 Found 732 hits with Last Name = 'lu' and Initial = 'ap'
Found 732 hits with Last Name = 'lu' and Initial = 'ap' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM315477
(US10172858, Table 1.1 | US10172858, Table 1.22)Show InChI InChI=1S/C15H15N7O/c1-7(2)22-14-11(13(16)18-6-19-14)12(21-22)8-3-4-10-9(5-8)20-15(17)23-10/h3-7H,1-2H3,(H2,17,20)(H2,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529395

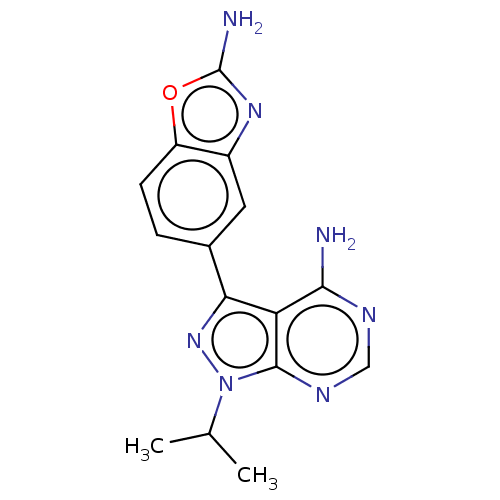
(CHEMBL4529672 | US11731973, Example 21)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 Show InChI InChI=1S/C15H16N8O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H4,16,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50453725

(CHEMBL5284183)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#7]-[#6](=O)-[#6]-[#6](-[#8])=O Show InChI InChI=1S/C18H29NO3/c1-14(2)7-5-8-15(3)9-6-10-16(4)11-12-19-17(20)13-18(21)22/h7,9,11H,5-6,8,10,12-13H2,1-4H3,(H,19,20)(H,21,22)/b15-9+,16-11+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50093374
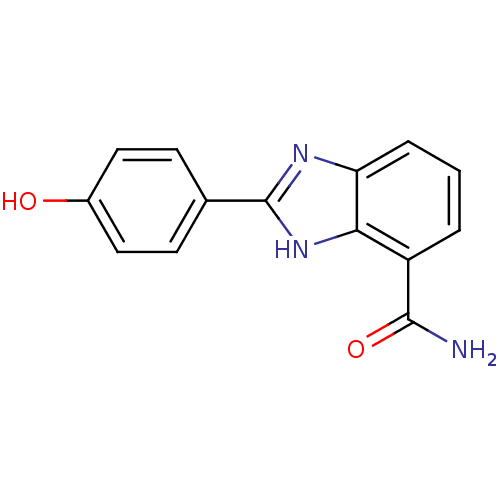
(2-(4-Hydroxy-phenyl)-1H-benzoimidazole-4-carboxyli...)Show InChI InChI=1S/C14H11N3O2/c15-13(19)10-2-1-3-11-12(10)17-14(16-11)8-4-6-9(18)7-5-8/h1-7,18H,(H2,15,19)(H,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529393

(CHEMBL4564479 | US11731973, Example 24)Show InChI InChI=1S/C15H15N7O/c1-7(2)22-13-9(6-18-14(16)20-13)12(21-22)8-3-4-11-10(5-8)19-15(17)23-11/h3-7H,1-2H3,(H2,17,19)(H2,16,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27119
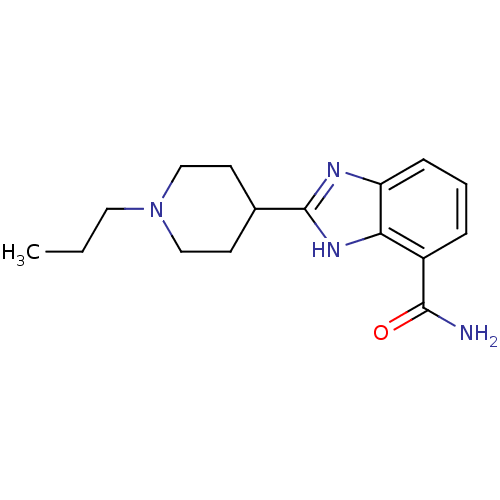
(2-(1-propylpiperidin-4-yl)-1H-1,3-benzodiazole-4-c...)Show InChI InChI=1S/C16H22N4O/c1-2-8-20-9-6-11(7-10-20)16-18-13-5-3-4-12(15(17)21)14(13)19-16/h3-5,11H,2,6-10H2,1H3,(H2,17,21)(H,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529398
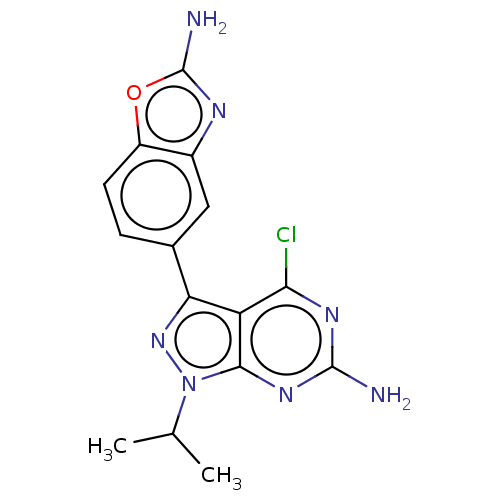
(CHEMBL4549761 | US11731973, Example 31)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(Cl)nc(N)nc12 Show InChI InChI=1S/C15H14ClN7O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529407

