

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520310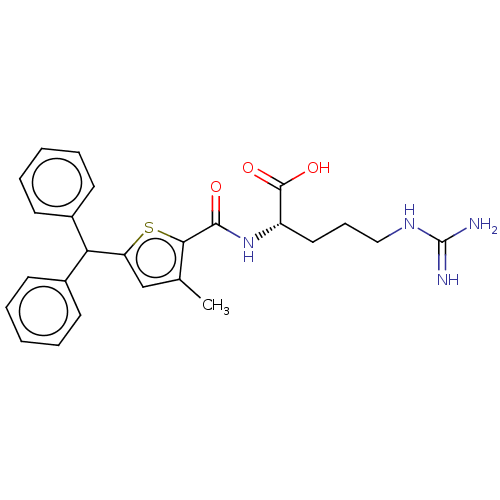 (CHEMBL4470864) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520310 (CHEMBL4470864) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520316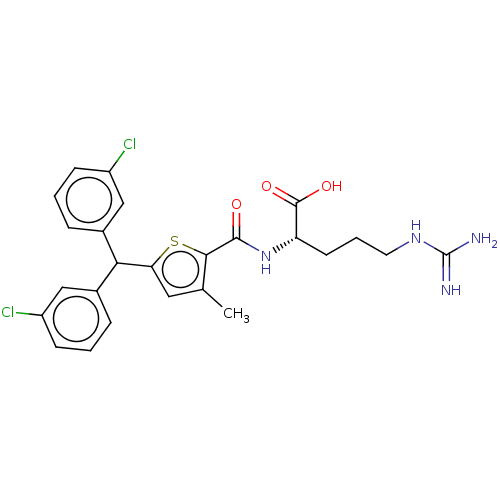 (CHEMBL4445758) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520316 (CHEMBL4445758) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520305 (CHEMBL4559976) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520309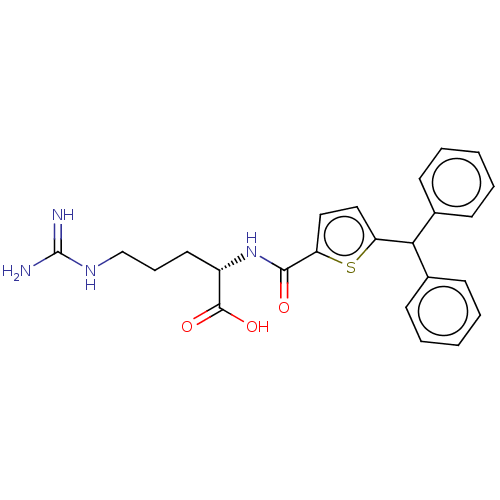 (CHEMBL4576800) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520305 (CHEMBL4559976) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520309 (CHEMBL4576800) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520303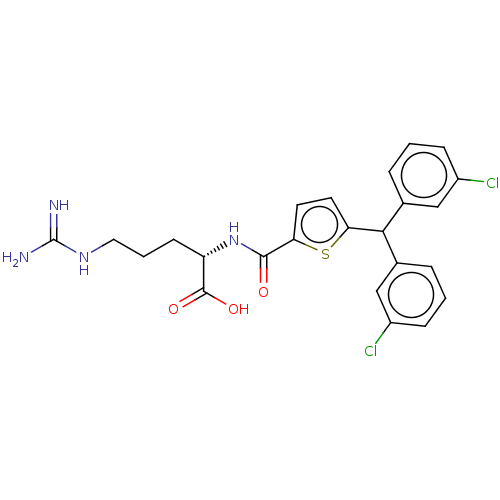 (CHEMBL4459830) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520303 (CHEMBL4459830) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520310 (CHEMBL4470864) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520310 (CHEMBL4470864) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM50333103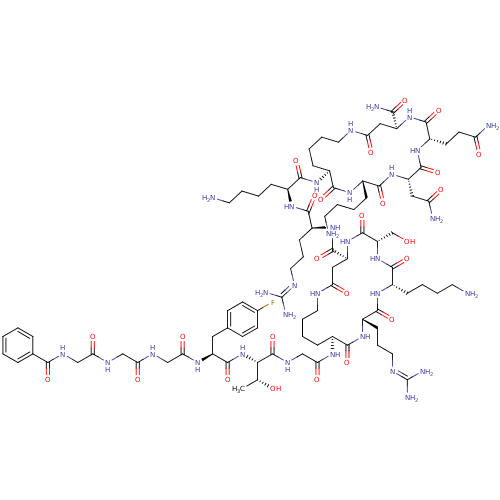 (CHEMBL1631931) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at NOR in mouse Neuro-2a cells assessed as inhibition of nociceptin-induced ERK phopshorylation administered for 15 mins before n... | J Med Chem 53: 8400-8408 (2010) Article DOI: 10.1021/jm101139f BindingDB Entry DOI: 10.7270/Q24Q7V77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520304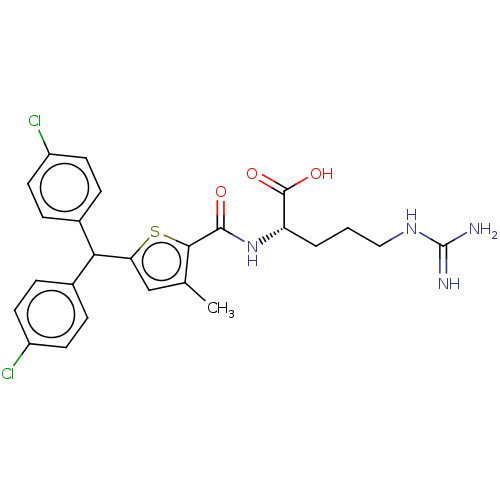 (CHEMBL4459627) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520304 (CHEMBL4459627) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520304 (CHEMBL4459627) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520304 (CHEMBL4459627) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM50333102 (CHEMBL1631930) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at NOR in mouse Neuro-2a cells assessed as inhibition of nociceptin-induced ERK phopshorylation