

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.574 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P1 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Rattus norvegicus) | BDBM50250631 (CHEMBL4093489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.772 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from rat S1P1 receptor after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50375611 (CHEMBL408120) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University Curated by ChEMBL | Assay Description Inhibition of PP1gamma by firefly bioluminescence assay | Bioorg Med Chem 16: 1747-55 (2008) Article DOI: 10.1016/j.bmc.2007.11.034 BindingDB Entry DOI: 10.7270/Q2SJ1MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50375608 (CHEMBL406220) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University Curated by ChEMBL | Assay Description Inhibition of PP1gamma by firefly bioluminescence assay | Bioorg Med Chem 16: 1747-55 (2008) Article DOI: 10.1016/j.bmc.2007.11.034 BindingDB Entry DOI: 10.7270/Q2SJ1MHT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50375616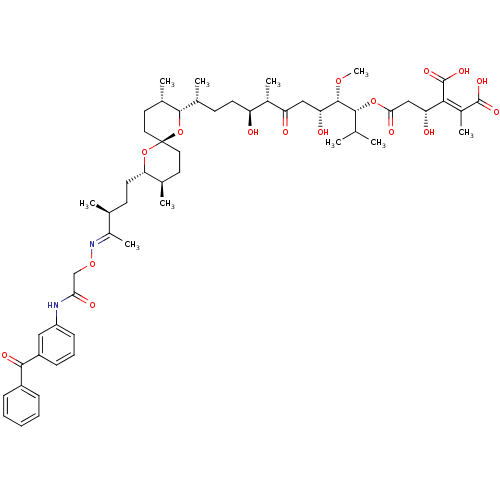 (CHEMBL405248) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University Curated by ChEMBL | Assay Description Inhibition of PP1gamma by firefly bioluminescence assay | Bioorg Med Chem 16: 1747-55 (2008) Article DOI: 10.1016/j.bmc.2007.11.034 BindingDB Entry DOI: 10.7270/Q2SJ1MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50375613 (CHEMBL261577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University Curated by ChEMBL | Assay Description Inhibition of PP1gamma by firefly bioluminescence assay | Bioorg Med Chem 16: 1747-55 (2008) Article DOI: 10.1016/j.bmc.2007.11.034 BindingDB Entry DOI: 10.7270/Q2SJ1MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50375612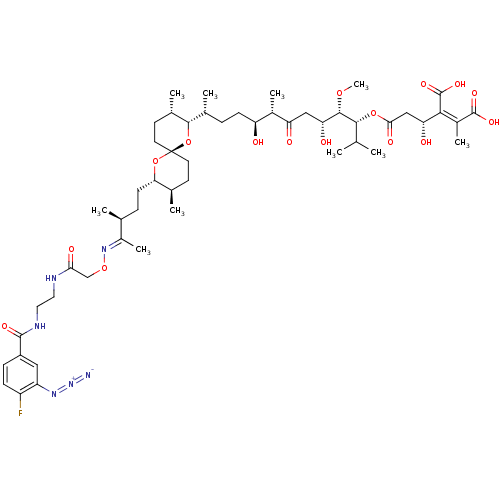 (CHEMBL427915) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University Curated by ChEMBL | Assay Description Inhibition of PP1gamma by firefly bioluminescence assay | Bioorg Med Chem 16: 1747-55 (2008) Article DOI: 10.1016/j.bmc.2007.11.034 BindingDB Entry DOI: 10.7270/Q2SJ1MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50375609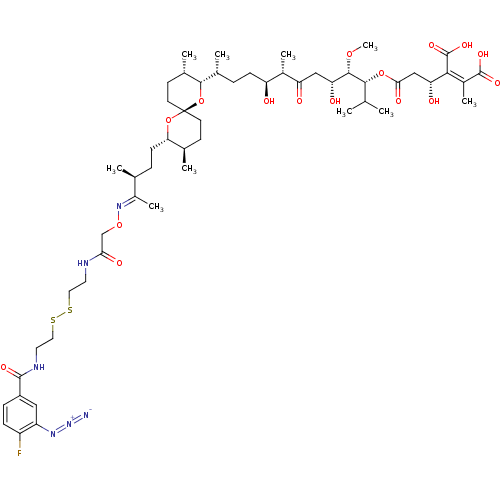 (CHEMBL406283) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 19.