

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-dependent protein kinase catalytic subunit alpha (Mus musculus (mouse)) | BDBM27250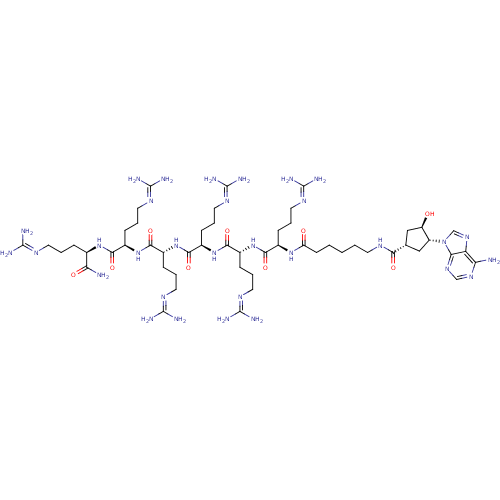 (6-{[(1S,3R,4R)-3-(6-amino-9H-purin-9-yl)-4-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Affinity of the compound for [3H]-N-propylnorapomorphine (NPA) Dopamine receptor D2 | J Med Chem 33: 445-50 (1990) BindingDB Entry DOI: 10.7270/Q2BK1B94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002338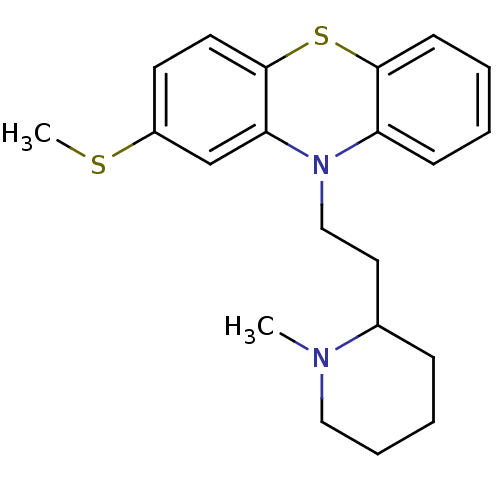 ((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Mus musculus (mouse)) | BDBM27249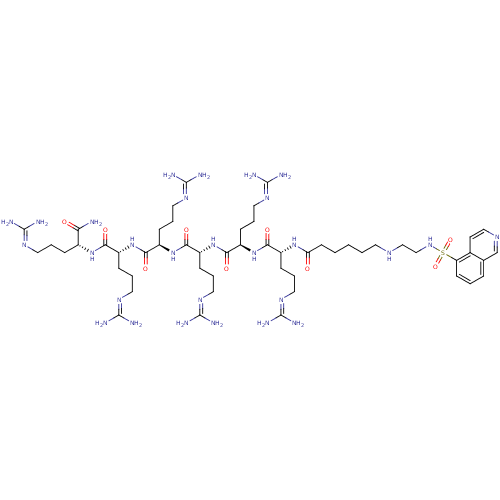 (ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Homo sapiens (Human)) | BDBM50199310 (2S,3S,4R,5R)-5-6-amino-9H-purin-9-yl)-N-12R,15R,18...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry Curated by ChEMBL | Assay Description Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide | J Med Chem 49: 7150-9 (2006) Article DOI: 10.1021/jm0605942 BindingDB Entry DOI: 10.7270/Q2542N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Homo sapiens (Human)) | BDBM50199310 (2S,3S,4R,5R)-5-6-amino-9H-purin-9-yl)-N-12R,15R,18...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry Curated by ChEMBL | Assay Description Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide | J Med Chem 49: 7150-9 (2006) Article DOI: 10.1021/jm0605942 BindingDB Entry DOI: 10.7270/Q2542N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Mus musculus (mouse)) | BDBM27227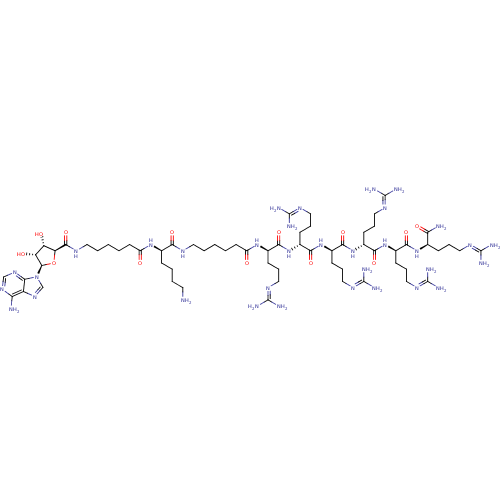 ((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Homo sapiens (Human)) | BDBM27248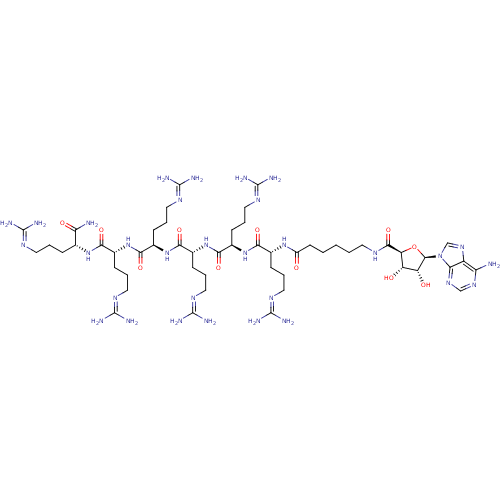 (6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry Curated by ChEMBL | Assay Description Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide | J Med Chem 49: 7150-9 (2006) Article DOI: 10.1021/jm0605942 BindingDB Entry DOI: 10.7270/Q2542N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Mus musculus (mouse)) | BDBM27248 (6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Homo sapiens (Human)) | BDBM27248 (6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry Curated by ChEMBL | Assay Description Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide | J Med Chem 49: 7150-9 (2006) Article DOI: 10.1021/jm0605942 BindingDB Entry DOI: 10.7270/Q2542N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010587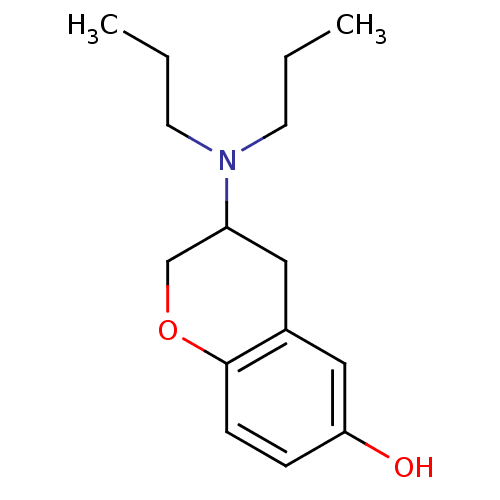 (3-Dipropylamino-chroman-6-ol | CHEMBL69414) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Affinity of the compound for [3H]-N-propylnorapomorphine (NPA) Dopamine receptor D2 | J Med Chem 33: 445-50 (1990) BindingDB Entry DOI: 10.7270/Q2BK1B94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Mus musculus (mouse)) | BDBM27226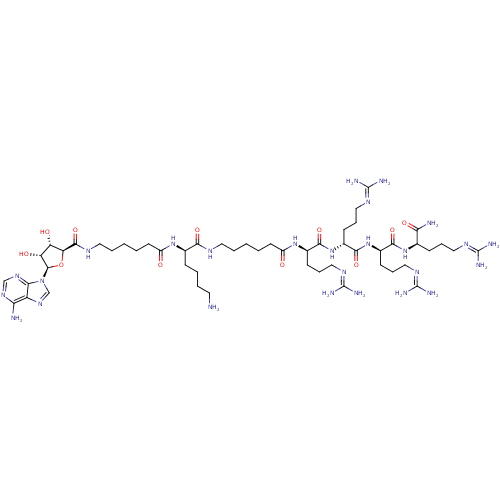 ((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM27227 ((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50002338 ((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description Affinity for displacement of [3H]-WB-4101 labeled Dopamine receptor D1 | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Mus musculus (mouse)) | BDBM27229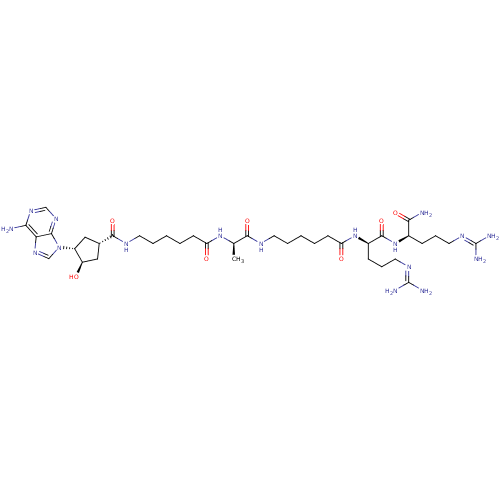 (6-{[(1S,3R,4R)-3-(6-amino-9H-purin-9-yl)-4-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration of the compound inhibiting specific binding of [3H]-haloperidol to Dopamine receptor D2 from rat striatal brain. | J Med Chem 33: 445-50 (1990) BindingDB Entry DOI: 10.7270/Q2BK1B94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM27249 (ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM27248 (6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM27250 (6-{[(1S,3R,4R)-3-(6-amino-9H-purin-9-yl)-4-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Mus musculus (mouse)) | BDBM27225 ((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM27226 ((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Mus musculus (mouse)) | BDBM27228 (6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Mus musculus (mouse)) | BDBM15211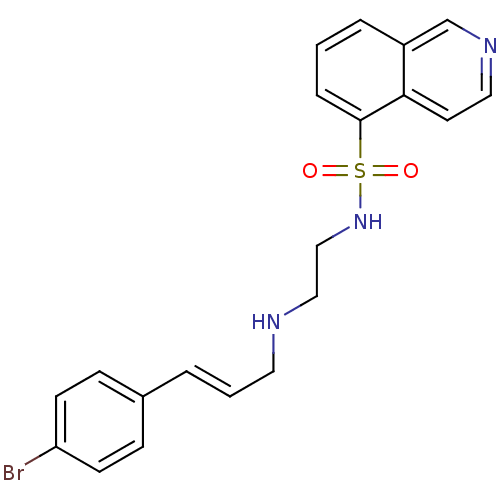 (CHEMBL104264 | H-89 | H89 | HT-89 (H-89) | N-(2-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010586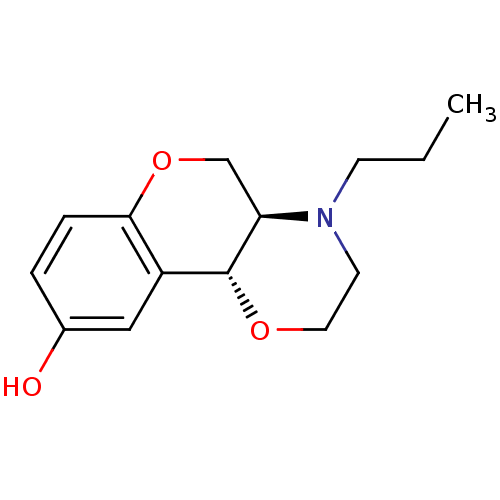 ((+)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4,9-diox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration of the compound inhibiting specific binding of [3H]-haloperidol to Dopamine receptor D2 from rat striatal brain. | J Med Chem 33: 445-50 (1990) BindingDB Entry DOI: 10.7270/Q2BK1B94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010586 ((+)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4,9-diox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 