 Found 158 hits with Last Name = 'kim' and Initial = 'sn'
Found 158 hits with Last Name = 'kim' and Initial = 'sn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50094903
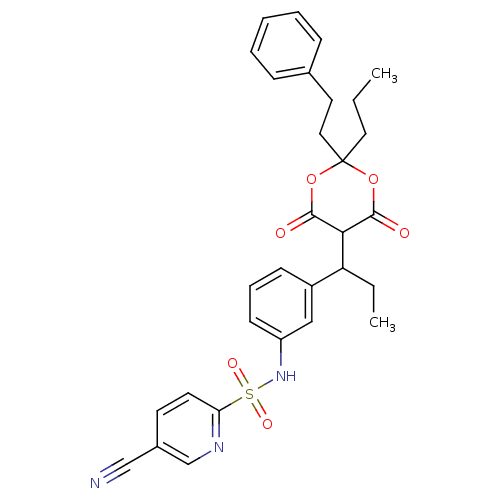
(5-Cyano-pyridine-2-sulfonic acid {3-[1-(6-hydroxy-...)Show SMILES CCCC1(CCc2ccccc2)OC(=O)C(C(CC)c2cccc(NS(=O)(=O)c3ccc(cn3)C#N)c2)C(=O)O1 |(.36,-8.1,;.78,-6.63,;2.27,-6.24,;2.67,-4.74,;1.17,-4.35,;.08,-5.42,;-1.4,-5.02,;-2.49,-6.11,;-3.97,-5.72,;-4.36,-4.21,;-3.27,-3.13,;-1.79,-3.53,;4.01,-5.51,;5.35,-4.74,;6.68,-5.51,;5.35,-3.22,;6.68,-2.43,;6.67,-.89,;8,-.12,;8.01,-3.2,;8.03,-4.74,;9.36,-5.54,;10.69,-4.77,;10.72,-3.2,;12.05,-2.43,;13.38,-3.23,;12.61,-4.56,;14.73,-4,;14.15,-1.87,;13.38,-.54,;14.12,.81,;15.66,.81,;16.46,-.52,;15.71,-1.87,;16.43,2.15,;17.18,3.49,;9.36,-2.43,;4,-2.43,;4,-.89,;2.67,-3.2,)| Show InChI InChI=1S/C30H31N3O6S/c1-3-16-30(17-15-21-9-6-5-7-10-21)38-28(34)27(29(35)39-30)25(4-2)23-11-8-12-24(18-23)33-40(36,37)26-14-13-22(19-31)20-32-26/h5-14,18,20,25,27,33H,3-4,15-17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity towards HIV protease |
Bioorg Med Chem Lett 10: 2625-7 (2000)
BindingDB Entry DOI: 10.7270/Q20R9NPF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50094906
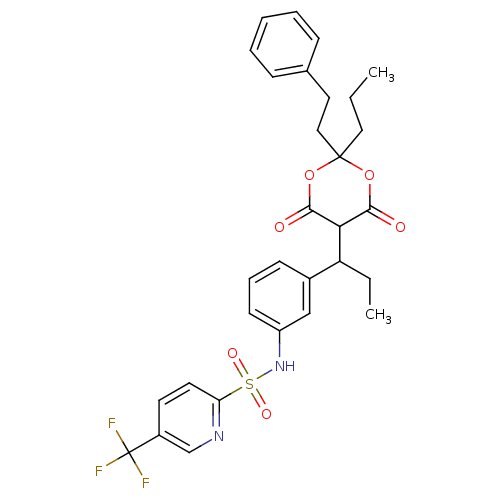
(5-Trifluoromethyl-pyridine-2-sulfonic acid {3-[1-(...)Show SMILES CCCC1(CCc2ccccc2)OC(O)=C(C(CC)c2cccc(NS(=O)(=O)c3ccc(cn3)C(F)(F)F)c2)C(=O)O1 |t:15| Show InChI InChI=1S/C30H31F3N2O6S/c1-3-16-29(17-15-20-9-6-5-7-10-20)40-27(36)26(28(37)41-29)24(4-2)21-11-8-12-23(18-21)35-42(38,39)25-14-13-22(19-34-25)30(31,32)33/h5-14,18-19,24,26,35H,3-4,15-17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity towards HIV protease |
Bioorg Med Chem Lett 10: 2625-7 (2000)
BindingDB Entry DOI: 10.7270/Q20R9NPF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50094901
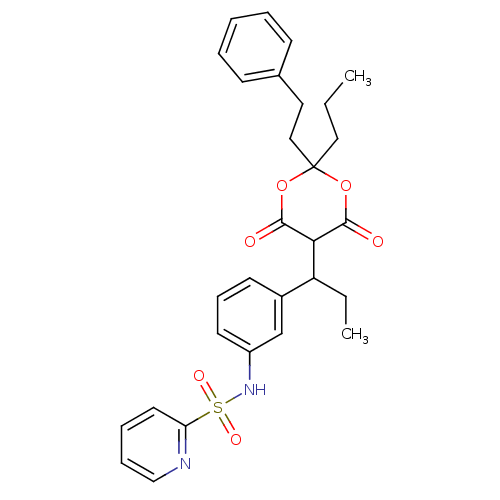
(CHEMBL90449 | Pyridine-2-sulfonic acid {3-[1-(6-hy...)Show SMILES CCCC1(CCc2ccccc2)OC(=O)C(C(CC)c2cccc(NS(=O)(=O)c3ccccn3)c2)C(=O)O1 |(-.81,-7.29,;-.39,-5.81,;1.1,-5.42,;1.5,-3.93,;.01,-3.53,;-1.09,-4.6,;-2.57,-4.21,;-3.66,-5.3,;-5.13,-4.9,;-5.53,-3.39,;-4.43,-2.32,;-2.96,-2.71,;2.85,-4.7,;4.18,-3.93,;5.51,-4.7,;4.18,-2.4,;5.51,-1.62,;5.51,-.08,;6.84,.69,;6.84,-2.39,;6.87,-3.93,;8.2,-4.72,;9.53,-3.95,;9.55,-2.39,;10.88,-1.62,;12.21,-2.41,;11.44,-3.74,;13.56,-3.18,;12.98,-1.06,;12.21,.27,;12.96,1.63,;14.5,1.63,;15.29,.3,;14.54,-1.06,;8.2,-1.62,;2.83,-1.62,;2.83,-.08,;1.5,-2.39,)| Show InChI InChI=1S/C29H32N2O6S/c1-3-17-29(18-16-21-11-6-5-7-12-21)36-27(32)26(28(33)37-29)24(4-2)22-13-10-14-23(20-22)31-38(34,35)25-15-8-9-19-30-25/h5-15,19-20,24,26,31H,3-4,16-18H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity towards HIV protease |
Bioorg Med Chem Lett 10: 2625-7 (2000)
BindingDB Entry DOI: 10.7270/Q20R9NPF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50094902
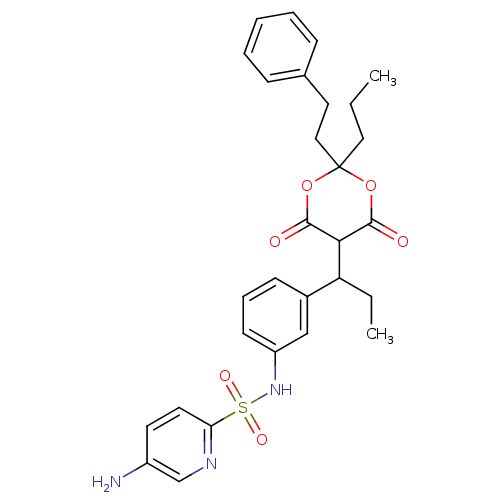
(5-Amino-pyridine-2-sulfonic acid {3-[1-(6-hydroxy-...)Show SMILES CCCC1(CCc2ccccc2)OC(O)=C(C(CC)c2cccc(NS(=O)(=O)c3ccc(N)cn3)c2)C(=O)O1 |t:15| Show InChI InChI=1S/C29H33N3O6S/c1-3-16-29(17-15-20-9-6-5-7-10-20)37-27(33)26(28(34)38-29)24(4-2)21-11-8-12-23(18-21)32-39(35,36)25-14-13-22(30)19-31-25/h5-14,18-19,24,26,32H,3-4,15-17,30H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity towards HIV protease |
Bioorg Med Chem Lett 10: 2625-7 (2000)
BindingDB Entry DOI: 10.7270/Q20R9NPF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50094907
(4-Cyano-N-{3-[1-(6-hydroxy-4-oxo-2-phenethyl-2-pro...)Show SMILES CCCC1(CCc2ccccc2)OC(=O)C(C(CC)c2cccc(NS(=O)(=O)c3ccc(cc3)C#N)c2)C(=O)O1 |(-.81,-7.27,;-.39,-5.8,;1.1,-5.41,;1.5,-3.92,;.01,-3.52,;-1.09,-4.6,;-2.56,-4.2,;-3.65,-5.29,;-5.12,-4.89,;-5.52,-3.38,;-4.42,-2.31,;-2.95,-2.71,;2.84,-4.69,;4.18,-3.92,;5.5,-4.69,;4.18,-2.4,;5.5,-1.61,;5.5,-.08,;6.82,.69,;6.83,-2.38,;6.85,-3.92,;8.18,-4.71,;9.51,-3.94,;9.53,-2.38,;10.86,-1.61,;12.19,-2.41,;11.42,-3.73,;13.54,-3.18,;12.96,-1.06,;12.19,.27,;12.93,1.62,;14.47,1.62,;15.26,.29,;14.52,-1.06,;15.26,2.97,;16.01,4.32,;8.18,-1.61,;2.83,-1.61,;2.83,-.08,;1.5,-2.38,)| Show InChI InChI=1S/C31H32N2O6S/c1-3-18-31(19-17-22-9-6-5-7-10-22)38-29(34)28(30(35)39-31)27(4-2)24-11-8-12-25(20-24)33-40(36,37)26-15-13-23(21-32)14-16-26/h5-16,20,27-28,33H,3-4,17-19H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity towards HIV protease |
Bioorg Med Chem Lett 10: 2625-7 (2000)
BindingDB Entry DOI: 10.7270/Q20R9NPF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50094904
(5-Trifluoromethyl-pyridine-2-sulfonic acid {3-[(R)...)Show SMILES CCC[C@@]1(CCc2ccccc2)OC(=O)C([C@H](CC)c2cccc(NS(=O)(=O)c3ccc(cn3)C(F)(F)F)c2)C(=O)O1 |wU:3.2,wD:16.19,3.3,(-.68,-8.28,;-.27,-6.8,;1.22,-6.42,;1.63,-4.93,;.14,-4.53,;-.96,-5.61,;-2.44,-5.2,;-3.52,-6.28,;-5.01,-5.89,;-5.41,-4.39,;-4.31,-3.31,;-2.82,-3.72,;1.63,-3.39,;2.96,-2.62,;2.96,-1.08,;4.3,-3.39,;5.63,-2.62,;5.63,-1.08,;6.96,-.31,;6.98,-3.39,;6.98,-4.93,;8.33,-5.72,;9.66,-4.95,;9.67,-3.39,;11.01,-2.62,;12.34,-3.41,;13.68,-4.18,;11.57,-4.74,;13.11,-2.06,;12.33,-.73,;13.09,.62,;14.63,.63,;15.42,-.71,;14.66,-2.05,;15.38,1.98,;16.06,3.2,;16.96,2.26,;14.79,3.48,;8.31,-2.62,;4.3,-4.93,;5.63,-5.7,;2.97,-5.7,)| Show InChI InChI=1S/C30H31F3N2O6S/c1-3-16-29(17-15-20-9-6-5-7-10-20)40-27(36)26(28(37)41-29)24(4-2)21-11-8-12-23(18-21)35-42(38,39)25-14-13-22(19-34-25)30(31,32)33/h5-14,18-19,24,26,35H,3-4,15-17H2,1-2H3/t24-,26?,29-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity towards HIV protease |
Bioorg Med Chem Lett 10: 2625-7 (2000)
BindingDB Entry DOI: 10.7270/Q20R9NPF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50094905

(5-Nitro-pyridine-2-sulfonic acid {3-[1-(6-hydroxy-...)Show SMILES CCCC1(CCc2ccccc2)OC([O-])=C(C(CC)c2cccc(NS(=O)(=O)c3ccc(cn3)[N+]([O-])=O)c2)C(=[OH+])O1 |t:15| Show InChI InChI=1S/C29H31N3O8S/c1-3-16-29(17-15-20-9-6-5-7-10-20)39-27(33)26(28(34)40-29)24(4-2)21-11-8-12-22(18-21)31-41(37,38)25-14-13-23(19-30-25)32(35)36/h5-14,18-19,24,31,33H,3-4,15-17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity towards HIV protease |
Bioorg Med Chem Lett 10: 2625-7 (2000)
BindingDB Entry DOI: 10.7270/Q20R9NPF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50383930
(CHEMBL2031955)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccc(-[#8])cc2)cc1-[#8] Show InChI InChI=1S/C24H28O3/c1-17(2)5-4-6-18(3)7-14-22-23(26)15-20(16-24(22)27)9-8-19-10-12-21(25)13-11-19/h5,7-13,15-16,25-27H,4,6,14H2,1-3H3/b9-8+,18-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085045
(5-((4-((6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-...)Show SMILES Cc1c(C)c2OC(C)(COc3ccc(Cc4sc(=O)[nH]c4O)cc3)CCc2c(C)c1O Show InChI InChI=1S/C24H27NO5S/c1-13-14(2)21-18(15(3)20(13)26)9-10-24(4,30-21)12-29-17-7-5-16(6-8-17)11-19-22(27)25-23(28)31-19/h5-8,26-27H,9-12H2,1-4H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50383925
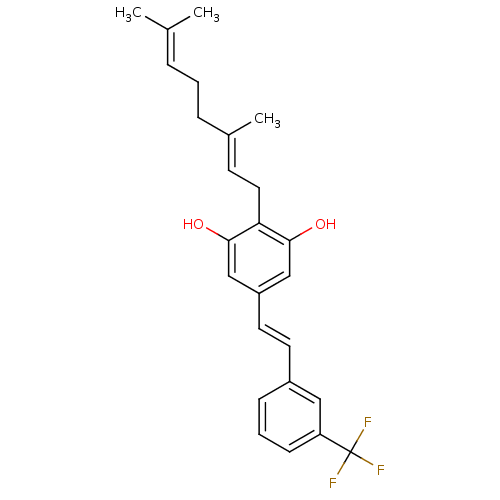
(CHEMBL2031858)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2cccc(c2)C(F)(F)F)cc1-[#8] Show InChI InChI=1S/C25H27F3O2/c1-17(2)6-4-7-18(3)10-13-22-23(29)15-20(16-24(22)30)12-11-19-8-5-9-21(14-19)25(26,27)28/h5-6,8-12,14-16,29-30H,4,7,13H2,1-3H3/b12-11+,18-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50383929
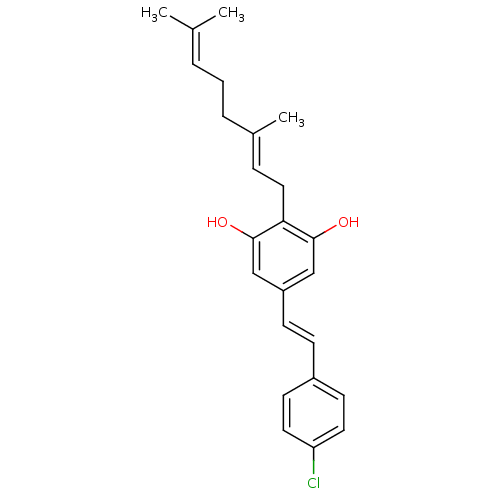
(CHEMBL2031953)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccc(Cl)cc2)cc1-[#8] Show InChI InChI=1S/C24H27ClO2/c1-17(2)5-4-6-18(3)7-14-22-23(26)15-20(16-24(22)27)9-8-19-10-12-21(25)13-11-19/h5,7-13,15-16,26-27H,4,6,14H2,1-3H3/b9-8+,18-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50383923
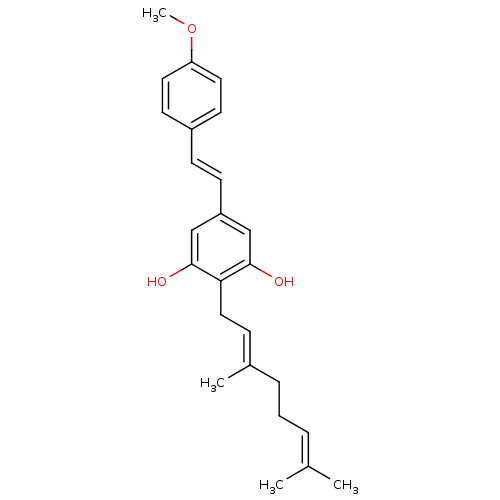
(CHEMBL2031856)Show SMILES [#6]-[#8]-c1ccc(\[#6]=[#6]\c2cc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c2)cc1 Show InChI InChI=1S/C25H30O3/c1-18(2)6-5-7-19(3)8-15-23-24(26)16-21(17-25(23)27)10-9-20-11-13-22(28-4)14-12-20/h6,8-14,16-17,26-27H,5,7,15H2,1-4H3/b10-9+,19-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50383931

