

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9277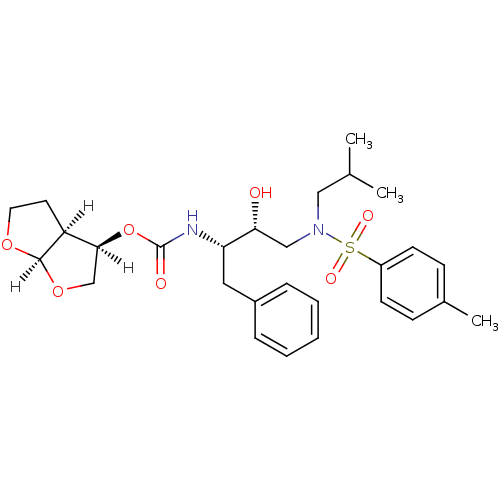 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9270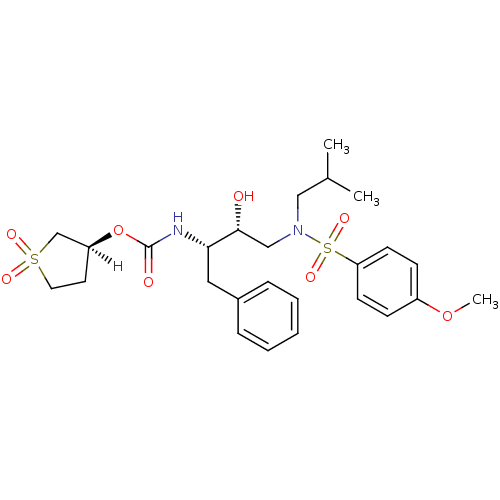 ((3S)-1,1-dioxo--thiolan-3-yl N-[(2S,3R)-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607 Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against HIV protease | Bioorg Med Chem Lett 8: 979-82 (1999) BindingDB Entry DOI: 10.7270/Q2D79BXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9271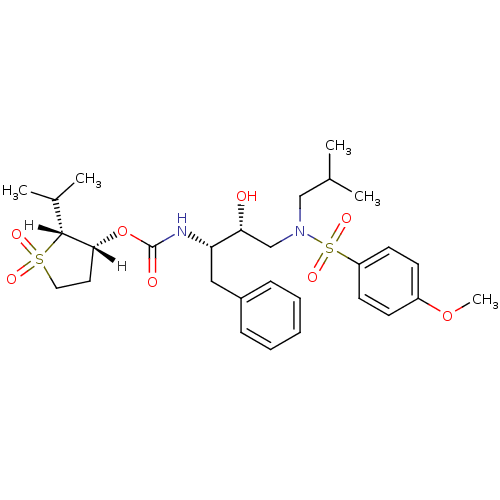 ((2R,3R)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9273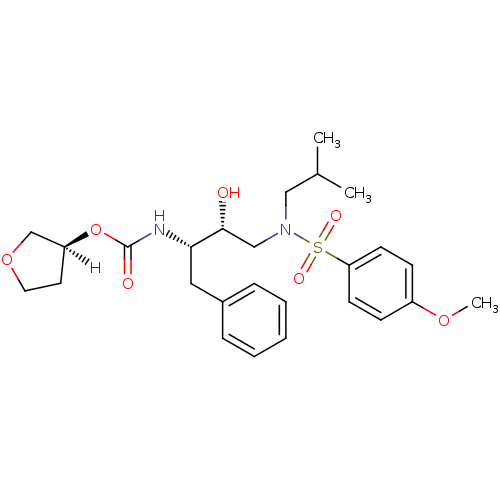 ((3S)-oxolan-3-yl N-[(2S,3R)-3-hydroxy-4-[(4-methox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9272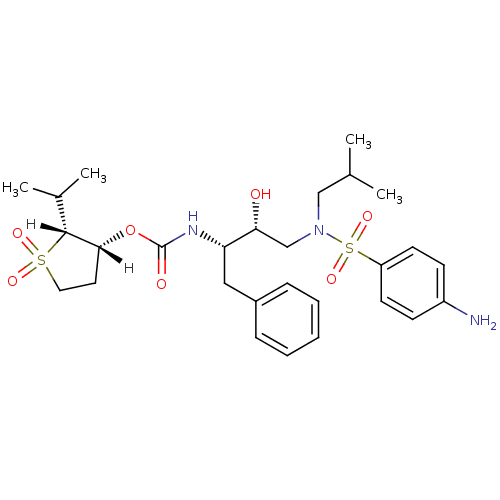 ((2R,3R)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9278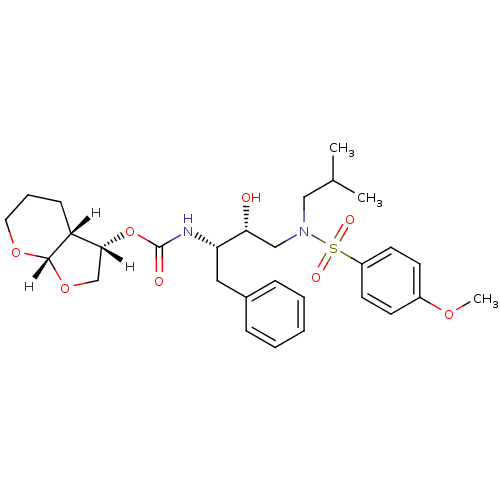 ((3S,3aR,7aS)-hexahydro-2H-furo[2,3-b]pyran-3-yl N-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | -49.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9269 ((3S)-thiolan-3-yl N-[(2S,3R)-3-hydroxy-4-[(4-metho...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | -49.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 8: 687-90 (1998) Article DOI: 10.1016/s0960-894x(98)00098-5 BindingDB Entry DOI: 10.7270/Q2J101DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069005 ((5R,7S)-1,8-Dioxa-spiro[4.5]decane-7-carboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607 Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against HIV protease | Bioorg Med Chem Lett 8: 979-82 (1999) BindingDB Entry DOI: 10.7270/Q2D79BXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069010 ((5S,7S)-1,8-Dioxa-spiro[4.5]decane-7-carboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607 Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against HIV protease | Bioorg Med Chem Lett 8: 979-82 (1999) BindingDB Entry DOI: 10.7270/Q2D79BXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069009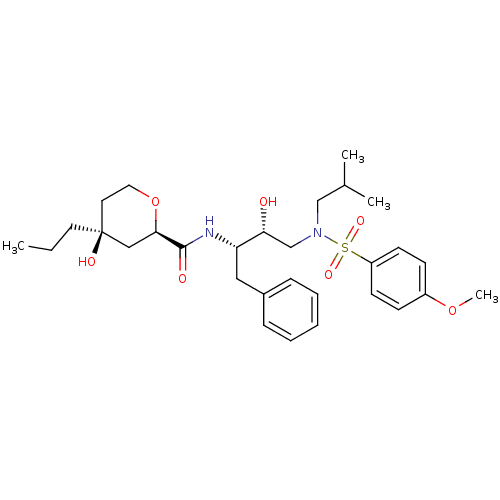 ((2R,4S)-4-Hydroxy-4-propyl-tetrahydro-pyran-2-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607 Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against HIV protease | Bioorg Med Chem Lett 8: 979-82 (1999) BindingDB Entry DOI: 