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University Curated by ChEMBL | Assay Description Inhibition of PP1gamma by firefly bioluminescence assay | Bioorg Med Chem 16: 1747-55 (2008) Article DOI: 10.1016/j.bmc.2007.11.034 BindingDB Entry DOI: 10.7270/Q2SJ1MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50375614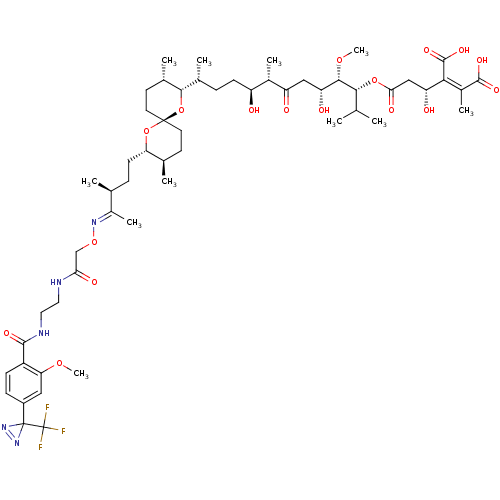 (CHEMBL259235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University Curated by ChEMBL | Assay Description Inhibition of PP1gamma by firefly bioluminescence assay | Bioorg Med Chem 16: 1747-55 (2008) Article DOI: 10.1016/j.bmc.2007.11.034 BindingDB Entry DOI: 10.7270/Q2SJ1MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50375610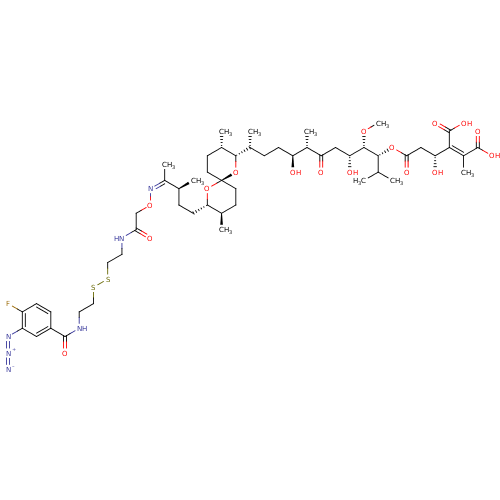 (CHEMBL436904) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 23.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University Curated by ChEMBL | Assay Description Inhibition of PP1gamma by firefly bioluminescence assay | Bioorg Med Chem 16: 1747-55 (2008) Article DOI: 10.1016/j.bmc.2007.11.034 BindingDB Entry DOI: 10.7270/Q2SJ1MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P4 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50375617 (CHEMBL261094) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University Curated by ChEMBL | Assay Description Inhibition of PP1gamma by firefly bioluminescence assay | Bioorg Med Chem 16: 1747-55 (2008) Article DOI: 10.1016/j.bmc.2007.11.034 BindingDB Entry DOI: 10.7270/Q2SJ1MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50375615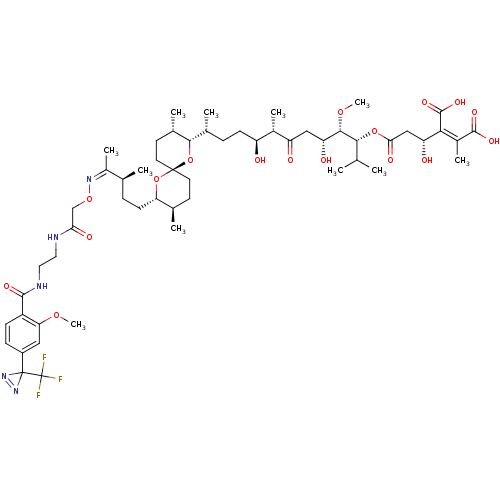 (CHEMBL261583) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University Curated by ChEMBL | Assay Description Inhibition of PP1gamma by firefly bioluminescence assay | Bioorg Med Chem 16: 1747-55 (2008) Article DOI: 10.1016/j.bmc.2007.11.034 BindingDB Entry DOI: 10.7270/Q2SJ1MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P2 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >5.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P3 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514126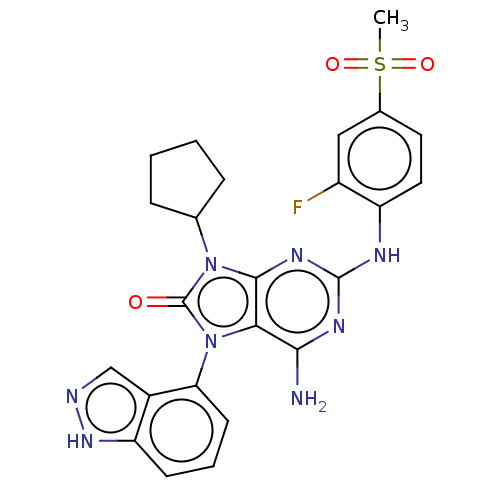 (US11052091, Example 5-1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514134 (US11052091, Example 5-16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581757 (US11512087, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514136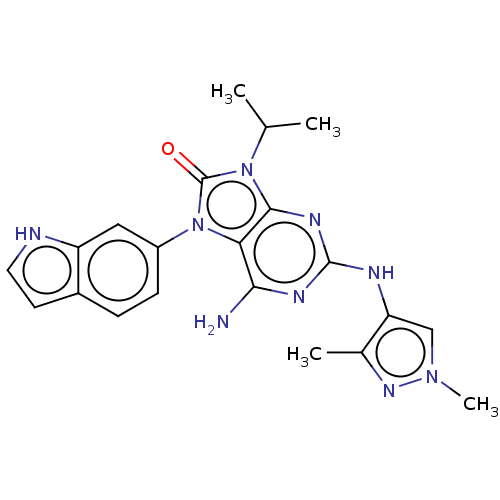 (US11052091, Example 5-18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514135 (US11052091, Example 5-17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514137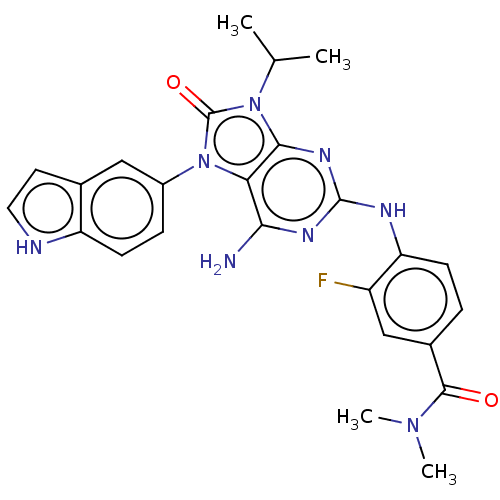 (US11052091, Example 5-19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581831 (US11512087, Example 13-1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581837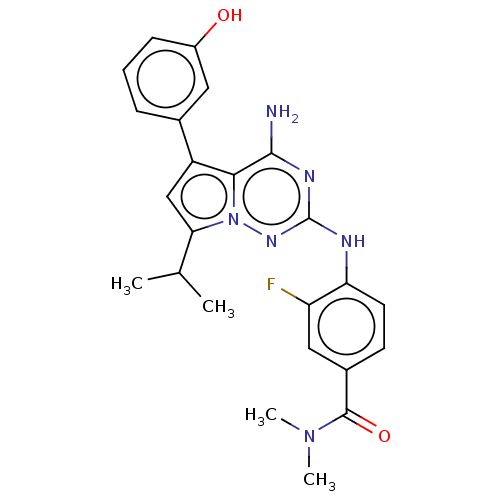 (US11512087, Example 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581838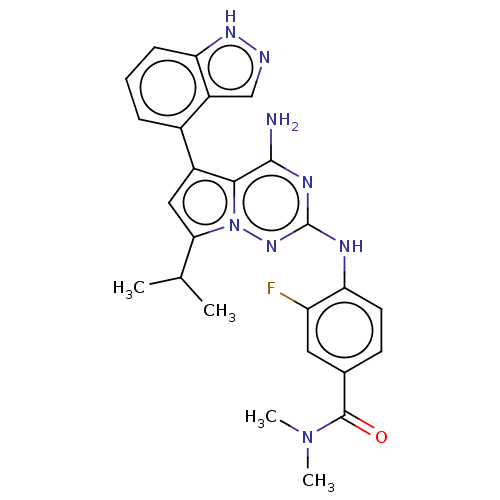 (US11512087, Example 26-1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581832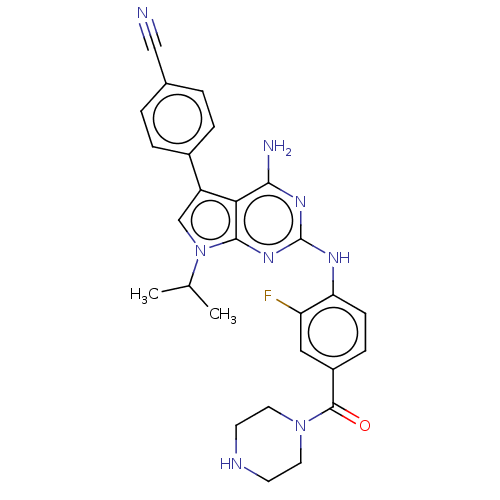 (US11512087, Example 13-9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514127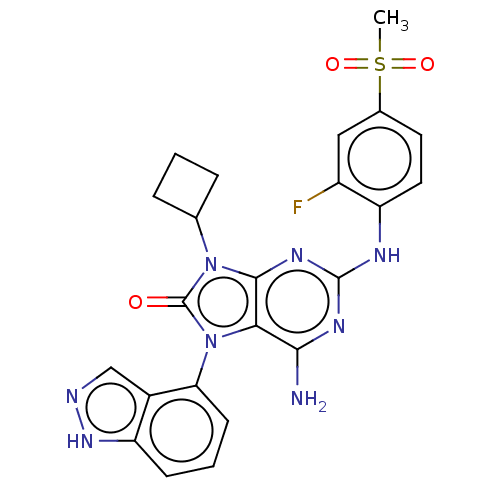 (US11052091, Example 5-2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514138 (US11052091, Example 5-21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514124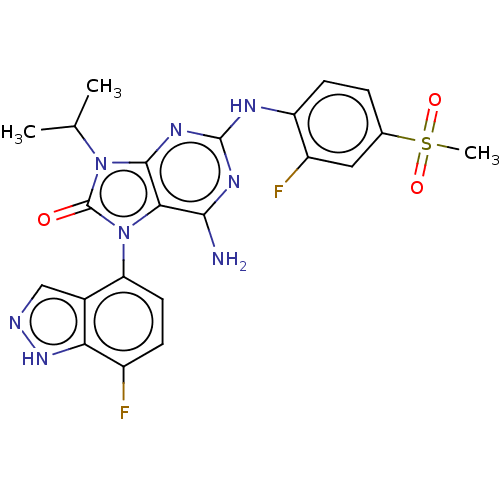 (US11052091, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581830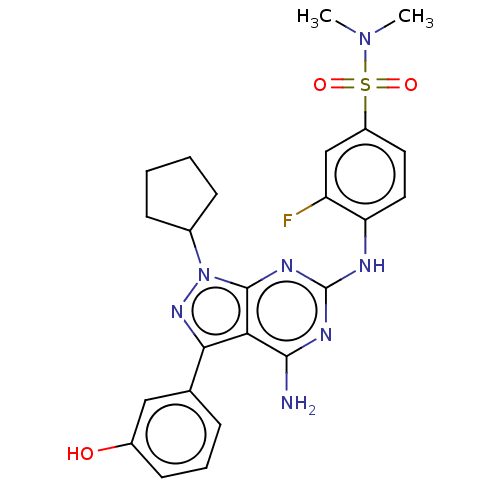 (US11512087, Example 