(CHEMBL2031956)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccoc2)cc1-[#8] Show InChI InChI=1S/C22H26O3/c1-16(2)5-4-6-17(3)7-10-20-21(23)13-19(14-22(20)24)9-8-18-11-12-25-15-18/h5,7-9,11-15,23-24H,4,6,10H2,1-3H3/b9-8+,17-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50383922
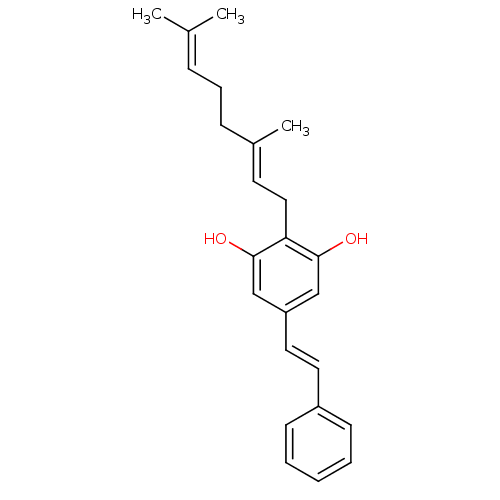
(AMORPHASTILBOL)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccccc2)cc1-[#8] Show InChI InChI=1S/C24H28O2/c1-18(2)8-7-9-19(3)12-15-22-23(25)16-21(17-24(22)26)14-13-20-10-5-4-6-11-20/h4-6,8,10-14,16-17,25-26H,7,9,15H2,1-3H3/b14-13+,19-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50383933