10.7270/Q2D79BXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069007 ((5R,7R)-1,8-Dioxa-spiro[4.5]decane-7-carboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607 Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against HIV protease | Bioorg Med Chem Lett 8: 979-82 (1999) BindingDB Entry DOI: 10.7270/Q2D79BXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069002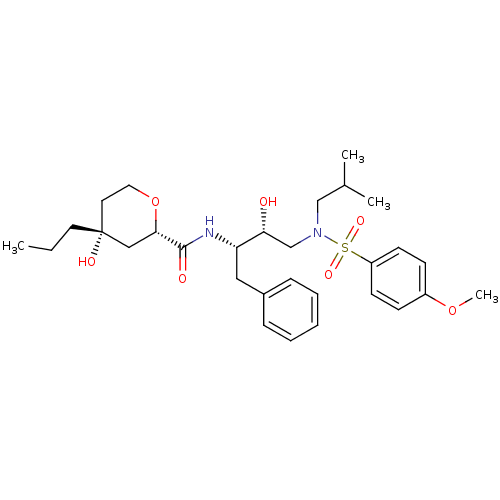 ((2S,4R)-4-Hydroxy-4-propyl-tetrahydro-pyran-2-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607 Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against HIV protease | Bioorg Med Chem Lett 8: 979-82 (1999) BindingDB Entry DOI: 10.7270/Q2D79BXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069004 ((2S,4S)-4-Hydroxy-4-propyl-tetrahydro-pyran-2-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607 Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against HIV protease | Bioorg Med Chem Lett 8: 979-82 (1999) BindingDB Entry DOI: 10.7270/Q2D79BXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069003 ((2R,4R)-4-Hydroxy-4-propyl-tetrahydro-pyran-2-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607 Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against HIV protease | Bioorg Med Chem Lett 8: 979-82 (1999) BindingDB Entry DOI: 10.7270/Q2D79BXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069006 (8-Oxa-spiro[4.5]decane-7-carboxylic acid {(1S,2R)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607 Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against HIV protease | Bioorg Med Chem Lett 8: 979-82 (1999) BindingDB Entry DOI: 10.7270/Q2D79BXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069001 ((5S,7R)-1,8-Dioxa-spiro[4.5]decane-7-carboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607 Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against HIV protease | Bioorg Med Chem Lett 8: 979-82 (1999) BindingDB Entry DOI: 10.7270/Q2D79BXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116303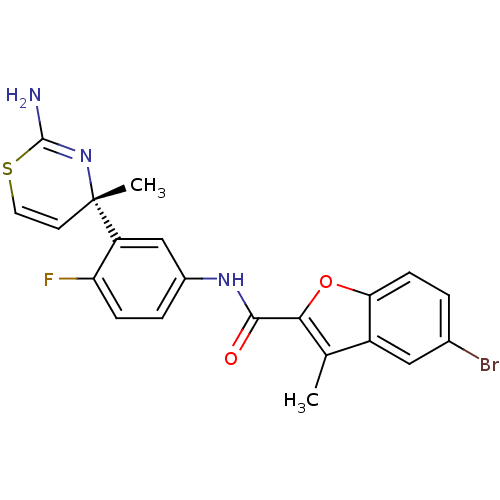 (US8637504, 81 | US9273053, 81 | US9650371, 81) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 μL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well ... | US Patent US9273053 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116303 (US8637504, 81 | US9273053, 81 | US9650371, 81) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 uL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well of... | US Patent US9650371 (2017) BindingDB Entry DOI: 10.7270/Q2M047HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50170264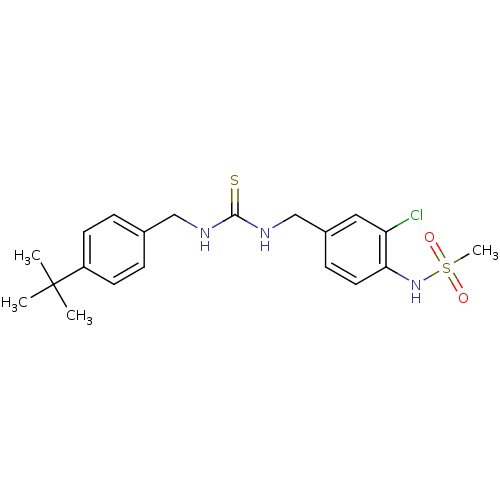 (CHEMBL361931 | N-{4-[3-(4-tert-Butyl-benzyl)-thiou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibitory effect on capsaicin (0.5 uM)-induced calcium uptake in rat DRG neurons upon incubation at RT for 10 minutes | J Med Chem 48: 5823-36 (2005) Article DOI: 10.1021/jm0502790 BindingDB Entry DOI: 10.7270/Q2057FGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM101023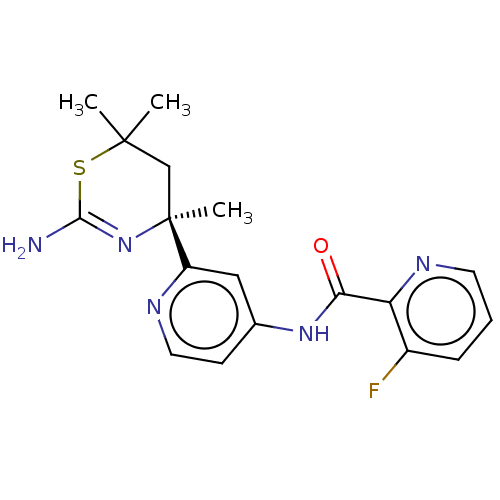 (US8507479, 655) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description Inhibition assay using human BACE-1. | US Patent US8541408 (2013) BindingDB Entry DOI: 10.7270/Q2KK99D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116274 (US8637504, 16 | US9270353, 16 | US9650371, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 uL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well of... | US Patent US9650371 (2017) BindingDB Entry DOI: 10.7270/Q2M047HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116274 (US8637504, 16 | US9270353, 16 | US9650371, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 μL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well ... | US Patent US9273053 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM101023 (US8507479, 655) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description Beta-Secretase inhibition assay. | US Patent US8507479 (2013) BindingDB Entry DOI: 10.7270/Q2KS6Q6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM101030 (US8507479, 674) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description Beta-Secretase inhibition assay. | US Patent US8507479 (2013) BindingDB Entry DOI: 10.7270/Q2KS6Q6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM101029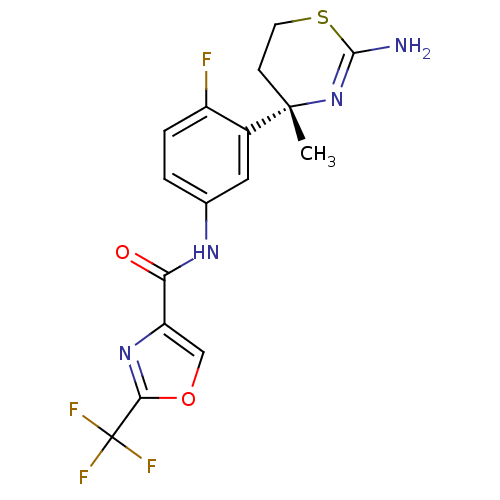 (US8507479, 669) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description Beta-Secretase inhibition assay. | US Patent US8507479 (2013) BindingDB Entry DOI: 10.7270/Q2KS6Q6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM101030 (US8507479, 674) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description Inhibition assay using human BACE-1. | US Patent US8541408 (2013) BindingDB Entry DOI: 10.7270/Q2KK99D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM101029 (US8507479, 669) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description Inhibition assay using human BACE-1. | US Patent US8541408 (2013) BindingDB Entry DOI: 10.7270/Q2KK99D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120379 (US8703785, 44) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Shionogi & Co., Ltd. US Patent | Assay Description First, 48.5 μl portions of a solution of substrate peptide (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) we... | US Patent US8703785 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116304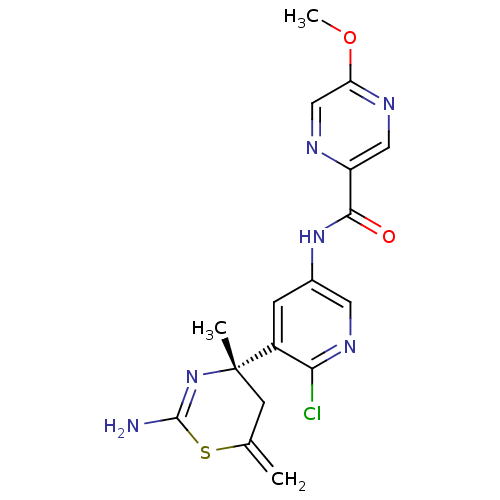 (US8637504, 99 | US9273053, 99 | US9650371, 99) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 uL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well of... | US Patent US9650371 (2017) BindingDB Entry DOI: 10.7270/Q2M047HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120377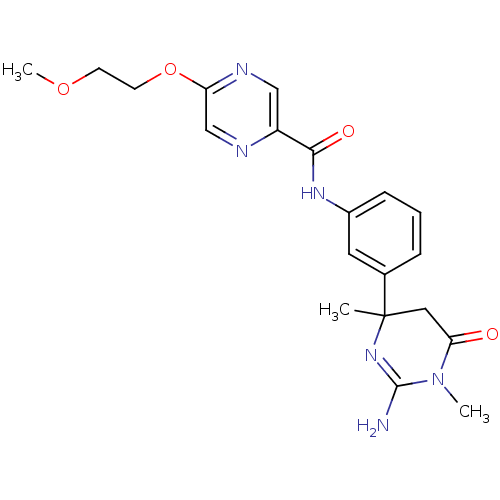 (US8703785, 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Shionogi & Co., Ltd. US Patent | Assay Description First, 48.5 μl portions of a solution of substrate peptide (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) we... | US Patent US8703785 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116304 (US8637504, 99 | US9273053, 99 | US9650371, 99) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 μL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well ... | US Patent US9273053 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116288 (US8637504, 51 | US9273053, 51 | US9650371, 51) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 uL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well of... | US Patent US9650371 (2017) BindingDB Entry DOI: 10.7270/Q2M047HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116288 (US8637504, 51 | US9273053, 51 | US9650371, 51) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 μL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well ... | US Patent US9273053 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116292 (US8637504, 59 | US9273053, 59 | US9650371, 59) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 μL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well ... | US Patent US9273053 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116292 (US8637504, 59 | US9273053, 59 | US9650371, 59) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 uL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well of... | US Patent US9650371 (2017) BindingDB Entry DOI: 10.7270/Q2M047HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM308068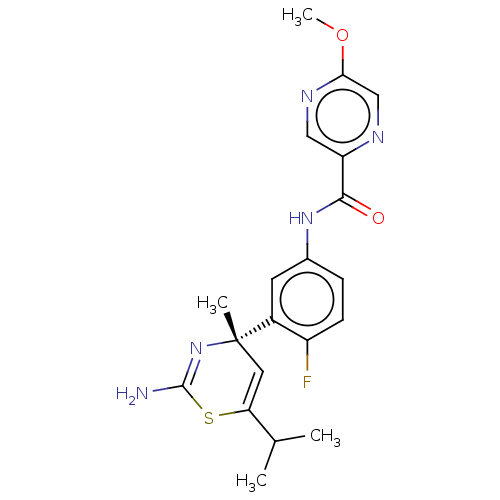 (Compound 200 | US9650371, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 uL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well of... | US Patent US9650371 (2017) BindingDB Entry DOI: 10.7270/Q2M047HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116276 (US8637504, 21 | US9273053, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 μL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well ... | US Patent US9273053 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM101026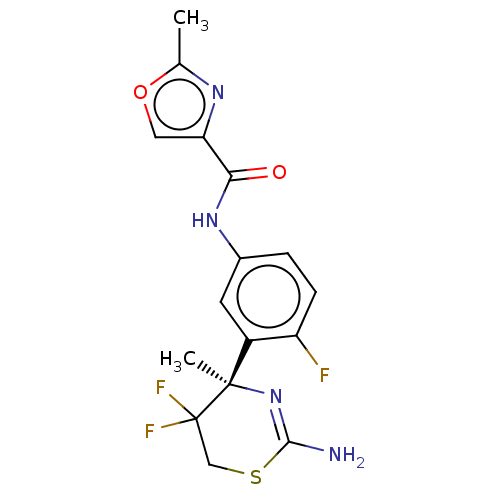 (US8507479, 664) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description Beta-Secretase inhibition assay. | US Patent US8507479 (2013) BindingDB Entry DOI: 10.7270/Q2KS6Q6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM101026 (US8507479, 664) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description Inhibition assay using human BACE-1. | US Patent US8541408 (2013) BindingDB Entry DOI: 10.7270/Q2KK99D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116305 (US8637504, 101 | US9273053, 101 | US9650371, 101) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 uL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well of... | US Patent US9650371 (2017) BindingDB Entry DOI: 10.7270/Q2M047HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116305 (US8637504, 101 | US9273053, 101 | US9650371, 101) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 μL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well ... | US Patent US9273053 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116302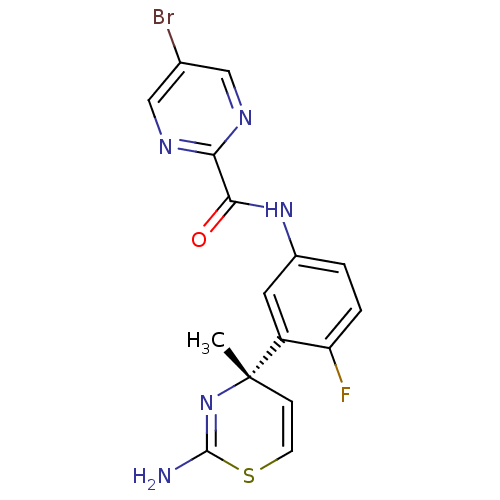 (US8637504, 79 | US9273053, 79 | US9650371, 79) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 uL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well