(CHEMBL4572049)Show SMILES CCCCn1nc(-c2ccc3oc(N)nc3c2)c2c(Cl)nc(N)nc12 Show InChI InChI=1S/C16H16ClN7O/c1-2-3-6-24-14-11(13(17)21-15(18)22-14)12(23-24)8-4-5-10-9(7-8)20-16(19)25-10/h4-5,7H,2-3,6H2,1H3,(H2,19,20)(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165486
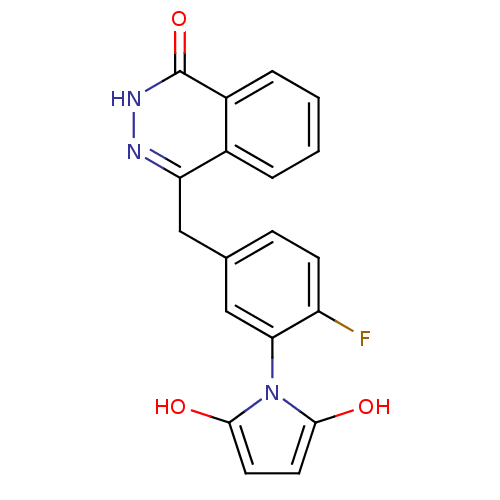
(1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Oc1ccc(O)n1-c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F |(35.28,-33.78,;34.95,-32.28,;35.97,-31.13,;35.19,-29.8,;33.69,-30.13,;32.54,-29.11,;33.55,-31.67,;32.22,-32.45,;30.88,-31.69,;29.56,-32.47,;28.22,-31.7,;28.22,-30.16,;29.56,-29.39,;29.55,-27.84,;28.2,-27.07,;28.19,-25.53,;26.87,-27.85,;25.53,-27.09,;24.21,-27.86,;24.21,-29.4,;25.54,-30.17,;26.88,-29.4,;29.56,-34,;30.89,-34.77,;32.23,-33.99,;33.56,-34.76,)| Show InChI InChI=1S/C19H14FN3O3/c20-14-6-5-11(10-16(14)23-17(24)7-8-18(23)25)9-15-12-3-1-2-4-13(12)19(26)22-21-15/h1-8,10,24-25H,9H2,(H,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529399
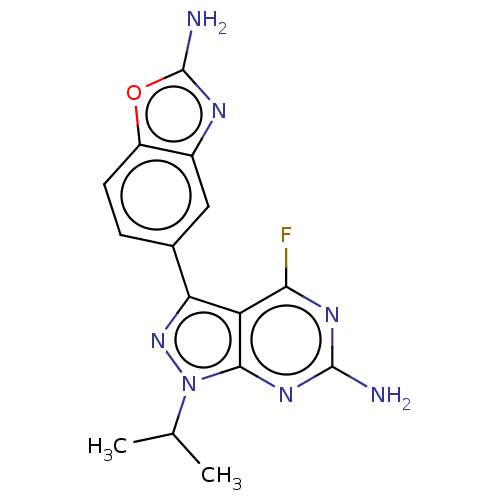
(CHEMBL4538004)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(F)nc(N)nc12 Show InChI InChI=1S/C15H14FN7O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529404
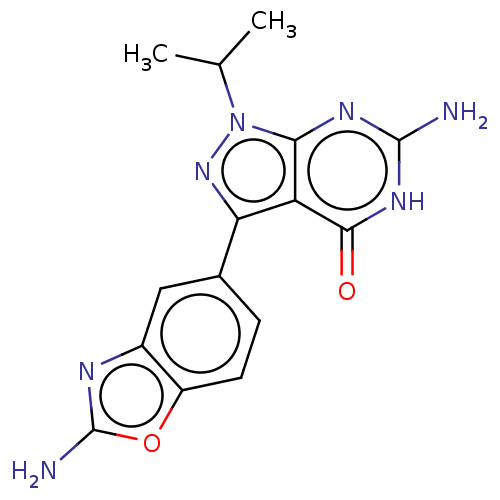
(CHEMBL4441763)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c1nc(N)[nH]c2=O Show InChI InChI=1S/C15H15N7O2/c1-6(2)22-12-10(13(23)20-14(16)19-12)11(21-22)7-3-4-9-8(5-7)18-15(17)24-9/h3-6H,1-2H3,(H2,17,18)(H3,16,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529405
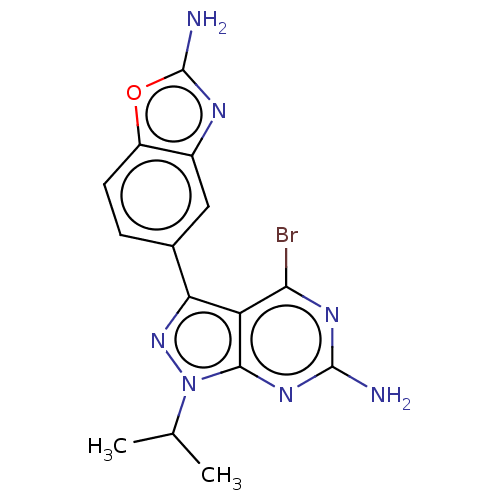
(CHEMBL4588610)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(Br)nc(N)nc12 Show InChI InChI=1S/C15H14BrN7O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529401
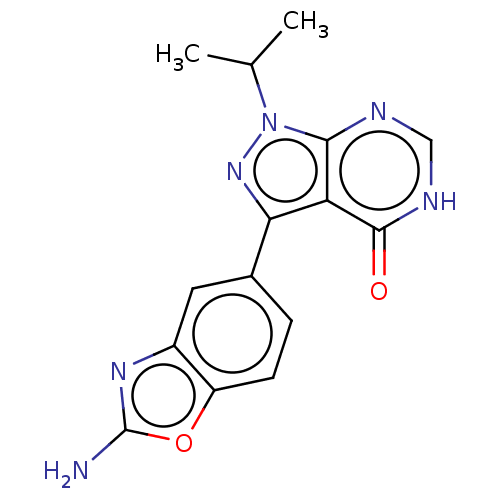
(CHEMBL4585005)Show InChI InChI=1S/C15H14N6O2/c1-7(2)21-13-11(14(22)18-6-17-13)12(20-21)8-3-4-10-9(5-8)19-15(16)23-10/h3-7H,1-2H3,(H2,16,19)(H,17,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM315477

