

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331048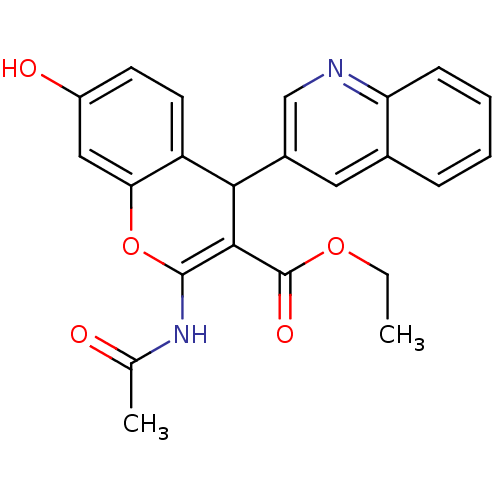 (CHEMBL1277437 | ethyl 2-acetamido-7-hydroxy-4-(qui...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449010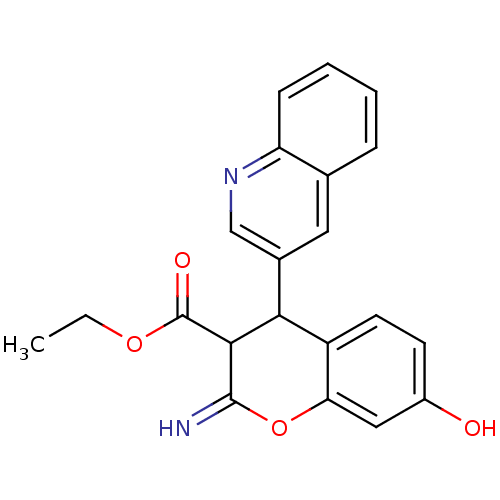 (CHEMBL3125960) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449026 (CHEMBL3125936) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449000 (CHEMBL3125942) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449033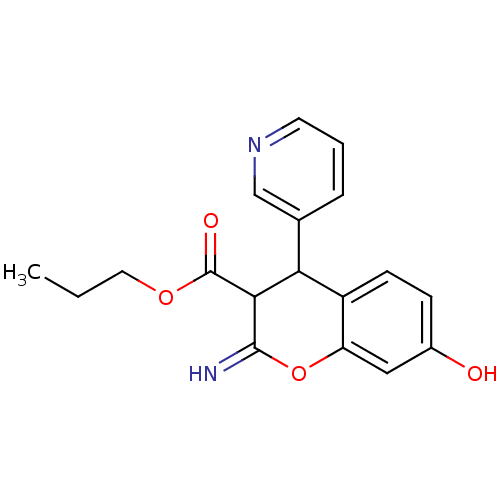 (CHEMBL3125949) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449029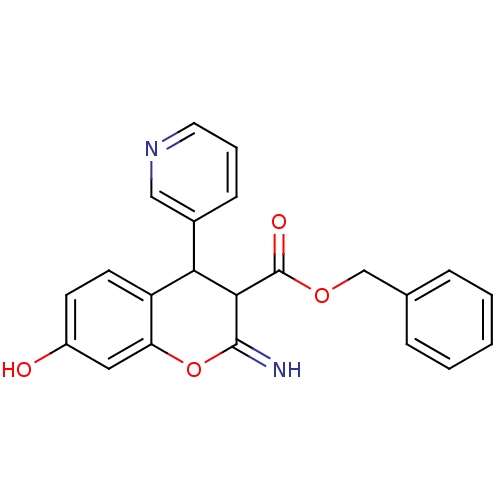 (CHEMBL3125954) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449011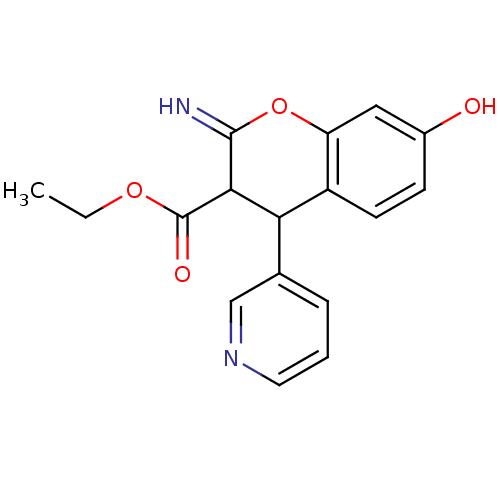 (CHEMBL1604159) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449032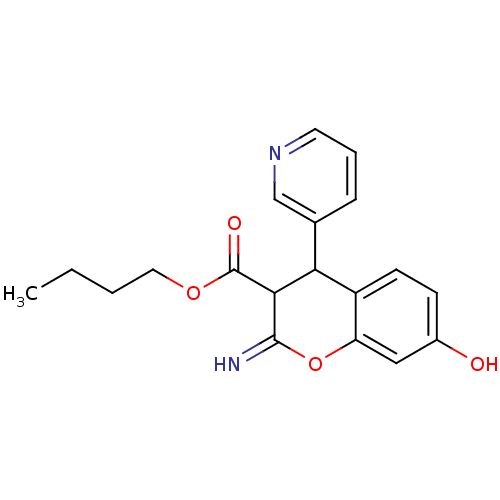 (CHEMBL3125950) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449008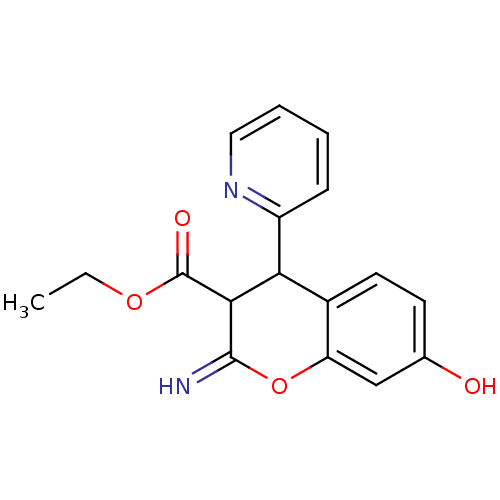 (CHEMBL3125965) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449007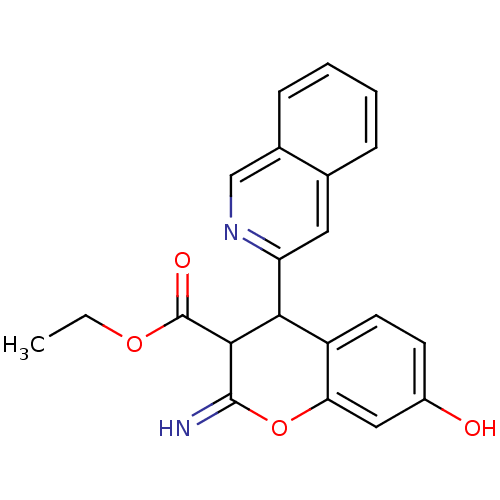 (CHEMBL3125966) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449004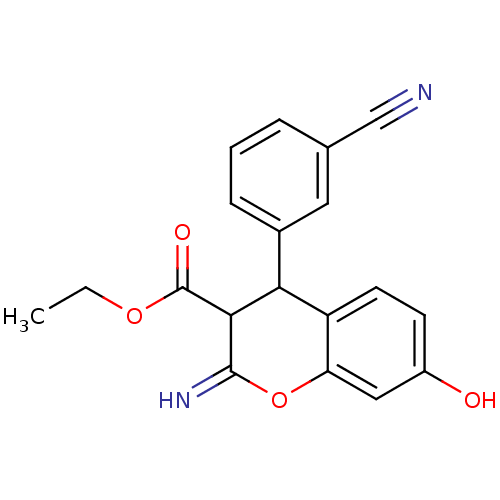 (CHEMBL3125937) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449001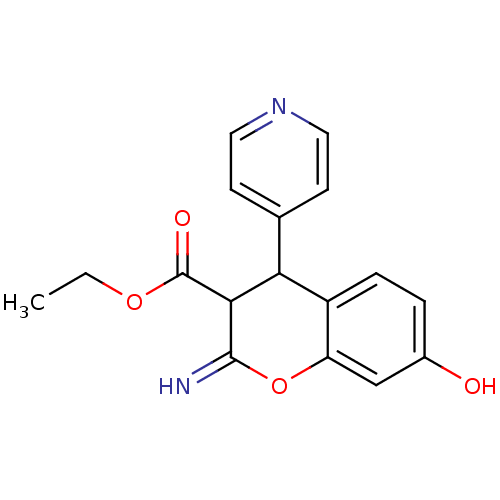 (CHEMBL3125941) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449030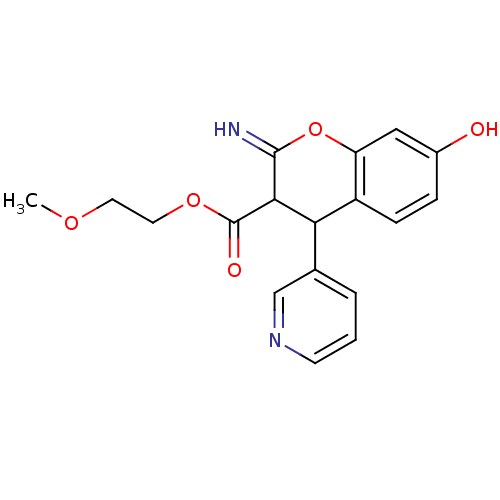 (CHEMBL3125952) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449034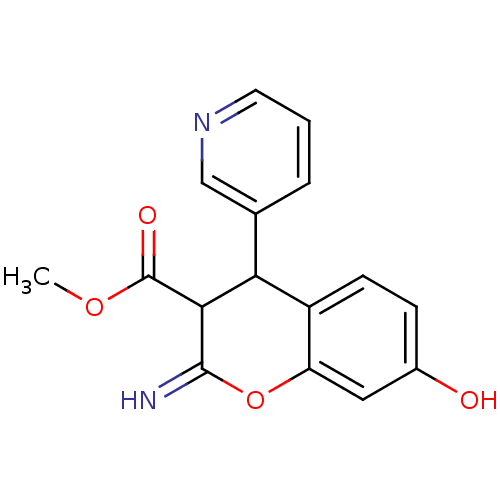 (CHEMBL3125948) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50448996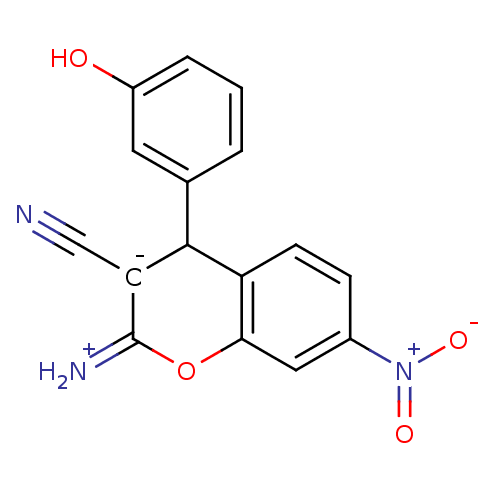 (CHEMBL3125931) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50448998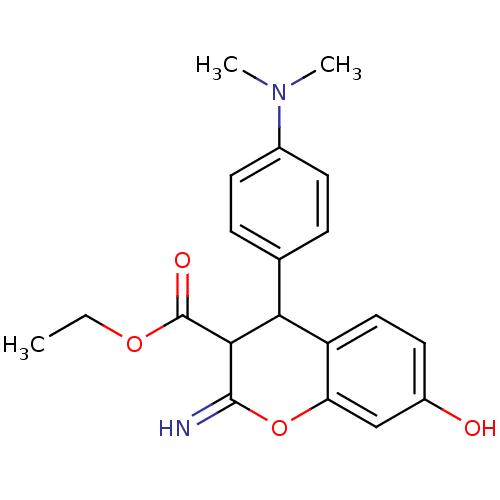 (CHEMBL3125944) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449028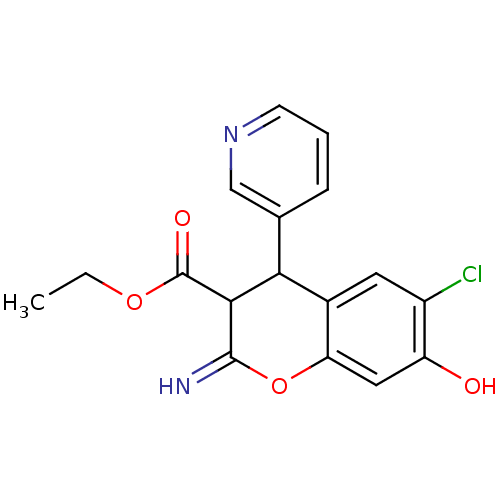 (CHEMBL3125932) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50448997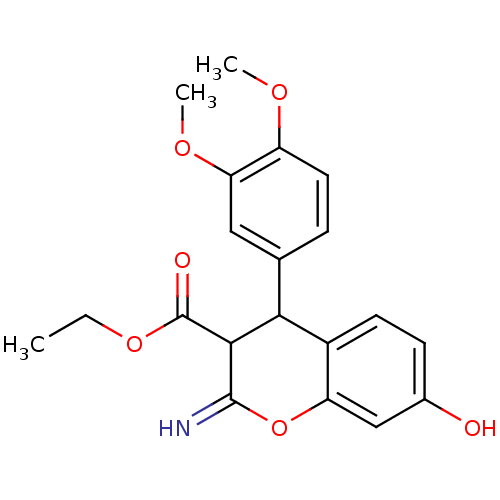 (CHEMBL3125945) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50448999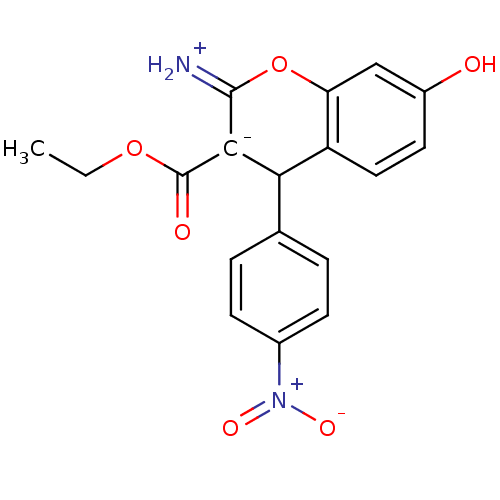 (CHEMBL1338272) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449027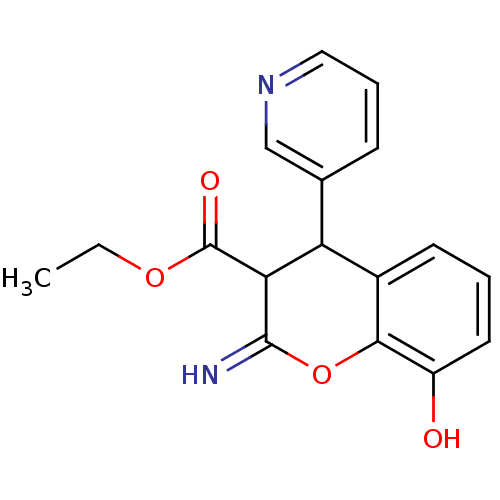 (CHEMBL3125934) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449002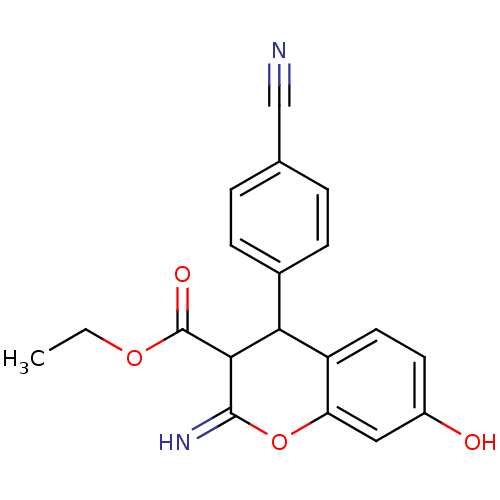 (CHEMBL3125940) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449031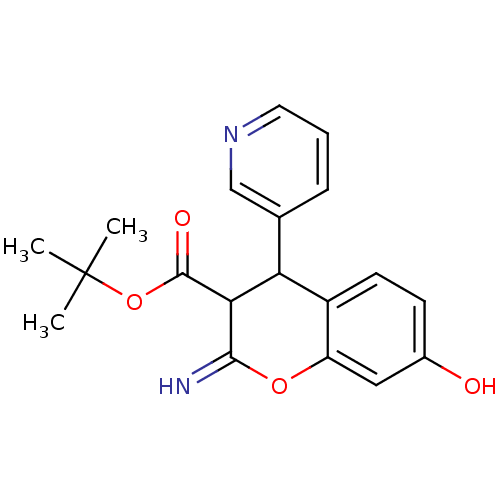 (CHEMBL3125951) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449003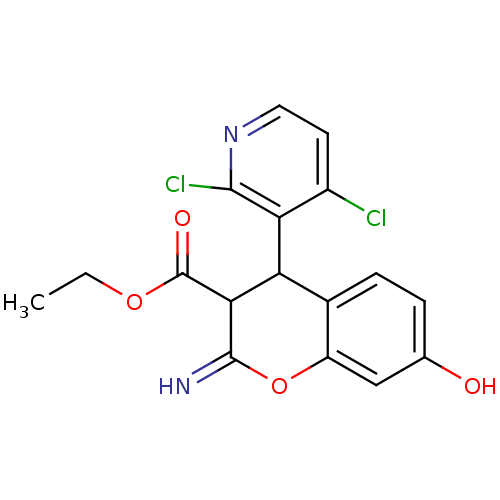 (CHEMBL3125938) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449005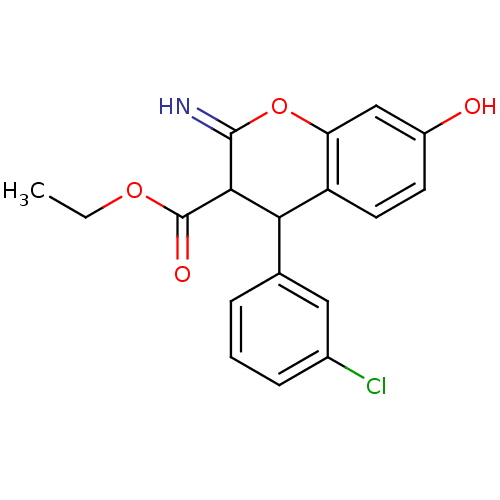 (CHEMBL1511289) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449006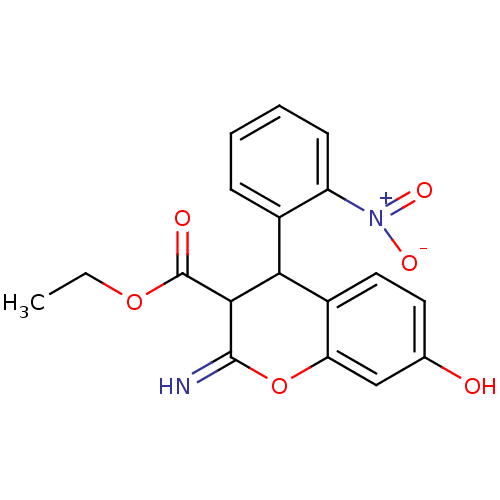 (CHEMBL3125967) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449009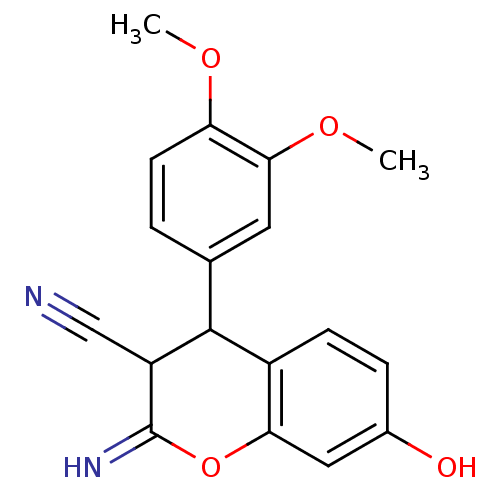 (CHEMBL1303063) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449024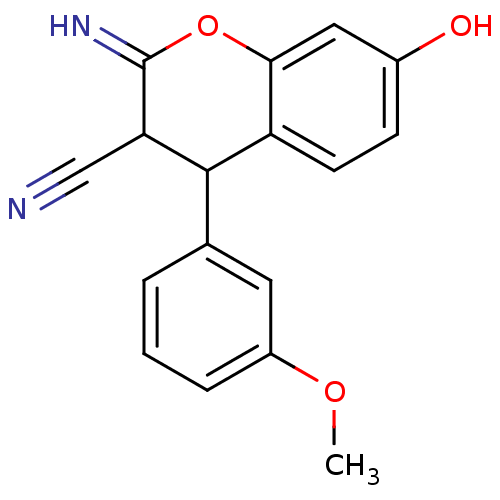 (CHEMBL3125961) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449025 (CHEMBL1276538) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449014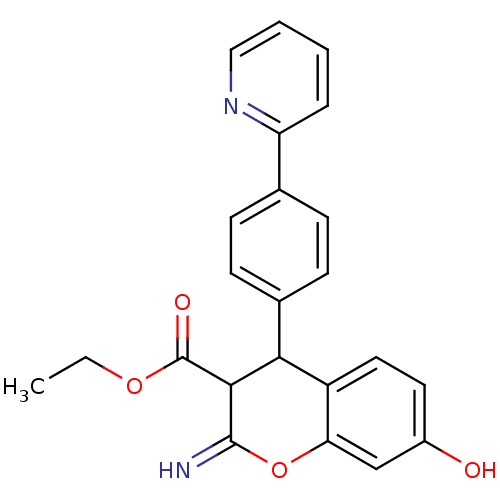 (CHEMBL3125943) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449013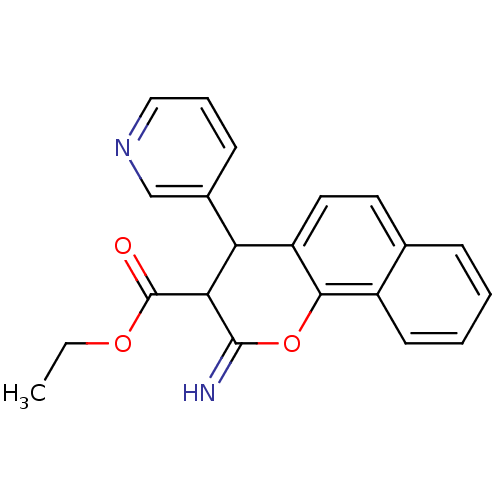 (CHEMBL3125935) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449012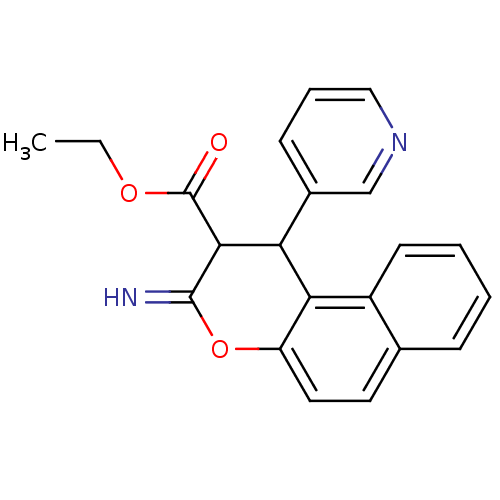 (CHEMBL1406709) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449023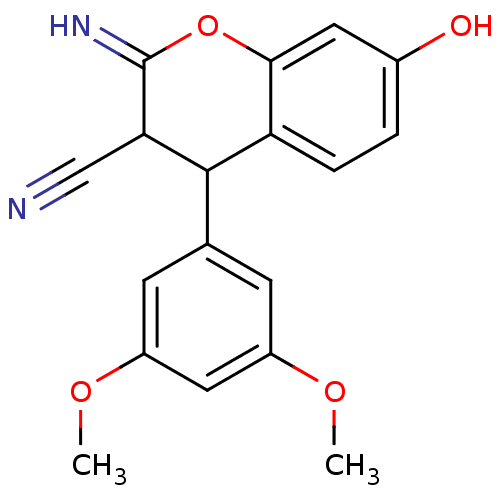 (CHEMBL3125962) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449022 (CHEMBL1403937) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449021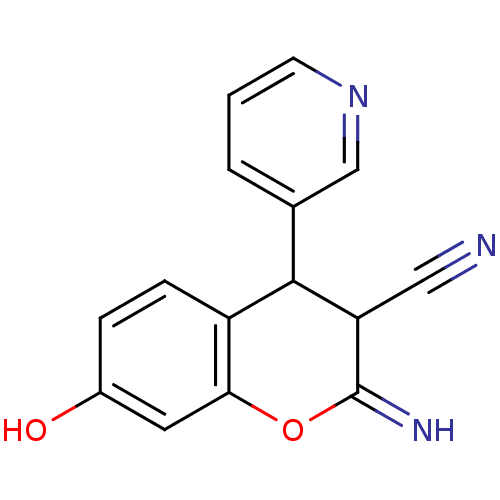 (CHEMBL1556216) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449020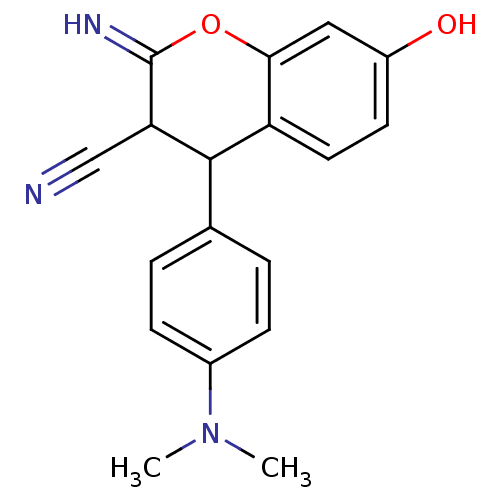 (CHEMBL3125963) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449019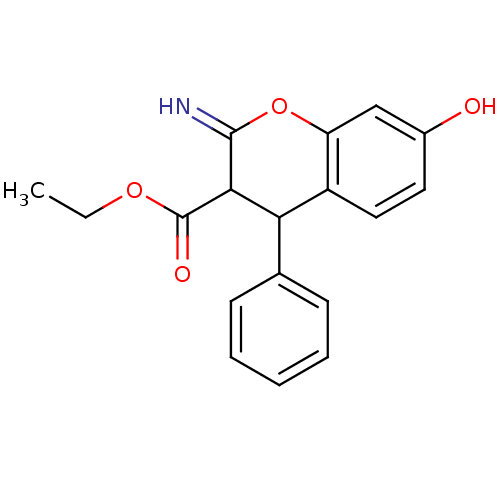 (CHEMBL1603587) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449018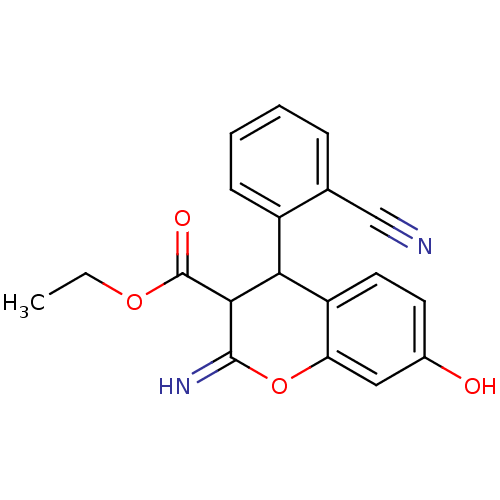 (CHEMBL3125964) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449015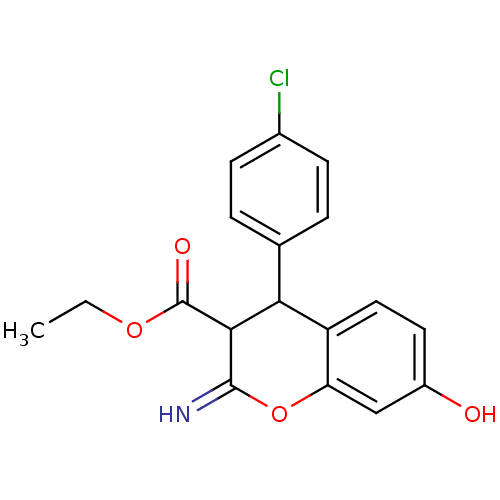 (CHEMBL3125939) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449016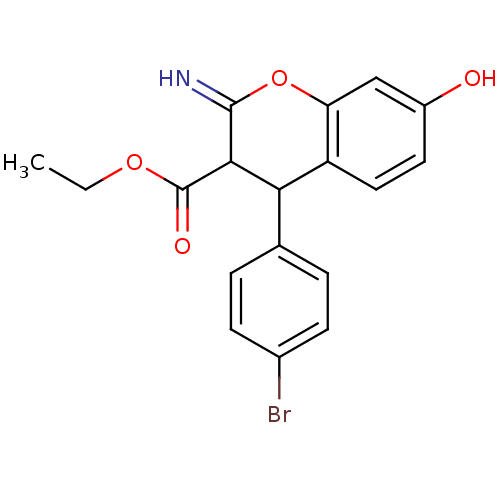 (CHEMBL1435420) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50449017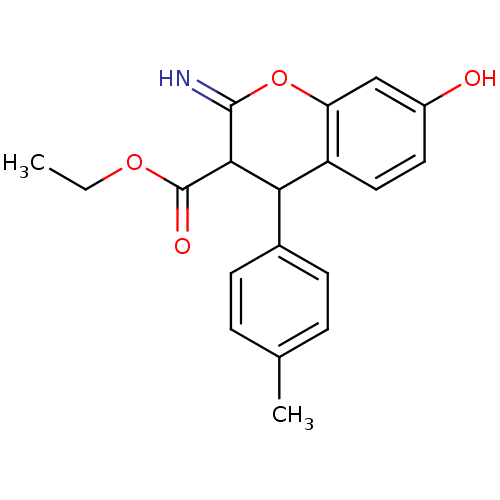 (CHEMBL1604644) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of IRAP in HEKT cells assessed as hydrolysis of L-leucine-4-methyl-7-coumarinylamide after 30 mins | J Med Chem 57: 1368-77 (2014) Article DOI: 10.1021/jm401540f BindingDB Entry DOI: 10.7270/Q2W66N8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50115589 (CHEMBL3609232) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.1 expressed in HEK293 cells assessed as inhibition of potassium currents after 10 mins by IonWorks Quattro patch cla... | J Med Chem 58: 6784-802 (2015) Article DOI: 10.1021/acs.jmedchem.5b00495 BindingDB Entry DOI: 10.7270/Q22V2HWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50115569 (CHEMBL3609231) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.1 expressed in HEK293 cells assessed as