(CHEMBL2031954)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccc(-[#6]=O)cc2)cc1-[#8] Show InChI InChI=1S/C25H28O3/c1-18(2)5-4-6-19(3)7-14-23-24(27)15-22(16-25(23)28)13-10-20-8-11-21(17-26)12-9-20/h5,7-13,15-17,27-28H,4,6,14H2,1-3H3/b13-10+,19-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50383928
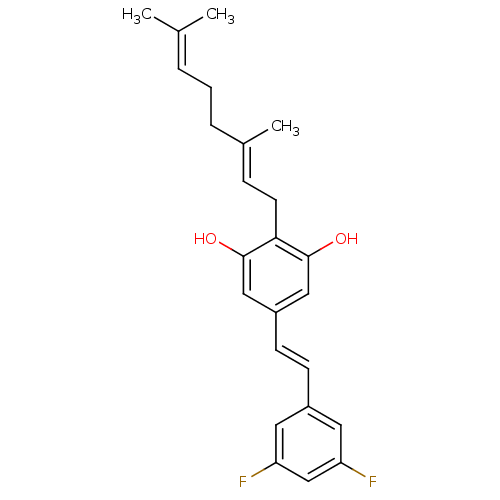
(CHEMBL2031952)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2cc(F)cc(F)c2)cc1-[#8] Show InChI InChI=1S/C24H26F2O2/c1-16(2)5-4-6-17(3)7-10-22-23(27)13-19(14-24(22)28)9-8-18-11-20(25)15-21(26)12-18/h5,7-9,11-15,27-28H,4,6,10H2,1-3H3/b9-8+,17-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50383932
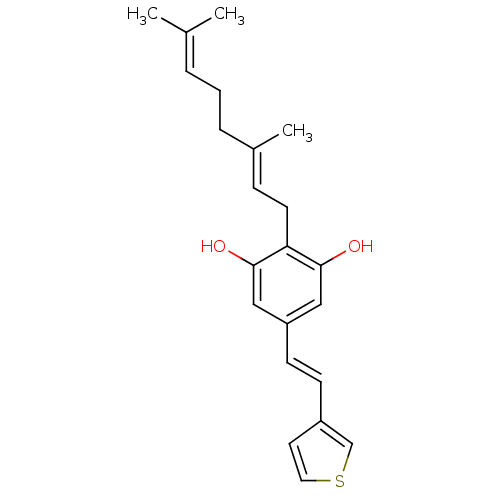
(CHEMBL2031957)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccsc2)cc1-[#8] Show InChI InChI=1S/C22H26O2S/c1-16(2)5-4-6-17(3)7-10-20-21(23)13-19(14-22(20)24)9-8-18-11-12-25-15-18/h5,7-9,11-15,23-24H,4,6,10H2,1-3H3/b9-8+,17-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50383926

(CHEMBL2031859)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccc(cc2)C#N)cc1-[#8] Show InChI InChI=1S/C25H27NO2/c1-18(2)5-4-6-19(3)7-14-23-24(27)15-22(16-25(23)28)13-10-20-8-11-21(17-26)12-9-20/h5,7-13,15-16,27-28H,4,6,14H2,1-3H3/b13-10+,19-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50383930

(CHEMBL2031955)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccc(-[#8])cc2)cc1-[#8] Show InChI InChI=1S/C24H28O3/c1-17(2)5-4-6-18(3)7-14-22-23(26)15-20(16-24(22)27)9-8-19-10-12-21(25)13-11-19/h5,7-13,15-16,25-27H,4,6,14H2,1-3H3/b9-8+,18-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARalpha by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50383925

(CHEMBL2031858)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2cccc(c2)C(F)(F)F)cc1-[#8] Show InChI InChI=1S/C25H27F3O2/c1-17(2)6-4-7-18(3)10-13-22-23(29)15-20(16-24(22)30)12-11-19-8-5-9-21(14-19)25(26,27)28/h5-6,8-12,14-16,29-30H,4,7,13H2,1-3H3/b12-11+,18-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARalpha by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50383922

(AMORPHASTILBOL)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccccc2)cc1-[#8] Show InChI InChI=1S/C24H28O2/c1-18(2)8-7-9-19(3)12-15-22-23(25)16-21(17-24(22)26)14-13-20-10-5-4-6-11-20/h4-6,8,10-14,16-17,25-26H,7,9,15H2,1-3H3/b14-13+,19-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARalpha by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344623
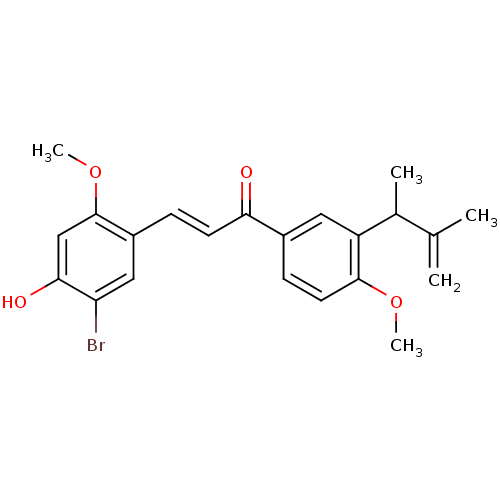
((E)-3-(5-Bromo-4-hydroxy-2-methoxyphenyl)-1-(4-met...)Show SMILES COc1cc(O)c(Br)cc1\C=C\C(=O)c1ccc(OC)c(c1)C(C)C(C)=C Show InChI InChI=1S/C22H23BrO4/c1-13(2)14(3)17-10-15(7-9-21(17)26-4)19(24)8-6-16-11-18(23)20(25)12-22(16)27-5/h6-12,14,25H,1H2,2-5H3/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50383931

(CHEMBL2031956)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccoc2)cc1-[#8] Show InChI InChI=1S/C22H26O3/c1-16(2)5-4-6-17(3)7-10-20-21(23)13-19(14-22(20)24)9-8-18-11-12-25-15-18/h5,7-9,11-15,23-24H,4,6,10H2,1-3H3/b9-8+,17-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARalpha by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50383928