(US10172858, Table 1.1 | US10172858, Table 1.22)Show InChI InChI=1S/C15H15N7O/c1-7(2)22-14-11(13(16)18-6-19-14)12(21-22)8-3-4-10-9(5-8)20-15(17)23-10/h3-7H,1-2H3,(H2,17,20)(H2,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529396

(CHEMBL4538629)Show InChI InChI=1S/C17H19N7O/c1-3-4-7-24-15-13(9(2)20-16(18)22-15)14(23-24)10-5-6-12-11(8-10)21-17(19)25-12/h5-6,8H,3-4,7H2,1-2H3,(H2,19,21)(H2,18,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529402

(CHEMBL4517253)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c1nc(C)[nH]c2=O Show InChI InChI=1S/C16H16N6O2/c1-7(2)22-14-12(15(23)19-8(3)18-14)13(21-22)9-4-5-11-10(6-9)20-16(17)24-11/h4-7H,1-3H3,(H2,17,20)(H,18,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529392
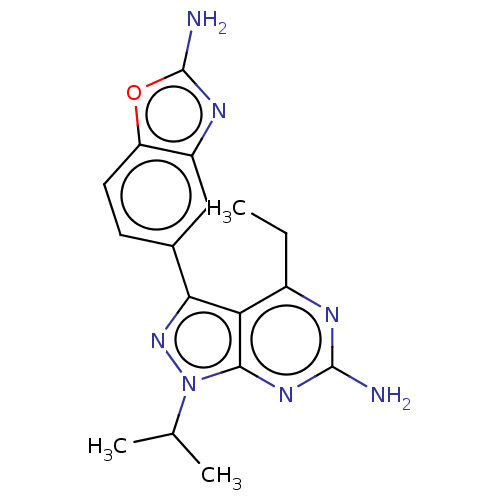
(CHEMBL4461815)Show SMILES CCc1nc(N)nc2n(nc(-c3ccc4oc(N)nc4c3)c12)C(C)C Show InChI InChI=1S/C17H19N7O/c1-4-10-13-14(9-5-6-12-11(7-9)21-17(19)25-12)23-24(8(2)3)15(13)22-16(18)20-10/h5-8H,4H2,1-3H3,(H2,19,21)(H2,18,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529394
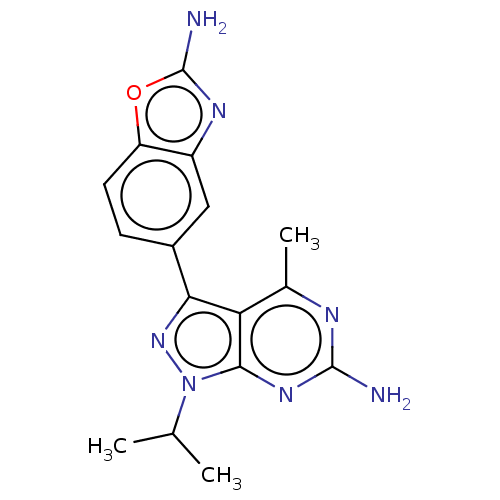
(CHEMBL4475845)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(C)nc(N)nc12 Show InChI InChI=1S/C16H17N7O/c1-7(2)23-14-12(8(3)19-15(17)21-14)13(22-23)9-4-5-11-10(6-9)20-16(18)24-11/h4-7H,1-3H3,(H2,18,20)(H2,17,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529392