inhibition of potassium currents after 10 mins by IonWorks Quattro patch cla... | J Med Chem 58: 6784-802 (2015) Article DOI: 10.1021/acs.jmedchem.5b00495 BindingDB Entry DOI: 10.7270/Q22V2HWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50115508 (CHEMBL3609227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.1 expressed in HEK293 cells assessed as inhibition of potassium currents after 10 mins by IonWorks Quattro patch cla... | J Med Chem 58: 6784-802 (2015) Article DOI: 10.1021/acs.jmedchem.5b00495 BindingDB Entry DOI: 10.7270/Q22V2HWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50115776 (CHEMBL3609037) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.1 expressed in HEK293 cells assessed as inhibition of potassium currents after 10 mins by IonWorks Quattro patch cla... | J Med Chem 58: 6784-802 (2015) Article DOI: 10.1021/acs.jmedchem.5b00495 BindingDB Entry DOI: 10.7270/Q22V2HWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50115603 (CHEMBL3609245) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.1 expressed in HEK293 cells assessed as inhibition of potassium currents after 10 mins by IonWorks Quattro patch cla... | J Med Chem 58: 6784-802 (2015) Article DOI: 10.1021/acs.jmedchem.5b00495 BindingDB Entry DOI: 10.7270/Q22V2HWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50115637 (CHEMBL3609074) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.1 expressed in HEK293 cells assessed as inhibition of potassium currents after 10 mins by IonWorks Quattro patch cla... | J Med Chem 58: 6784-802 (2015) Article DOI: 10.1021/acs.jmedchem.5b00495 BindingDB Entry DOI: 10.7270/Q22V2HWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50115638 (CHEMBL3609075) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.1 expressed in HEK293 cells assessed as inhibition of potassium currents after 10 mins by IonWorks Quattro patch cla... | J Med Chem 58: 6784-802 (2015) Article DOI: 10.1021/acs.jmedchem.5b00495 BindingDB Entry DOI: 10.7270/Q22V2HWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50115632 (CHEMBL3609069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.1 expressed in HEK293 cells assessed as inhibition of potassium currents after 10 mins by IonWorks Quattro patch cla... | J Med Chem 58: 6784-802 (2015) Article DOI: 10.1021/acs.jmedchem.5b00495 BindingDB Entry DOI: 10.7270/Q22V2HWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50115504 (CHEMBL3609223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.1 expressed in HEK293 cells assessed as inhibition of potassium currents after 10 mins by IonWorks Quattro patch cla... | J Med Chem 58: 6784-802 (2015) Article DOI: 10.1021/acs.jmedchem.5b00495 BindingDB Entry DOI: 10.7270/Q22V2HWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50115501 (CHEMBL3609220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.1 expressed in HEK293 cells assessed as inhibition of potassium currents after 10 mins by IonWorks Quattro patch cla... | J Med Chem 58: 6784-802 (2015) Article DOI: 10.1021/acs.jmedchem.5b00495 BindingDB Entry DOI: 10.7270/Q22V2HWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 720 total ) | Next | Last >> |