(CHEMBL2031952)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2cc(F)cc(F)c2)cc1-[#8] Show InChI InChI=1S/C24H26F2O2/c1-16(2)5-4-6-17(3)7-10-22-23(27)13-19(14-24(22)28)9-8-18-11-20(25)15-21(26)12-18/h5,7-9,11-15,27-28H,4,6,10H2,1-3H3/b9-8+,17-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARalpha by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344610

((E)-4-(3-(5-Bromo-4-hydroxy-2-methoxyphenyl)acrylo...)Show SMILES COc1cc(O)c(Br)cc1\C=C\C(=O)c1ccc(OC(=O)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C23H16Br2O5/c1-29-22-13-21(27)19(25)12-16(22)6-11-20(26)14-4-9-18(10-5-14)30-23(28)15-2-7-17(24)8-3-15/h2-13,27H,1H3/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50383927

(CHEMBL2031860)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccc(F)cc2)cc1-[#8] Show InChI InChI=1S/C24H27FO2/c1-17(2)5-4-6-18(3)7-14-22-23(26)15-20(16-24(22)27)9-8-19-10-12-21(25)13-11-19/h5,7-13,15-16,26-27H,4,6,14H2,1-3H3/b9-8+,18-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARgamma by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344609
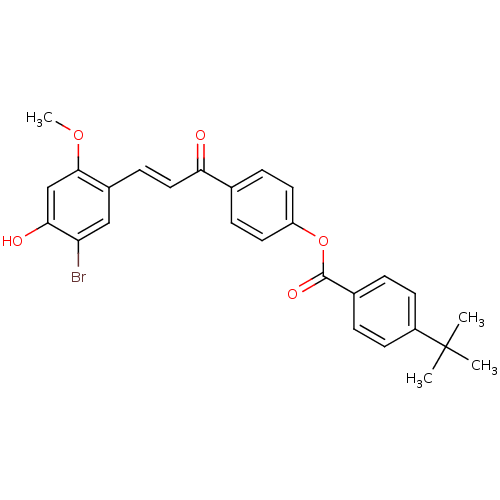
((E)-4-(3-(5-Bromo-4-hydroxy-2-methoxyphenyl)acrylo...)Show SMILES COc1cc(O)c(Br)cc1\C=C\C(=O)c1ccc(OC(=O)c2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C27H25BrO5/c1-27(2,3)20-10-5-18(6-11-20)26(31)33-21-12-7-17(8-13-21)23(29)14-9-19-15-22(28)24(30)16-25(19)32-4/h5-16,30H,1-4H3/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50383927

(CHEMBL2031860)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccc(F)cc2)cc1-[#8] Show InChI InChI=1S/C24H27FO2/c1-17(2)5-4-6-18(3)7-14-22-23(26)15-20(16-24(22)27)9-8-19-10-12-21(25)13-11-19/h5,7-13,15-16,26-27H,4,6,14H2,1-3H3/b9-8+,18-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARalpha by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50383923

(CHEMBL2031856)Show SMILES [#6]-[#8]-c1ccc(\[#6]=[#6]\c2cc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c2)cc1 Show InChI InChI=1S/C25H30O3/c1-18(2)6-5-7-19(3)8-15-23-24(26)16-21(17-25(23)27)10-9-20-11-13-22(28-4)14-12-20/h6,8-14,16-17,26-27H,5,7,15H2,1-4H3/b10-9+,19-8+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARalpha by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344625
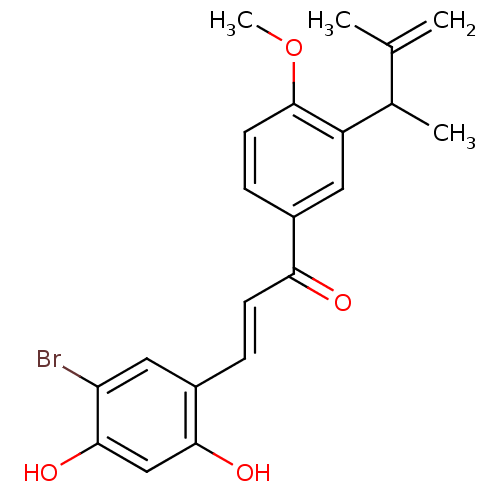
((E)-3-(5-Bromo-2,4-dihydroxyphenyl)-1-(4-methoxy-3...)Show SMILES COc1ccc(cc1C(C)C(C)=C)C(=O)\C=C\c1cc(Br)c(O)cc1O Show InChI InChI=1S/C21H21BrO4/c1-12(2)13(3)16-9-14(6-8-21(16)26-4)18(23)7-5-15-10-17(22)20(25)11-19(15)24/h5-11,13,24-25H,1H2,2-4H3/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344597
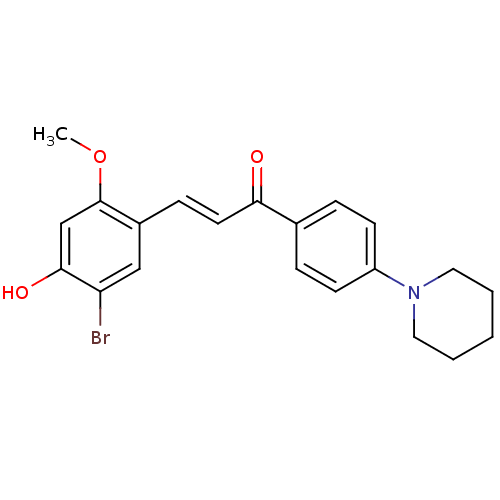
((E)-3-(5-Bromo-4-hydroxy-2-methoxyphenyl)-1-(4-(pi...)Show SMILES COc1cc(O)c(Br)cc1\C=C\C(=O)c1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C21H22BrNO3/c1-26-21-14-20(25)18(22)13-16(21)7-10-19(24)15-5-8-17(9-6-15)23-11-3-2-4-12-23/h5-10,13-14,25H,2-4,11-12H2,1H3/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344608
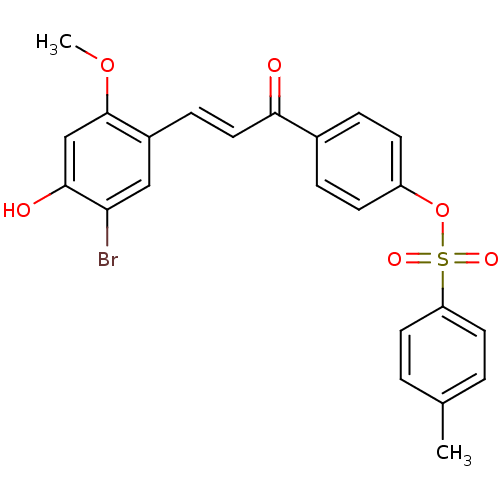
((E)-4-(3-(5-Bromo-4-hydroxy-2-methoxyphenyl)acrylo...)Show SMILES COc1cc(O)c(Br)cc1\C=C\C(=O)c1ccc(OS(=O)(=O)c2ccc(C)cc2)cc1 Show InChI InChI=1S/C23H19BrO6S/c1-15-3-10-19(11-4-15)31(27,28)30-18-8-5-16(6-9-18)21(25)12-7-17-13-20(24)22(26)14-23(17)29-2/h3-14,26H,1-2H3/b12-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50383933