(CHEMBL4461815)Show SMILES CCc1nc(N)nc2n(nc(-c3ccc4oc(N)nc4c3)c12)C(C)C Show InChI InChI=1S/C17H19N7O/c1-4-10-13-14(9-5-6-12-11(7-9)21-17(19)25-12)23-24(8(2)3)15(13)22-16(18)20-10/h5-8H,4H2,1-3H3,(H2,19,21)(H2,18,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529395

(CHEMBL4529672 | US11731973, Example 21)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 Show InChI InChI=1S/C15H16N8O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H4,16,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529403
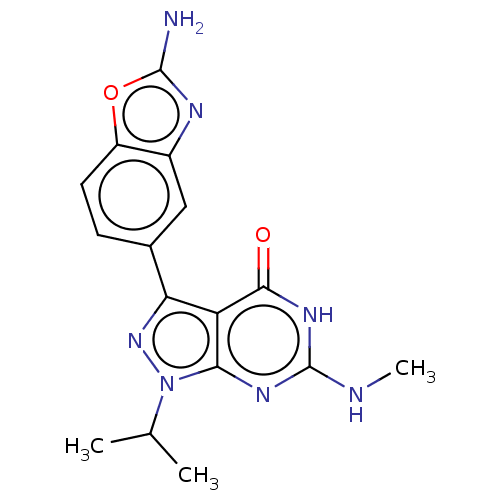
(CHEMBL4456417)Show SMILES CNc1nc2n(nc(-c3ccc4oc(N)nc4c3)c2c(=O)[nH]1)C(C)C Show InChI InChI=1S/C16H17N7O2/c1-7(2)23-13-11(14(24)21-16(18-3)20-13)12(22-23)8-4-5-10-9(6-8)19-15(17)25-10/h4-7H,1-3H3,(H2,17,19)(H2,18,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529406
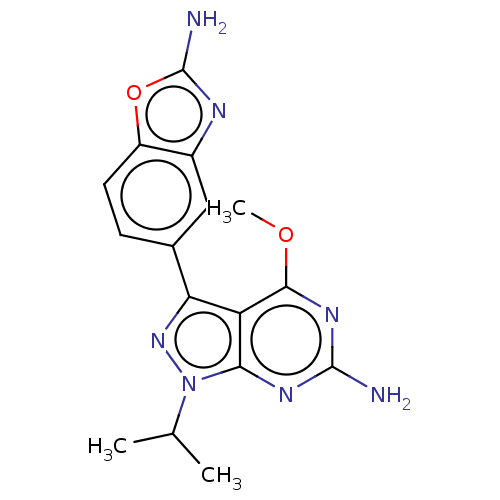
(CHEMBL4525459)Show SMILES COc1nc(N)nc2n(nc(-c3ccc4oc(N)nc4c3)c12)C(C)C Show InChI InChI=1S/C16H17N7O2/c1-7(2)23-13-11(14(24-3)21-15(17)20-13)12(22-23)8-4-5-10-9(6-8)19-16(18)25-10/h4-7H,1-3H3,(H2,18,19)(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529406

