
Article DOI: 10.1021/jm030432v
BindingDB Entry DOI: 10.7270/Q21C20MJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500519
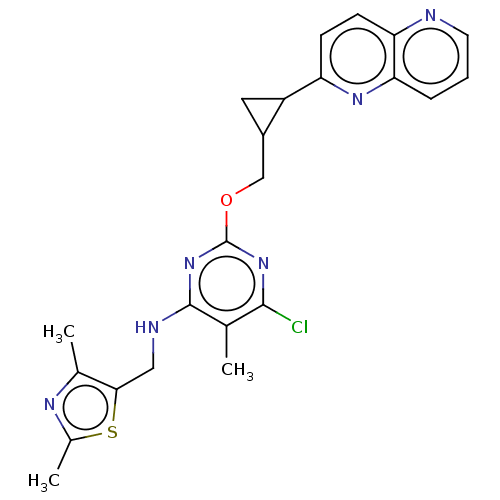
(CHEMBL3746917)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ncccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C23H23ClN6OS/c1-12-21(24)29-23(30-22(12)26-10-20-13(2)27-14(3)32-20)31-11-15-9-16(15)17-6-7-18-19(28-17)5-4-8-25-18/h4-8,15-16H,9-11H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500535

(CHEMBL3747450)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cn(C)cn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C19H23ClN6OS/c1-10-17(20)24-19(25-18(10)21-6-16-11(2)23-12(3)28-16)27-8-13-5-14(13)15-7-26(4)9-22-15/h7,9,13-14H,5-6,8H2,1-4H3,(H,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM144757
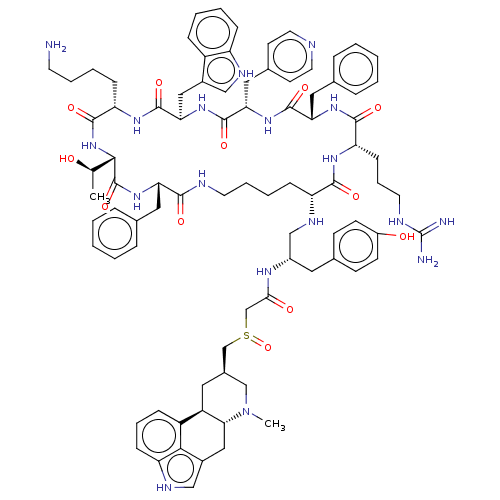
(US8952128, 15)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccncc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC1=O)NC[C@H](Cc1ccc(O)cc1)NC(=O)CS(=O)C[C@@H]1C[C@H]2[C@@H](Cc3c[nH]c4cccc2c34)N(C)C1 |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S.
US Patent
| Assay Description
Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... |
US Patent US8952128 (2015)
BindingDB Entry DOI: 10.7270/Q2057DNG |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM144761
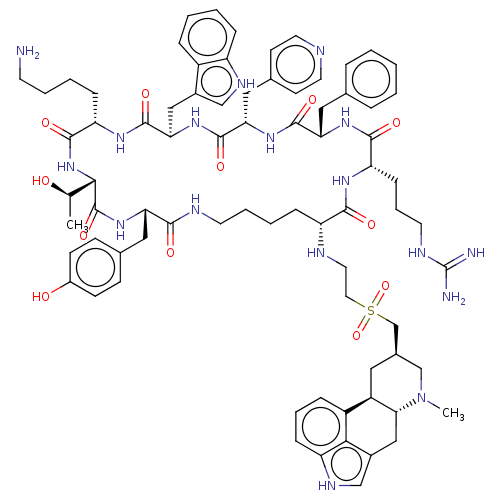
(US8952128, 20)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccncc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)NCCS(=O)(=O)C[C@@H]1C[C@H]2[C@@H](Cc3c[nH]c4cccc2c34)N(C)C1 |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S.
US Patent
| Assay Description
Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... |
US Patent US8952128 (2015)
BindingDB Entry DOI: 10.7270/Q2057DNG |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM144762

(US8952128, 21)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccncc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC1=O)NC[C@H](Cc1ccc(O)cc1)NC(=O)CS(=O)(=O)C[C@@H]1C[C@H]2[C@@H](Cc3c[nH]c4cccc2c34)N(C)C1 |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S.
US Patent
| Assay Description
Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... |
US Patent US8952128 (2015)
BindingDB Entry DOI: 10.7270/Q2057DNG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data