(CHEMBL2031954)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccc(-[#6]=O)cc2)cc1-[#8] Show InChI InChI=1S/C25H28O3/c1-18(2)5-4-6-19(3)7-14-23-24(27)15-22(16-25(23)28)13-10-20-8-11-21(17-26)12-9-20/h5,7-13,15-17,27-28H,4,6,14H2,1-3H3/b13-10+,19-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARalpha by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344616
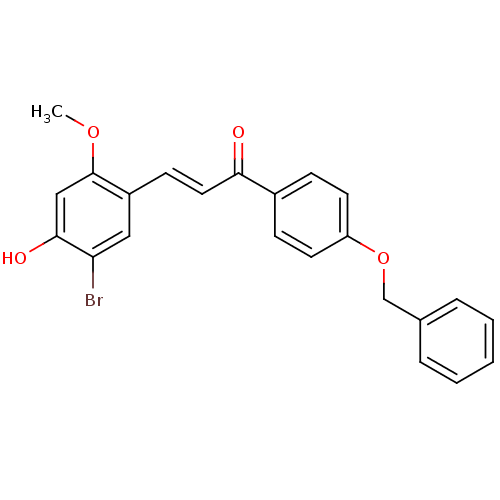
((E)-1-(4-(Benzyloxy)phenyl)-3-(5-bromo-4-hydroxy-2...)Show SMILES COc1cc(O)c(Br)cc1\C=C\C(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C23H19BrO4/c1-27-23-14-22(26)20(24)13-18(23)9-12-21(25)17-7-10-19(11-8-17)28-15-16-5-3-2-4-6-16/h2-14,26H,15H2,1H3/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344627
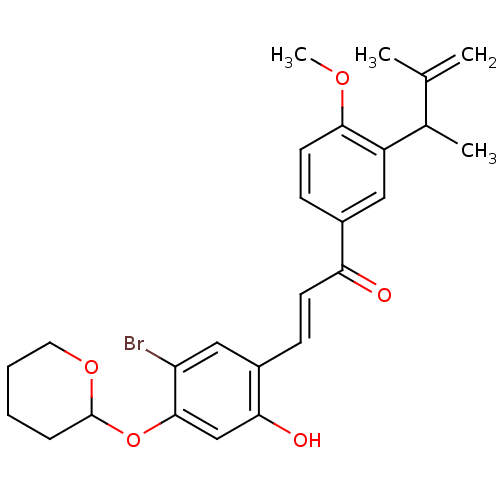
((E)-3-(5-bromo-2-hydroxy-4-(tetrahydro-2H-pyran-2-...)Show SMILES COc1ccc(cc1C(C)C(C)=C)C(=O)\C=C\c1cc(Br)c(OC2CCCCO2)cc1O Show InChI InChI=1S/C26H29BrO5/c1-16(2)17(3)20-13-18(9-11-24(20)30-4)22(28)10-8-19-14-21(27)25(15-23(19)29)32-26-7-5-6-12-31-26/h8-11,13-15,17,26,29H,1,5-7,12H2,2-4H3/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344586
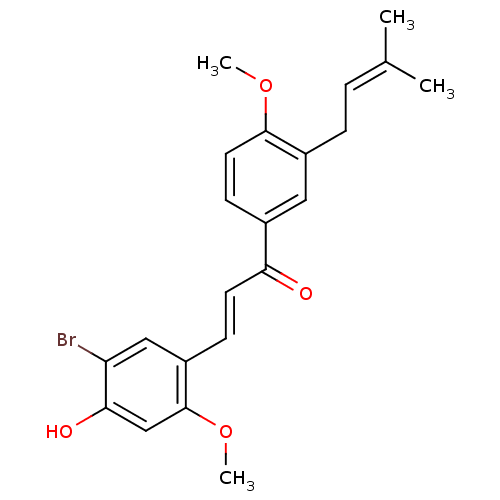
((E)-3-(5-Bromo-4-hydroxy-2-methoxyphenyl)-1-(4-met...)Show SMILES [#6]-[#8]-c1ccc(cc1-[#6]\[#6]=[#6](/[#6])-[#6])-[#6](=O)\[#6]=[#6]\c1cc(Br)c(-[#8])cc1-[#8]-[#6] Show InChI InChI=1S/C22H23BrO4/c1-14(2)5-6-16-11-15(8-10-21(16)26-3)19(24)9-7-17-12-18(23)20(25)13-22(17)27-4/h5,7-13,25H,6H2,1-4H3/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50383924