(CHEMBL4525459)Show SMILES COc1nc(N)nc2n(nc(-c3ccc4oc(N)nc4c3)c12)C(C)C Show InChI InChI=1S/C16H17N7O2/c1-7(2)23-13-11(14(24-3)21-15(17)20-13)12(22-23)8-4-5-10-9(6-8)19-16(18)25-10/h4-7H,1-3H3,(H2,18,19)(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529393

(CHEMBL4564479 | US11731973, Example 24)Show InChI InChI=1S/C15H15N7O/c1-7(2)22-13-9(6-18-14(16)20-13)12(21-22)8-3-4-11-10(5-8)19-15(17)23-11/h3-7H,1-2H3,(H2,17,19)(H2,16,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529398

(CHEMBL4549761 | US11731973, Example 31)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(Cl)nc(N)nc12 Show InChI InChI=1S/C15H14ClN7O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529394

(CHEMBL4475845)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(C)nc(N)nc12 Show InChI InChI=1S/C16H17N7O/c1-7(2)23-14-12(8(3)19-15(17)21-14)13(22-23)9-4-5-11-10(6-9)20-16(18)24-11/h4-7H,1-3H3,(H2,18,20)(H2,17,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529405

(CHEMBL4588610)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(Br)nc(N)nc12 Show InChI InChI=1S/C15H14BrN7O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529408

(CHEMBL4451788)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c1nc([nH]c2=O)N(C)C Show InChI InChI=1S/C17H19N7O2/c1-8(2)24-14-12(15(25)21-17(20-14)23(3)4)13(22-24)9-5-6-11-10(7-9)19-16(18)26-11/h5-8H,1-4H3,(H2,18,19)(H,20,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529396

(CHEMBL4538629)Show InChI InChI=1S/C17H19N7O/c1-3-4-7-24-15-13(9(2)20-16(18)22-15)14(23-24)10-5-6-12-11(8-10)21-17(19)25-12/h5-6,8H,3-4,7H2,1-2H3,(H2,19,21)(H2,18,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529409
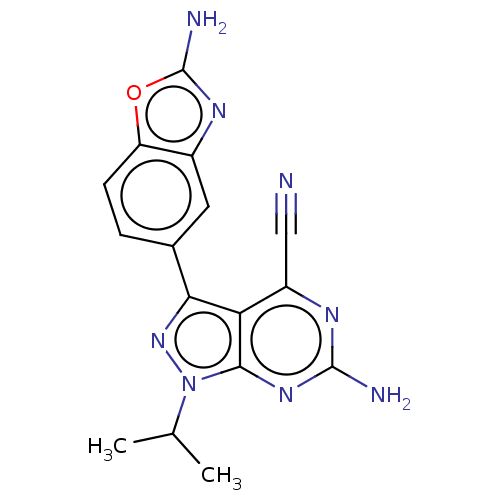
(CHEMBL4440554)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(nc(N)nc12)C#N Show InChI InChI=1S/C16H14N8O/c1-7(2)24-14-12(10(6-17)20-15(18)22-14)13(23-24)8-3-4-11-9(5-8)21-16(19)25-11/h3-5,7H,1-2H3,(H2,19,21)(H2,18,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529397
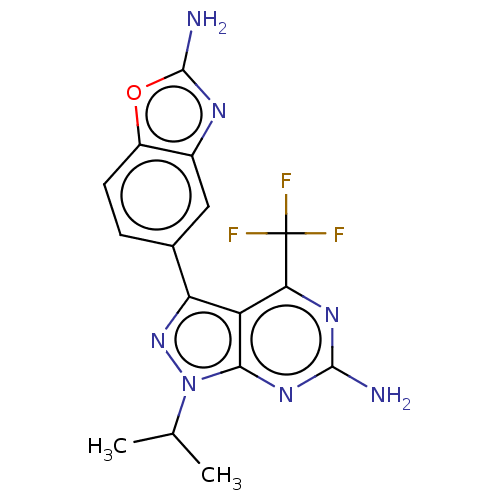
(CHEMBL4475002)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(nc(N)nc12)C(F)(F)F Show InChI InChI=1S/C16H14F3N7O/c1-6(2)26-13-10(12(16(17,18)19)23-14(20)24-13)11(25-26)7-3-4-9-8(5-7)22-15(21)27-9/h3-6H,1-2H3,(H2,21,22)(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529399