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581795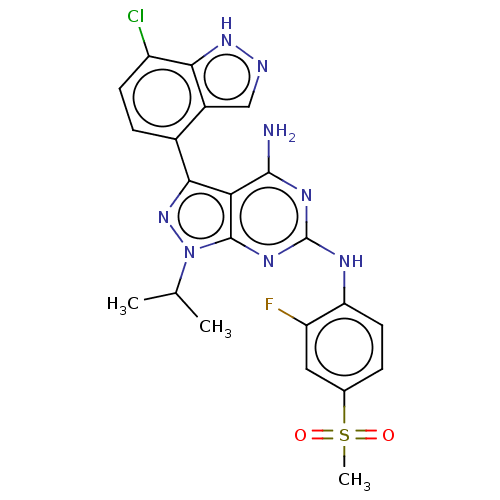 (US11512087, Example 4-1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581780 (US11512087, Example 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514128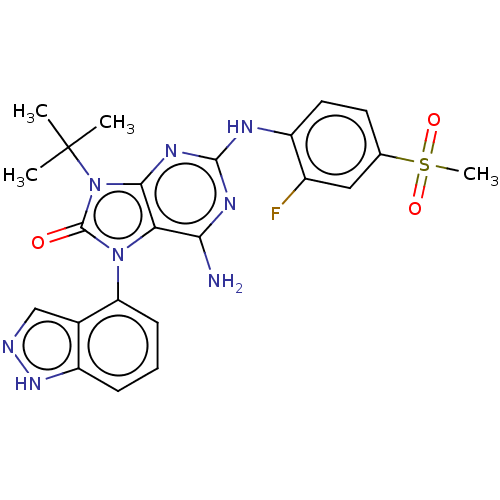 (US11052091, Example 5-3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514129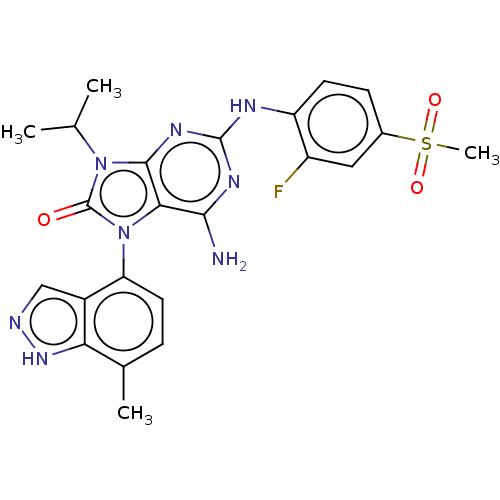 (US11052091, Example 5-4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581829 (US11512087, Example 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514139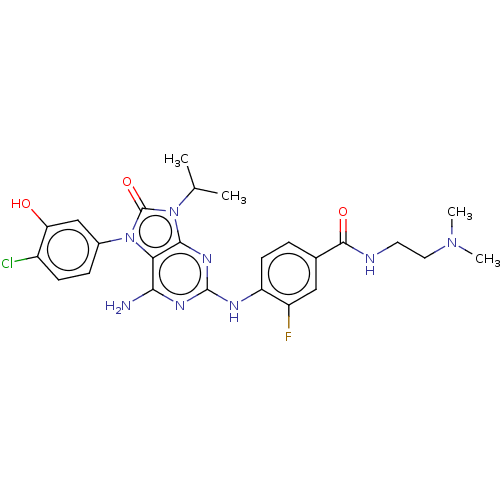 (US11052091, Example 5-22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514121 (US11052091, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514130 (US11052091, Example 5-5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581828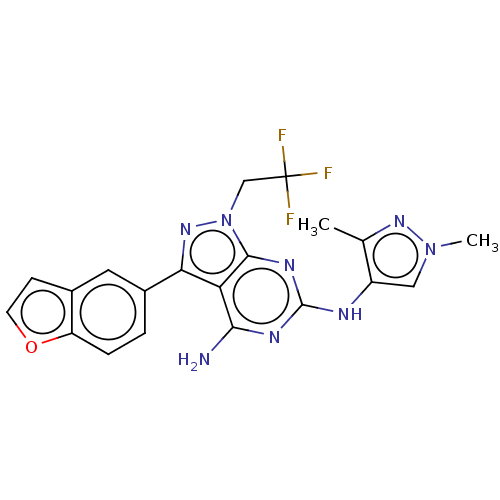 (US11512087, Example 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581835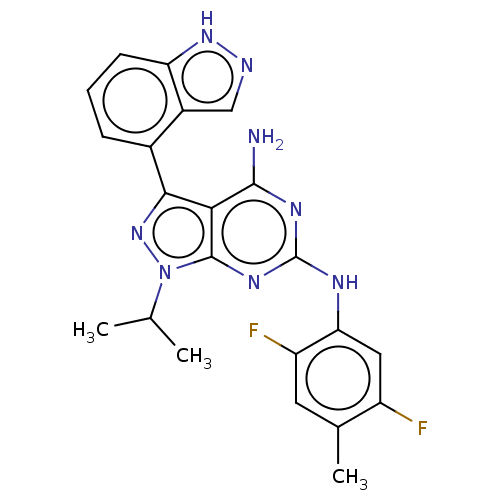 (US11512087, Example 18-1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581806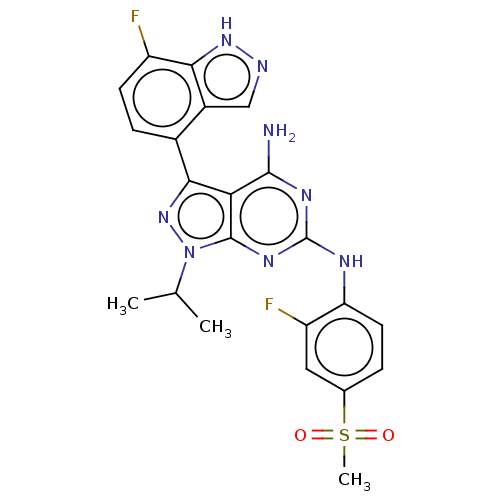 (US11512087, Example 