(CHEMBL2031857)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccc(cc2)C(F)(F)F)cc1-[#8] Show InChI InChI=1S/C25H27F3O2/c1-17(2)5-4-6-18(3)7-14-22-23(29)15-20(16-24(22)30)9-8-19-10-12-21(13-11-19)25(26,27)28/h5,7-13,15-16,29-30H,4,6,14H2,1-3H3/b9-8+,18-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARalpha by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344591
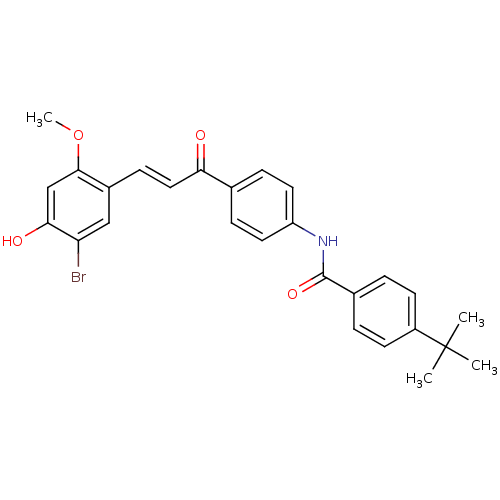
((E)-N-(4-(3-(5-bromo-4-hydroxy-2-methoxyphenyl)acr...)Show SMILES COc1cc(O)c(Br)cc1\C=C\C(=O)c1ccc(NC(=O)c2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C27H26BrNO4/c1-27(2,3)20-10-5-18(6-11-20)26(32)29-21-12-7-17(8-13-21)23(30)14-9-19-15-22(28)24(31)16-25(19)33-4/h5-16,31H,1-4H3,(H,29,32)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344600
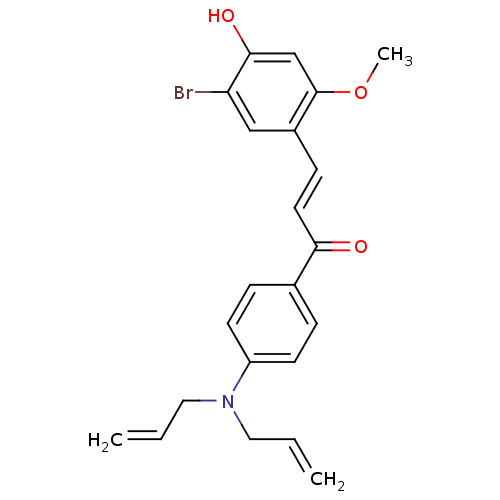
((E)-3-(5-Bromo-4-hydroxy-2-methoxyphenyl)-1-(4-(di...)Show SMILES COc1cc(O)c(Br)cc1\C=C\C(=O)c1ccc(cc1)N(CC=C)CC=C Show InChI InChI=1S/C22H22BrNO3/c1-4-12-24(13-5-2)18-9-6-16(7-10-18)20(25)11-8-17-14-19(23)21(26)15-22(17)27-3/h4-11,14-15,26H,1-2,12-13H2,3H3/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50383926