(CHEMBL4538004)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(F)nc(N)nc12 Show InChI InChI=1S/C15H14FN7O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529404

(CHEMBL4441763)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c1nc(N)[nH]c2=O Show InChI InChI=1S/C15H15N7O2/c1-6(2)22-12-10(13(23)20-14(16)19-12)11(21-22)7-3-4-9-8(5-7)18-15(17)24-9/h3-6H,1-2H3,(H2,17,18)(H3,16,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50529400
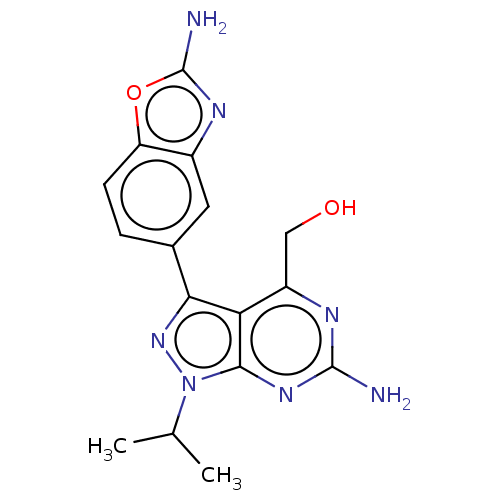
(CHEMBL4522109)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(CO)nc(N)nc12 Show InChI InChI=1S/C16H17N7O2/c1-7(2)23-14-12(10(6-24)19-15(17)21-14)13(22-23)8-3-4-11-9(5-8)20-16(18)25-11/h3-5,7,24H,6H2,1-2H3,(H2,18,20)(H2,17,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529407

(CHEMBL4572049)Show SMILES CCCCn1nc(-c2ccc3oc(N)nc3c2)c2c(Cl)nc(N)nc12 Show InChI InChI=1S/C16H16ClN7O/c1-2-3-6-24-14-11(13(17)21-15(18)22-14)12(23-24)8-4-5-10-9(7-8)20-16(19)25-10/h4-5,7H,2-3,6H2,1H3,(H2,19,20)(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529402

(CHEMBL4517253)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c1nc(C)[nH]c2=O Show InChI InChI=1S/C16H16N6O2/c1-7(2)22-14-12(15(23)19-8(3)18-14)13(21-22)9-4-5-11-10(6-9)20-16(17)24-11/h4-7H,1-3H3,(H2,17,20)(H,18,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529401

(CHEMBL4585005)Show InChI InChI=1S/C15H14N6O2/c1-7(2)21-13-11(14(22)18-6-17-13)12(20-21)8-3-4-10-9(5-8)19-15(16)23-10/h3-7H,1-2H3,(H2,16,19)(H,17,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529408

(CHEMBL4451788)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c1nc([nH]c2=O)N(C)C Show InChI InChI=1S/C17H19N7O2/c1-8(2)24-14-12(15(25)21-17(20-14)23(3)4)13(22-24)9-5-6-11-10(7-9)19-16(18)26-11/h5-8H,1-4H3,(H2,18,19)(H,20,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529397

(CHEMBL4475002)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(nc(N)nc12)C(F)(F)F Show InChI InChI=1S/C16H14F3N7O/c1-6(2)26-13-10(12(16(17,18)19)23-14(20)24-13)11(25-26)7-3-4-9-8(5-7)22-15(21)27-9/h3-6H,1-2H3,(H2,21,22)(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529403

(CHEMBL4456417)Show SMILES CNc1nc2n(nc(-c3ccc4oc(N)nc4c3)c2c(=O)[nH]1)C(C)C Show InChI InChI=1S/C16H17N7O2/c1-7(2)23-13-11(14(24)21-16(18-3)20-13)12(22-23)8-4-5-10-9(6-8)19-15(17)25-10/h4-7H,1-3H3,(H2,17,19)(H2,18,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529400

(CHEMBL4522109)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(CO)nc(N)nc12 Show InChI InChI=1S/C16H17N7O2/c1-7(2)23-14-12(10(6-24)19-15(17)21-14)13(22-23)8-3-4-11-9(5-8)20-16(18)25-11/h3-5,7,24H,6H2,1-2H3,(H2,18,20)(H2,17,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50529409

(CHEMBL4440554)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(nc(N)nc12)C#N Show InChI InChI=1S/C16H14N8O/c1-7(2)24-14-12(10(6-17)20-15(18)22-14)13(23-24)8-3-4-11-9(5-8)21-16(19)25-11/h3-5,7H,1-2H3,(H2,19,21)(H2,18,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50236612

(CHEMBL4071465)Show SMILES Cc1cc(COc2ccc(cc2)S(=O)(=O)N[C@@H]2CCC[C@]2(O)C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C23H25N3O6S/c1-15-13-16(19-5-2-3-6-20(19)24-15)14-32-17-8-10-18(11-9-17)33(30,31)26-21-7-4-12-23(21,28)22(27)25-29/h2-3,5-6,8-11,13,21,26,28-29H,4,7,12,14H2,1H3,(H,25,27)/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TACE catalytic domain (unknown origin) using Abz-LAQAVRSSSR-Dpa as substrate preincubated for 10 mins followed by substrate... |
Bioorg Med Chem Lett 27: 1848-1853 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.035
BindingDB Entry DOI: 10.7270/Q28S4S59 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50236612

(CHEMBL4071465)Show SMILES Cc1cc(COc2ccc(cc2)S(=O)(=O)N[C@@H]2CCC[C@]2(O)C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C23H25N3O6S/c1-15-13-16(19-5-2-3-6-20(19)24-15)14-32-17-8-10-18(11-9-17)33(30,31)26-21-7-4-12-23(21,28)22(27)25-29/h2-3,5-6,8-11,13,21,26,28-29H,4,7,12,14H2,1H3,(H,25,27)/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]- PDBu from Protein kinase C gamma C1b domain |
Bioorg Med Chem Lett 27: 1848-1853 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.035
BindingDB Entry DOI: 10.7270/Q28S4S59 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50236605

(CHEMBL4071782)Show SMILES Cc1cc(COc2ccc(cc2)S(=O)(=O)N[C@@H]2CCOC[C@]2(C)C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C24H27N3O6S/c1-16-13-17(20-5-3-4-6-21(20)25-16)14-33-18-7-9-19(10-8-18)34(30,31)27-22-11-12-32-15-24(22,2)23(28)26-29/h3-10,13,22,27,29H,11-12,14-15H2,1-2H3,(H,26,28)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]- SCH 23390 from the dopamine receptor D1 of bovine striatal membrane |
Bioorg Med Chem Lett 27: 1848-1853 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.035
BindingDB Entry DOI: 10.7270/Q28S4S59 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50236633
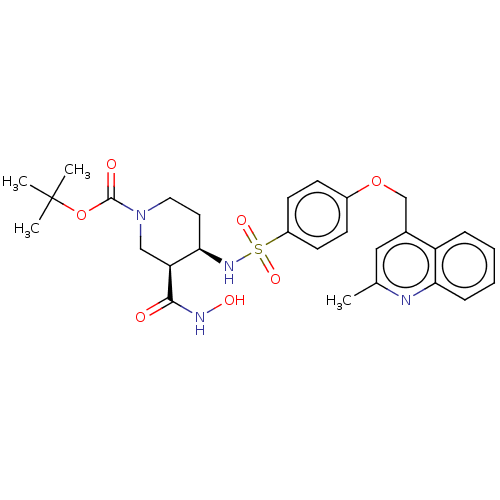
(CHEMBL4094122)Show SMILES Cc1cc(COc2ccc(cc2)S(=O)(=O)N[C@@H]2CCN(C[C@@H]2C(=O)NO)C(=O)OC(C)(C)C)c2ccccc2n1 |r| Show InChI InChI=1S/C28H34N4O7S/c1-18-15-19(22-7-5-6-8-24(22)29-18)17-38-20-9-11-21(12-10-20)40(36,37)31-25-13-14-32(16-23(25)26(33)30-35)27(34)39-28(2,3)4/h5-12,15,23,25,31,35H,13-14,16-17H2,1-4H3,(H,30,33)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TACE catalytic domain (unknown origin) using Abz-LAQAVRSSSR-Dpa as substrate preincubated for 10 mins followed by substrate... |
Bioorg Med Chem Lett 27: 1848-1853 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.035
BindingDB Entry DOI: 10.7270/Q28S4S59 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50236633