administered for 15 mins before n... | J Med Chem 53: 8400-8408 (2010) Article DOI: 10.1021/jm101139f BindingDB Entry DOI: 10.7270/Q24Q7V77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520301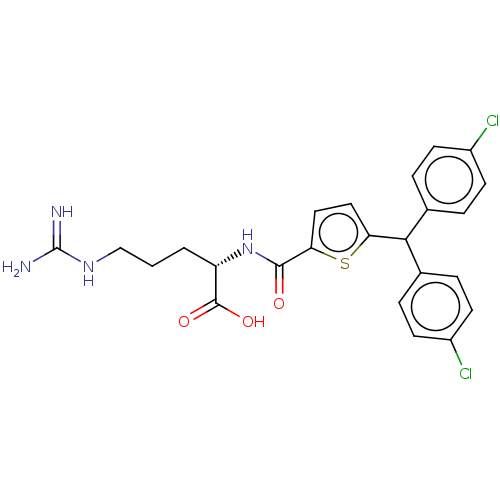 (CHEMBL4558167) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520321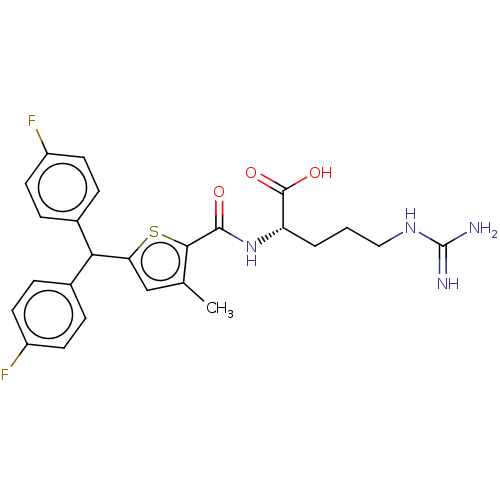 (CHEMBL4473192) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM50333100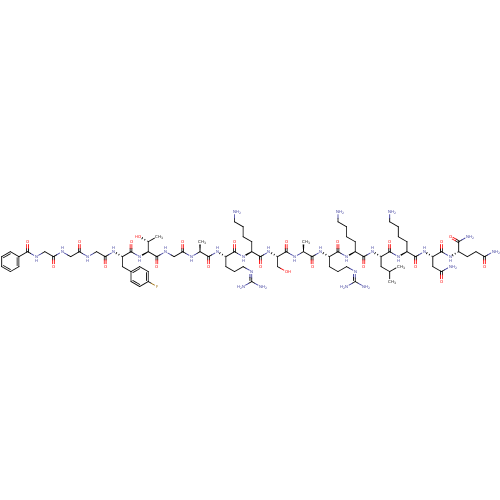 (BzLGlyGGF(4-F)TGARKSARKLKNQ-NH2 | CHEMBL1631928) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at NOR in mouse Neuro-2a cells assessed as inhibition of nociceptin-induced ERK phopshorylation administered for 15 mins before n... | J Med Chem 53: 8400-8408 (2010) Article DOI: 10.1021/jm101139f BindingDB Entry DOI: 10.7270/Q24Q7V77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520301 (CHEMBL4558167) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520321 (CHEMBL4473192) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM50333099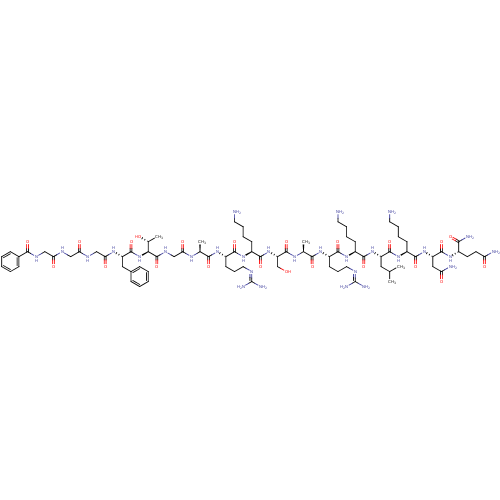 (BzLGlyGGFTGARKSARKLKNQ-NH2 | CHEMBL1631927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at NOR in mouse Neuro-2a cells assessed as inhibition of nociceptin-induced ERK phopshorylation administered for 15 mins before n... | J Med Chem 53: 8400-8408 (2010) Article DOI: 10.1021/jm101139f BindingDB Entry DOI: 10.7270/Q24Q7V77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520312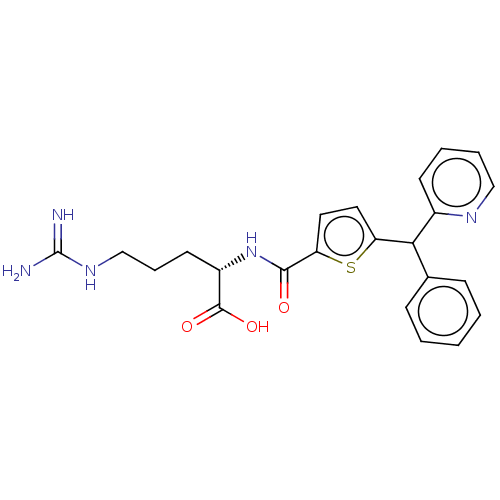 (CHEMBL4559463) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520312 (CHEMBL4559463) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520309 (CHEMBL4576800) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520309 (CHEMBL4576800) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520306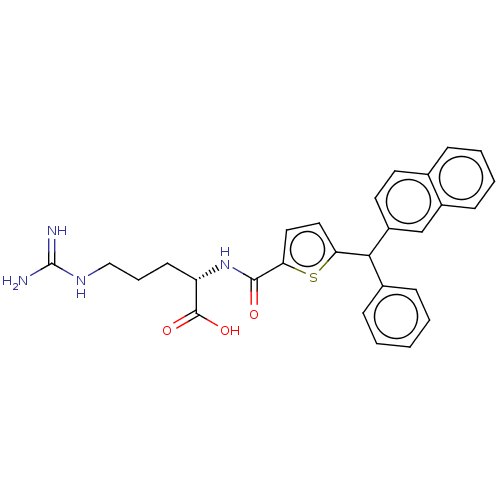 (CHEMBL4455385) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520306 (CHEMBL4455385) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520324 (CHEMBL4472149) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520326 (CHEMBL4562412) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520324 (CHEMBL4472149) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM50417550 (CHEMBL1631919) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at NOR in mouse Neuro-2a cells assessed as inhibition of nociceptin-induced ERK phopshorylation administered for 15 mins before n... | J Med Chem 53: 8400-8408 (2010) Article DOI: 10.1021/jm101139f BindingDB Entry DOI: 10.7270/Q24Q7V77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM50417551 (CHEMBL1631932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at NOR in mouse Neuro-2a cells assessed as inhibition of nociceptin-induced ERK phopshorylation administered for 15 mins before n... | J Med Chem 53: 8400-8408 (2010) Article DOI: 10.1021/jm101139f BindingDB Entry DOI: 10.7270/Q24Q7V77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM50417552 (CHEMBL1631934) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at NOR in mouse Neuro-2a cells assessed as inhibition of nociceptin-induced ERK phopshorylation administered for 15 mins before n... | J Med Chem 53: 8400-8408 (2010) Article DOI: 10.1021/jm101139f BindingDB Entry DOI: 10.7270/Q24Q7V77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM50417553 (CHEMBL1631933) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at NOR in mouse Neuro-2a cells assessed as inhibition of nociceptin-induced ERK phopshorylation administered for 15 mins before n... | J Med Chem 53: 8400-8408 (2010) Article DOI: 10.1021/jm101139f BindingDB Entry DOI: 10.7270/Q24Q7V77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520326 (CHEMBL4562412) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440863 (CHEMBL2431716) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for 20 mins followed by... | ACS Med Chem Lett 7: 1179-1184 (2016) Article DOI: 10.1021/acsmedchemlett.6b00306 BindingDB Entry DOI: 10.7270/Q2PN97MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440863 (CHEMBL2431716) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Agonist