(CHEMBL2031859)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(\[#6]=[#6]\c2ccc(cc2)C#N)cc1-[#8] Show InChI InChI=1S/C25H27NO2/c1-18(2)5-4-6-19(3)7-14-23-24(27)15-22(16-25(23)28)13-10-20-8-11-21(17-26)12-9-20/h5,7-13,15-16,27-28H,4,6,14H2,1-3H3/b13-10+,19-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPARalpha by TR-FRET analysis |
Bioorg Med Chem Lett 22: 4122-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.062
BindingDB Entry DOI: 10.7270/Q2TM7C5Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344599
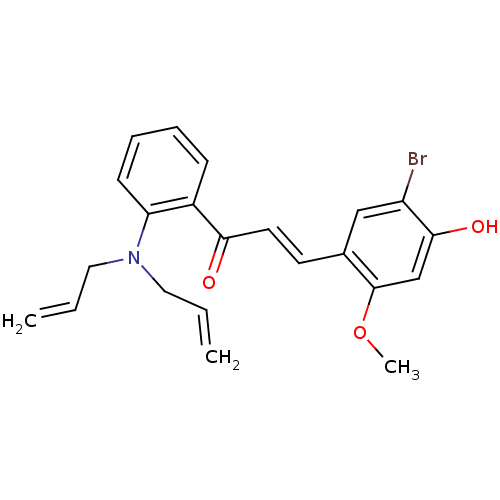
((E)-3-(5-Bromo-4-hydroxy-2-methoxyphenyl)-1-(2-(di...)Show SMILES COc1cc(O)c(Br)cc1\C=C\C(=O)c1ccccc1N(CC=C)CC=C Show InChI InChI=1S/C22H22BrNO3/c1-4-12-24(13-5-2)19-9-7-6-8-17(19)20(25)11-10-16-14-18(23)21(26)15-22(16)27-3/h4-11,14-15,26H,1-2,12-13H2,3H3/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
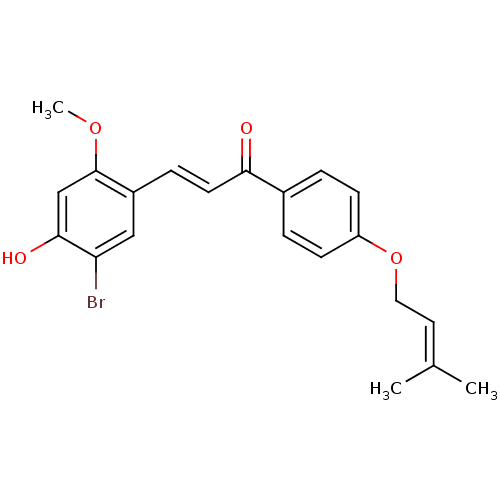
(Homo sapiens (Human)) | BDBM50344617
((E)-3-(5-Bromo-4-hydroxy-2-methoxyphenyl)-1-(4-(3-...)Show SMILES [#6]-[#8]-c1cc(-[#8])c(Br)cc1\[#6]=[#6]\[#6](=O)-c1ccc(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])cc1 Show InChI InChI=1S/C21H21BrO4/c1-14(2)10-11-26-17-7-4-15(5-8-17)19(23)9-6-16-12-18(22)20(24)13-21(16)25-3/h4-10,12-13,24H,11H2,1-3H3/b9-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B assessed as hydrolysis of p-nitrophenyl phosphate after 30 mins |
Bioorg Med Chem Lett 19: 5155-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.054
BindingDB Entry DOI: 10.7270/Q24X58R7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using pNPP substrate at 37 degC measured after 30 mins |
Bioorg Med Chem Lett 24: 3337-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.099
BindingDB Entry DOI: 10.7270/Q29888N1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
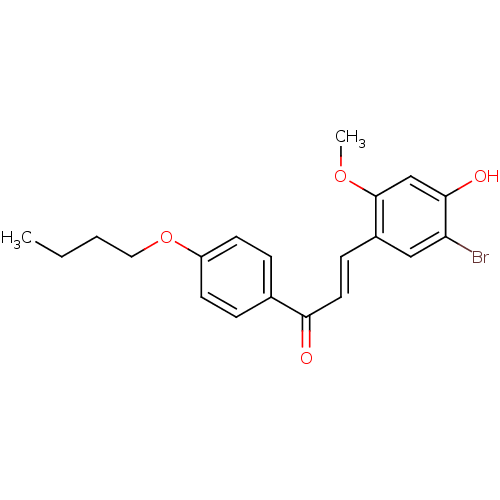
(Homo sapiens (Human)) | BDBM50344618
((E)-3-(5-Bromo-4-hydroxy-2-methoxyphenyl)-1-(4-but...)Show InChI InChI=1S/C20H21BrO4/c1-3-4-11-25-16-8-5-14(6-9-16)18(22)10-7-15-12-17(21)19(23)13-20(15)24-2/h5-10,12-13,23H,3-4,11H2,1-2H3/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
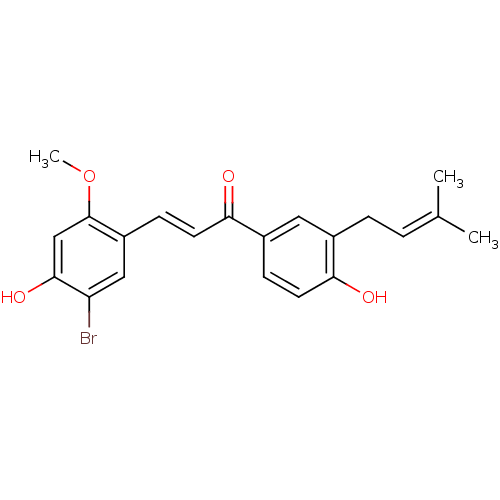
(Homo sapiens (Human)) | BDBM50344589
((E)-3-(5-Bromo-4-hydroxy-2-methoxyphenyl)-1-(4-hyd...)Show SMILES [#6]-[#8]-c1cc(-[#8])c(Br)cc1\[#6]=[#6]\[#6](=O)-c1ccc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6])c1 Show InChI InChI=1S/C21H21BrO4/c1-13(2)4-5-14-10-15(6-8-18(14)23)19(24)9-7-16-11-17(22)20(25)12-21(16)26-3/h4,6-12,23,25H,5H2,1-3H3/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344598
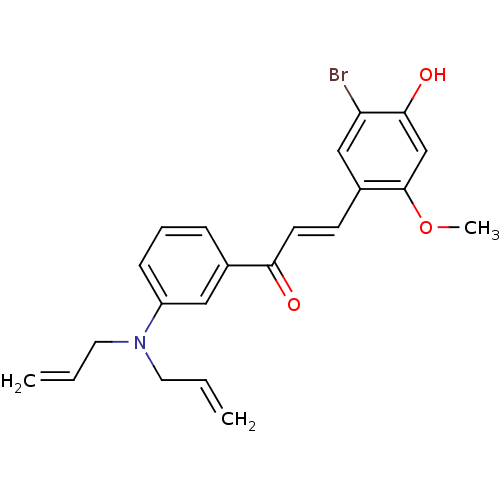
((E)-3-(5-Bromo-4-hydroxy-2-methoxyphenyl)-1-(3-(di...)Show SMILES COc1cc(O)c(Br)cc1\C=C\C(=O)c1cccc(c1)N(CC=C)CC=C Show InChI InChI=1S/C22H22BrNO3/c1-4-11-24(12-5-2)18-8-6-7-16(13-18)20(25)10-9-17-14-19(23)21(26)15-22(17)27-3/h4-10,13-15,26H,1-2,11-12H2,3H3/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50046694
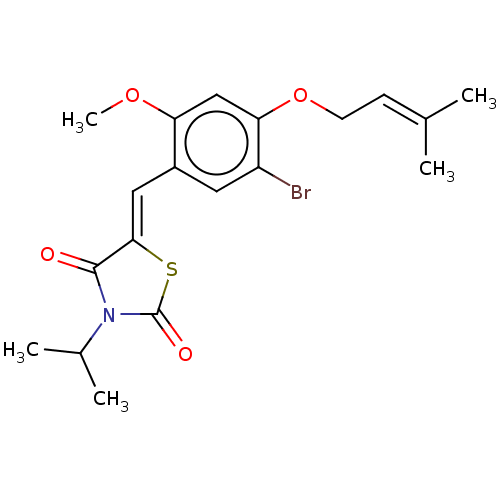
(CHEMBL3310437)Show SMILES [#6]-[#8]-c1cc(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c(Br)cc1\[#6]=[#6]-1/[#16]-[#6](=O)-[#7](-[#6](-[#6])-[#6])-[#6]-1=O Show InChI InChI=1S/C19H22BrNO4S/c1-11(2)6-7-25-16-10-15(24-5)13(8-14(16)20)9-17-18(22)21(12(3)4)19(23)26-17/h6,8-10,12H,7H2,1-5H3/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using pNPP substrate at 37 degC measured after 30 mins |
Bioorg Med Chem Lett 24: 3337-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.099
BindingDB Entry DOI: 10.7270/Q29888N1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50344611
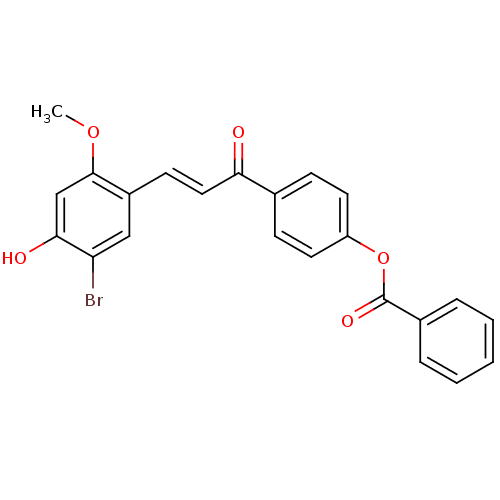
((E)-4-(3-(5-Bromo-4-hydroxy-2-methoxyphenyl)acrylo...)Show SMILES COc1cc(O)c(Br)cc1\C=C\C(=O)c1ccc(OC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C23H17BrO5/c1-28-22-14-21(26)19(24)13-17(22)9-12-20(25)15-7-10-18(11-8-15)29-23(27)16-5-3-2-4-6-16/h2-14,26H,1H3/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem Lett 21: 3755-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.057
BindingDB Entry DOI: 10.7270/Q23J3D96 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data