(CHEMBL4094122)Show SMILES Cc1cc(COc2ccc(cc2)S(=O)(=O)N[C@@H]2CCN(C[C@@H]2C(=O)NO)C(=O)OC(C)(C)C)c2ccccc2n1 |r| Show InChI InChI=1S/C28H34N4O7S/c1-18-15-19(22-7-5-6-8-24(22)29-18)17-38-20-9-11-21(12-10-20)40(36,37)31-25-13-14-32(16-23(25)26(33)30-35)27(34)39-28(2,3)4/h5-12,15,23,25,31,35H,13-14,16-17H2,1-4H3,(H,30,33)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TACE catalytic domain (unknown origin) using Abz-LAQAVRSSSR-Dpa as substrate preincubated for 10 mins followed by substrate... |
Bioorg Med Chem Lett 27: 1848-1853 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.035
BindingDB Entry DOI: 10.7270/Q28S4S59 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50236605

(CHEMBL4071782)Show SMILES Cc1cc(COc2ccc(cc2)S(=O)(=O)N[C@@H]2CCOC[C@]2(C)C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C24H27N3O6S/c1-16-13-17(20-5-3-4-6-21(20)25-16)14-33-18-7-9-19(10-8-18)34(30,31)27-22-11-12-32-15-24(22,2)23(28)26-29/h3-10,13,22,27,29H,11-12,14-15H2,1-2H3,(H,26,28)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant mammalian protein Farnesyltransferase expressed in baculovirus |
Bioorg Med Chem Lett 27: 1848-1853 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.035
BindingDB Entry DOI: 10.7270/Q28S4S59 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50236573
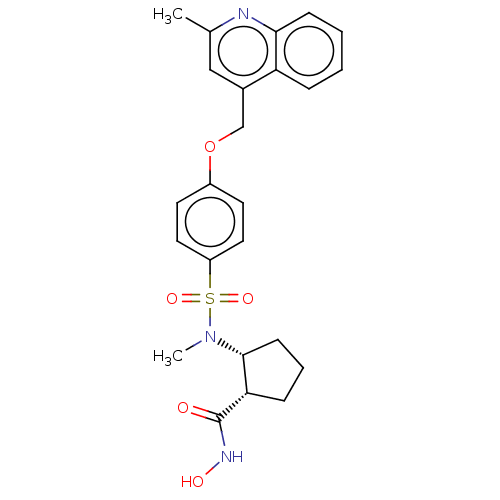
(CHEMBL4082009)Show SMILES CN([C@@H]1CCC[C@@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1 |r| Show InChI InChI=1S/C24H27N3O5S/c1-16-14-17(20-6-3-4-8-22(20)25-16)15-32-18-10-12-19(13-11-18)33(30,31)27(2)23-9-5-7-21(23)24(28)26-29/h3-4,6,8,10-14,21,23,29H,5,7,9,15H2,1-2H3,(H,26,28)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit high-affinity uptake of [3H]NE into parietal and occipital cortex in rat |
Bioorg Med Chem Lett 27: 1848-1853 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.035
BindingDB Entry DOI: 10.7270/Q28S4S59 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data