4-3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514131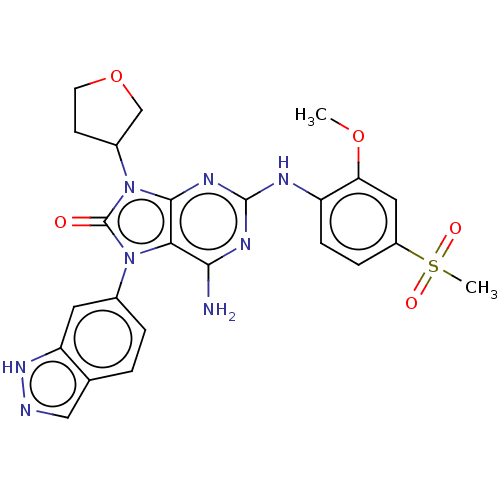 (US11052091, Example 5-8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514122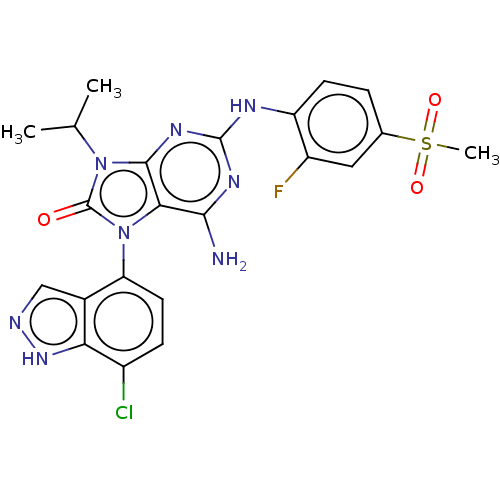 (US11052091, Example 2-1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581805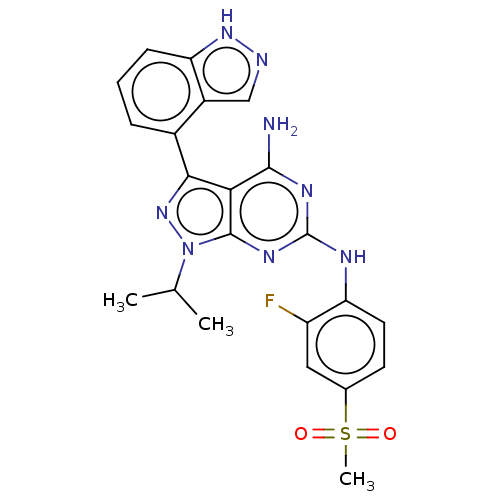 (US11512087, Example 4-2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581836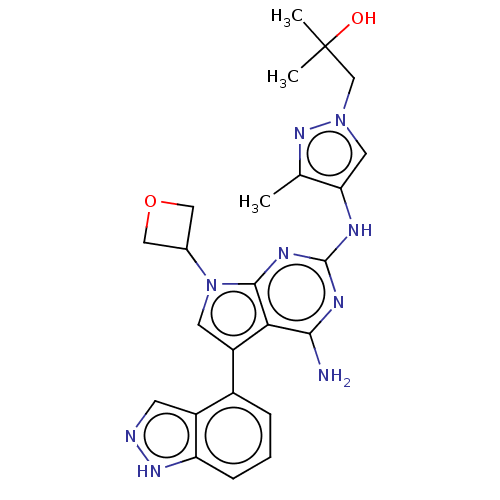 (US11512087, Example 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514123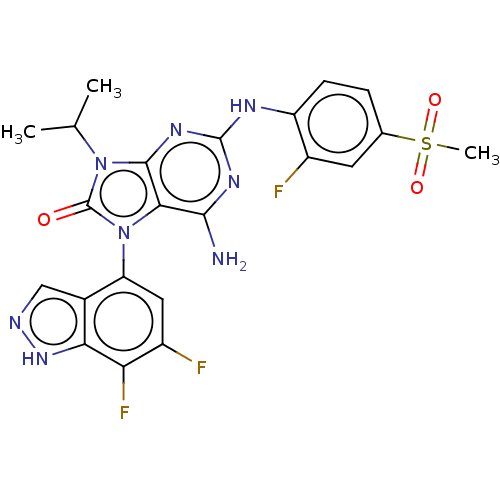 (US11052091, Example 2-2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514125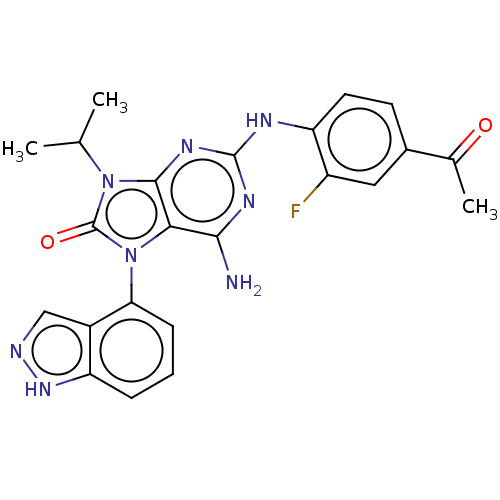 (US11052091, Example 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581833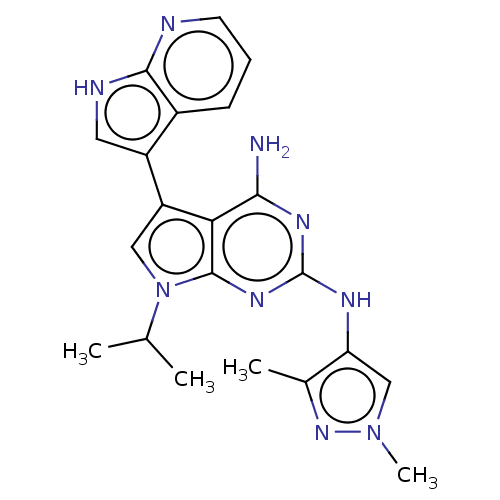 (US11512087, Example 14-4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514140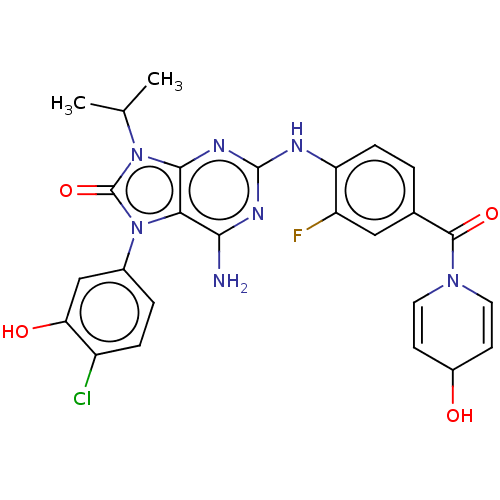 (US11052091, Example 5-27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514132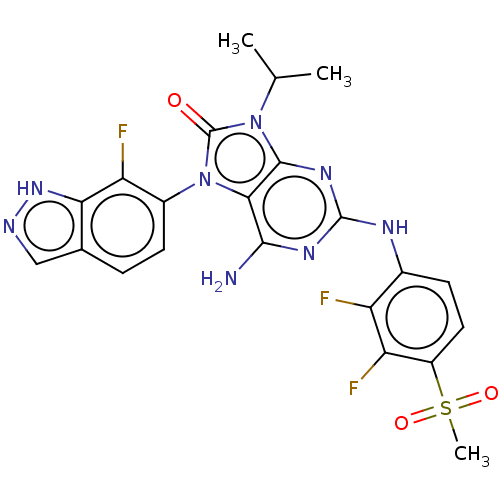 (US11052091, Example 5-10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 186 total ) | Next | Last >> |