of... | US Patent US9650371 (2017) BindingDB Entry DOI: 10.7270/Q2M047HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116302 (US8637504, 79 | US9273053, 79 | US9650371, 79) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 μL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well ... | US Patent US9273053 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116297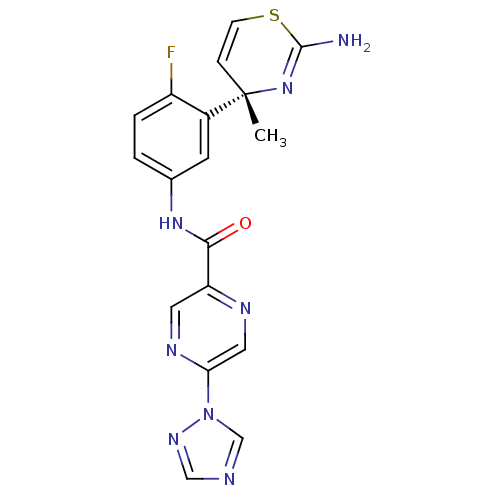 (US8637504, 70 | US9273053, 70 | US9650371, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 μL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well ... | US Patent US9273053 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116306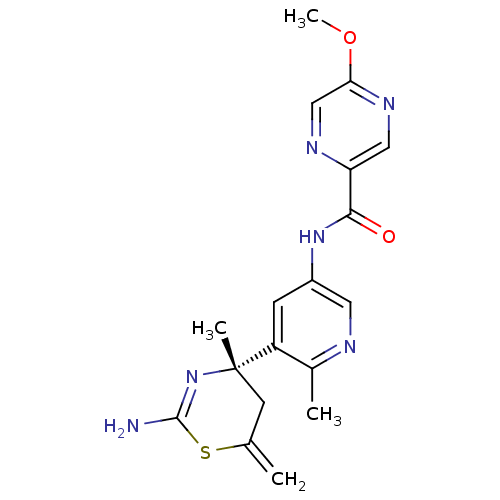 (US8637504, 102 | US9273053, 102 | US9650371, 102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 uL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well of... | US Patent US9650371 (2017) BindingDB Entry DOI: 10.7270/Q2M047HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116297 (US8637504, 70 | US9273053, 70 | US9650371, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 uL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well of... | US Patent US9650371 (2017) BindingDB Entry DOI: 10.7270/Q2M047HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116306 (US8637504, 102 | US9273053, 102 | US9650371, 102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 μL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well ... | US Patent US9273053 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM20321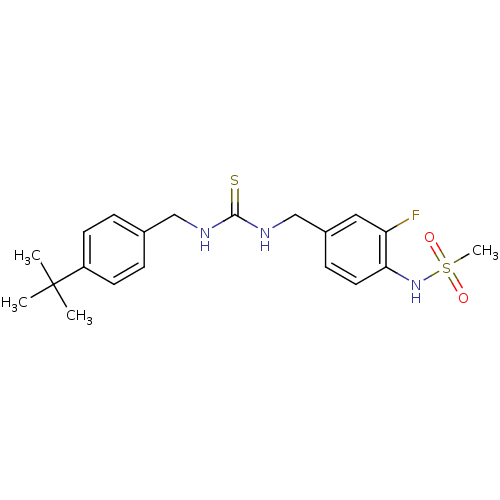 (3-[(4-tert-butylphenyl)methyl]-1-[(3-fluoro-4-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of [Ca2+] uptake in dorsal root ganglion (DRG) neurons from neonatal Sprague-Dawley rat | Bioorg Med Chem Lett 13: 4389-93 (2003) BindingDB Entry DOI: 10.7270/Q2KH0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20321 (3-[(4-tert-butylphenyl)methyl]-1-[(3-fluoro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibitory effect on capsaicin (0.5 uM)-induced calcium uptake in rat DRG neurons upon incubation at RT for 10 minutes | J Med Chem 48: 5823-36 (2005) Article DOI: 10.1021/jm0502790 BindingDB Entry DOI: 10.7270/Q2057FGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3428 total ) | Next | Last >> |