activity at human PAR2 expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for 20 mins followed by... | ACS Med Chem Lett 7: 1179-1184 (2016) Article DOI: 10.1021/acsmedchemlett.6b00306 BindingDB Entry DOI: 10.7270/Q2PN97MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520317 (CHEMBL4551058) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520307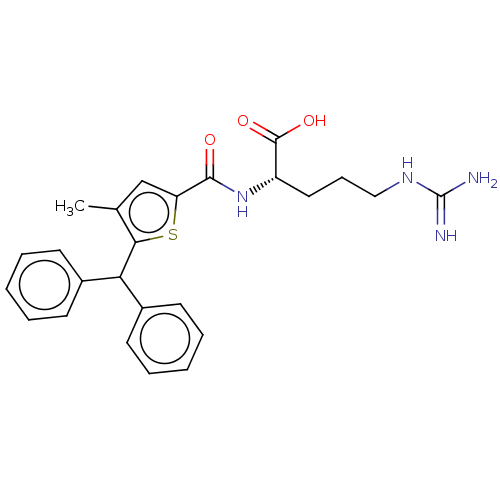 (CHEMBL4472196) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520307 (CHEMBL4472196) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520317 (CHEMBL4551058) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520320 (CHEMBL4458380) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520320 (CHEMBL4458380) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440862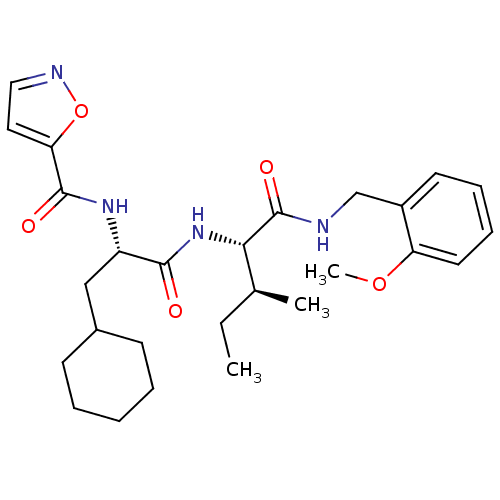 (CHEMBL2431717) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human HT-29 cells assessed as inhibition of 2f-LIGRLO-NH2-induced ca2+ release preincubated for 15 mins measured after... | Bioorg Med Chem Lett 26: 986-91 (2016) Article DOI: 10.1016/j.bmcl.2015.12.048 BindingDB Entry DOI: 10.7270/Q2PG1TKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50520318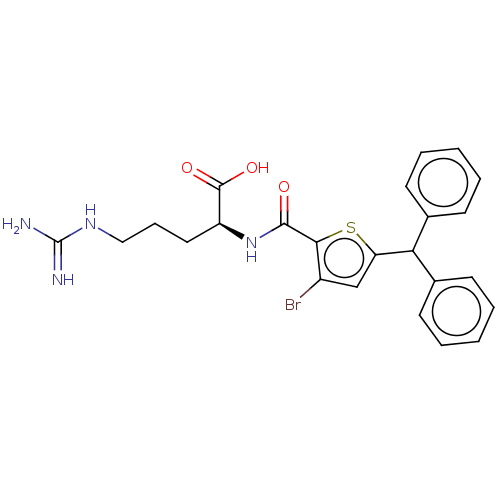 (CHEMBL4592883) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440862 (CHEMBL2431717) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human HT-29 cells assessed as inhibition of 2f-LIGRLO-NH2-induced ca2+ release preincubated for 15 mins measured after... | Bioorg Med Chem Lett 26: 986-91 (2016) Article DOI: 10.1016/j.bmcl.2015.12.048 BindingDB Entry DOI: 10.7270/Q2PG1TKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50322650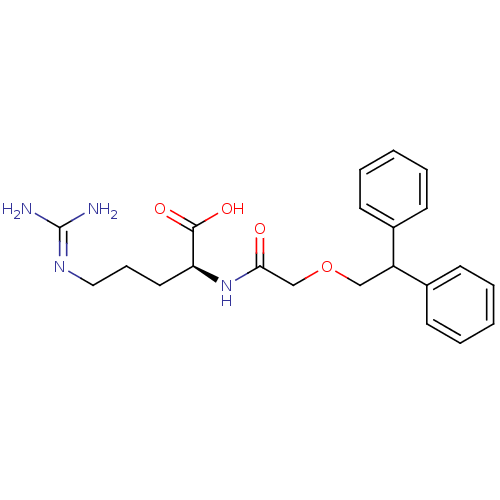 ((S)-2-(2-(2,2-diphenylethoxy)acetamido)-5-guanidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... | J Med Chem 63: 529-541 (2020) Article DOI: 10.1021/acs.jmedchem.9b00927 BindingDB Entry DOI: 10.7270/Q2377D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 177 total ) | Next | Last >> |