409 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Affinity of the compound for [3H]-N-propylnorapomorphine (NPA) Dopamine receptor D2 | J Med Chem 33: 445-50 (1990) BindingDB Entry DOI: 10.7270/Q2BK1B94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010586 ((+)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4,9-diox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 409 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration of the compound inhibiting specific binding of [3H]-haloperidol to Dopamine receptor D2 from rat striatal brain. | J Med Chem 33: 445-50 (1990) BindingDB Entry DOI: 10.7270/Q2BK1B94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15211 (CHEMBL104264 | H-89 | H89 | HT-89 (H-89) | N-(2-{[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 662 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM27225 ((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 774 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010587 (3-Dipropylamino-chroman-6-ol | CHEMBL69414) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration of the compound inhibiting specific binding of [3H]-haloperidol to Dopamine receptor D2 from rat striatal brain. | J Med Chem 33: 445-50 (1990) BindingDB Entry DOI: 10.7270/Q2BK1B94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019909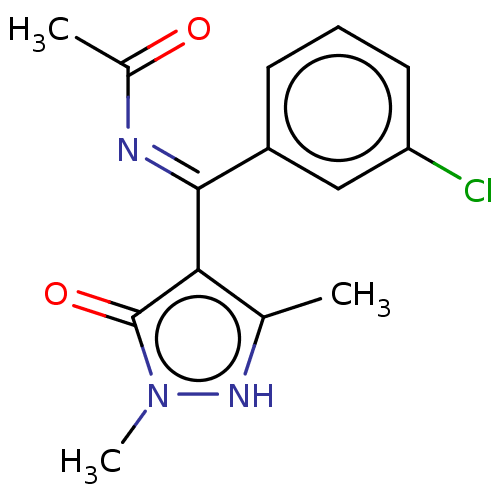 (CHEMBL3144632 | N-[(3-Chloro-phenyl)-(5-hydroxy-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019916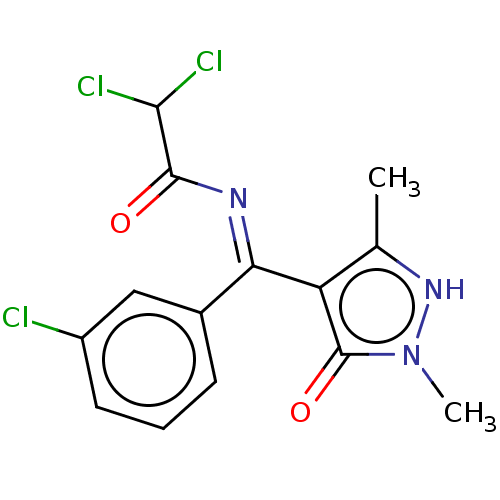 (2,2-Dichloro-N-[(3-chloro-phenyl)-(5-hydroxy-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50002338 ((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description Affinity for displacement of [3H]-clonidine labeled Dopamine receptor D1 | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM27224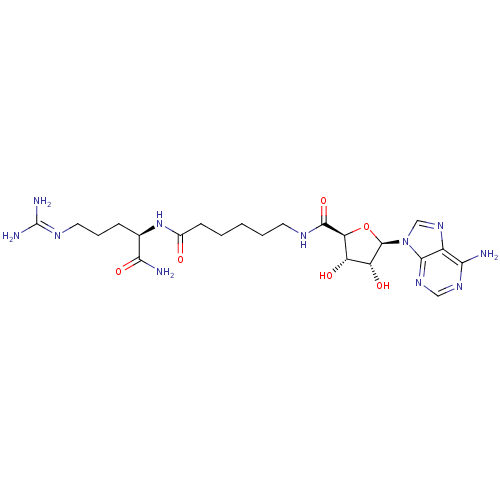 (6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM27229 (6-{[(1S,3R,4R)-3-(6-amino-9H-purin-9-yl)-4-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Mus musculus (mouse)) | BDBM27224 (6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Mus musculus (mouse)) | BDBM27222 (6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM27228 (6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia | Assay Description The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... | J Med Chem 52: 308-21 (2009) Article DOI: 10.1021/jm800797n BindingDB Entry DOI: 10.7270/Q2RX99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019908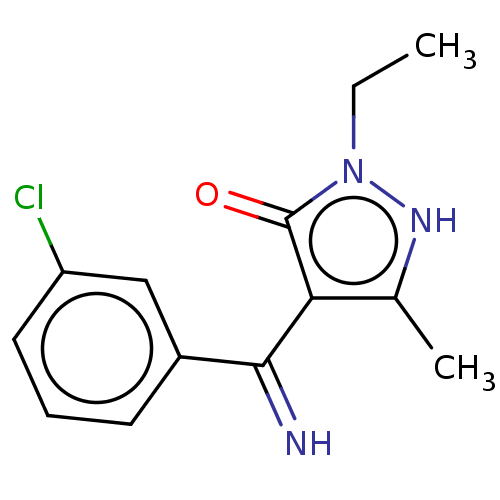 (4-[(3-Chloro-phenyl)-imino-methyl]-2-ethyl-5-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019912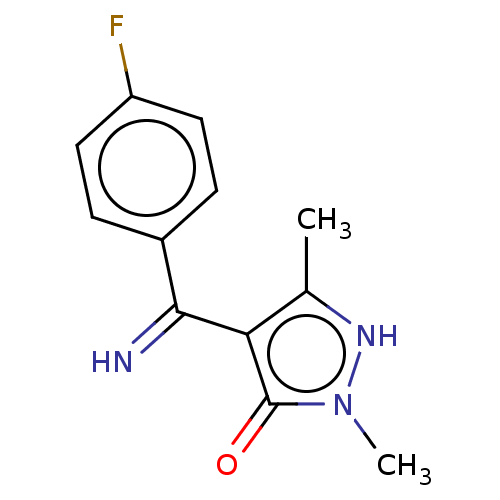 (4-[(4-Fluoro-phenyl)-imino-methyl]-2,5-dimethyl-2H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50019913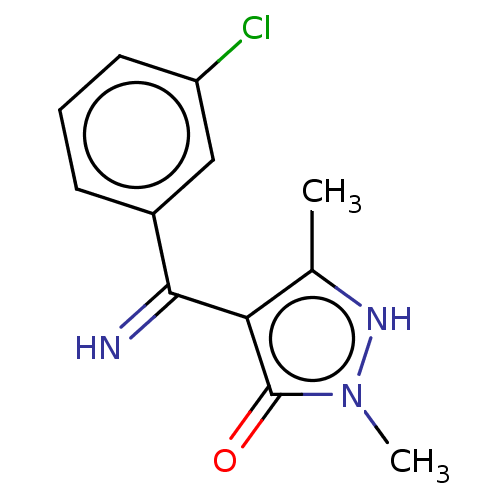 (4-[(3-Chloro-phenyl)-imino-methyl]-2,5-dimethyl-2H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description Affinity for displacement of [3H]-clonidine labeled Dopamine receptor D1 | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019914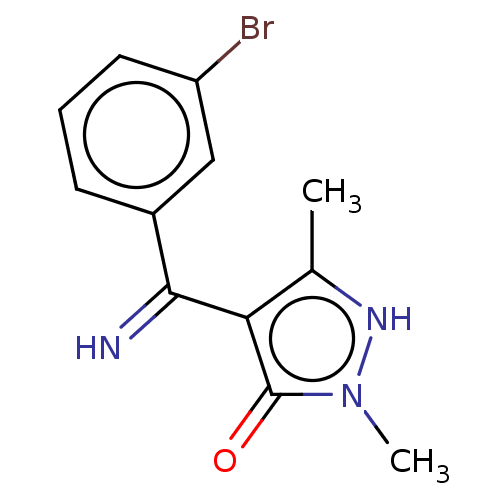 (4-[(3-Bromo-phenyl)-imino-methyl]-2,5-dimethyl-2H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019915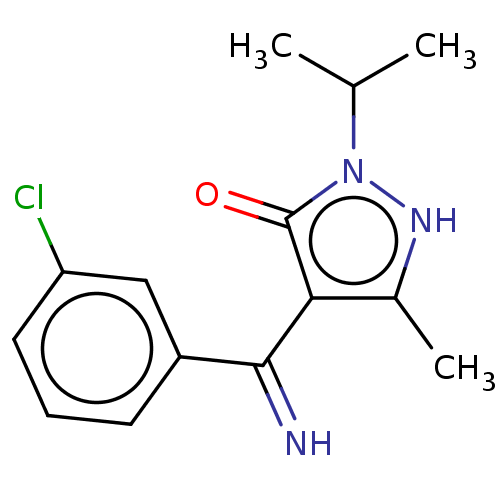 (4-[(3-Chloro-phenyl)-imino-methyl]-2-isopropyl-5-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019913 (4-[(3-Chloro-phenyl)-imino-methyl]-2,5-dimethyl-2H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019918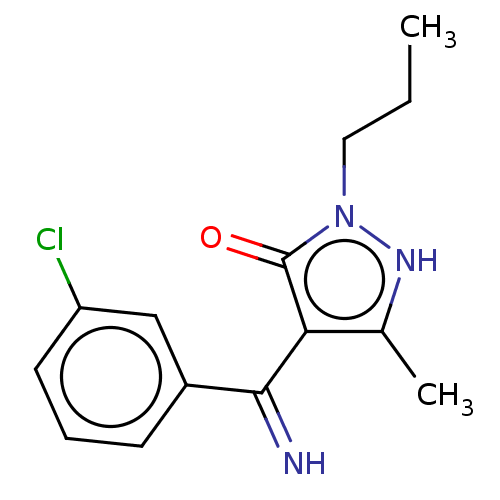 (4-[(3-Chloro-phenyl)-imino-methyl]-5-methyl-2-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019917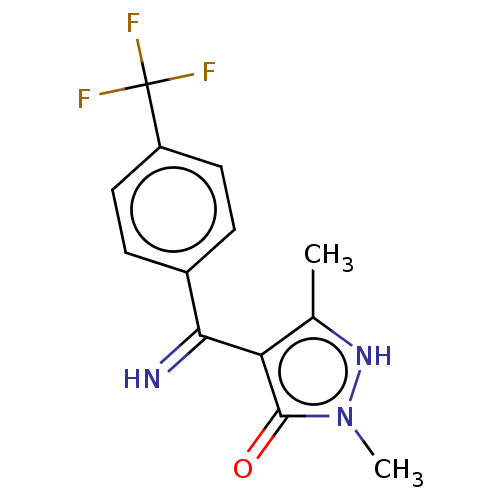 (4-[Imino-(4-trifluoromethyl-phenyl)-methyl]-2,5-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019910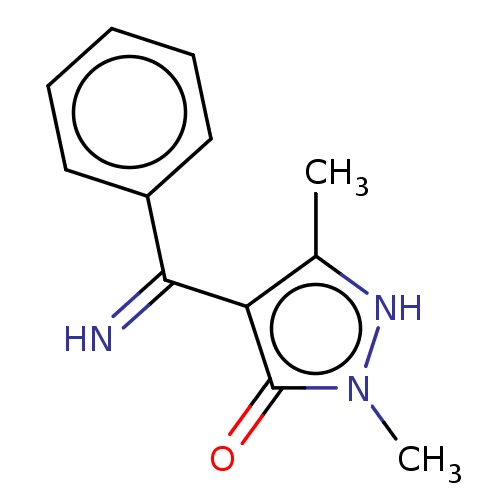 (4-(Imino-phenyl-methyl)-2,5-dimethyl-2H-pyrazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019911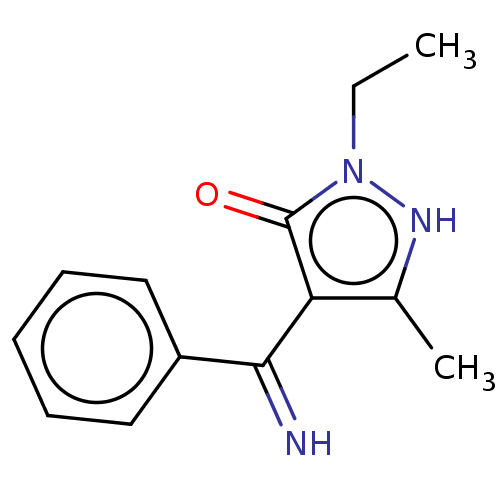 (2-Ethyl-4-(imino-phenyl-methyl)-5-methyl-2H-pyrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50019913 (4-[(3-Chloro-phenyl)-imino-methyl]-2,5-dimethyl-2H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description Affinity for displacement of [3H]-SCH-23,390 labeled Dopamine receptor D1 | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50019913 (4-[(3-Chloro-phenyl)-imino-methyl]-2,5-dimethyl-2H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description Affinity for displacement of [3H]-WB-4101 labeled Dopamine receptor D1 | J Med Chem 30: 1807-12 (1987) BindingDB Entry DOI: 10.7270/Q29K497N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |