 Found 247 hits with Last Name = 'ma' and Initial = 'gl'
Found 247 hits with Last Name = 'ma' and Initial = 'gl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Rattus norvegicus) | BDBM50003025
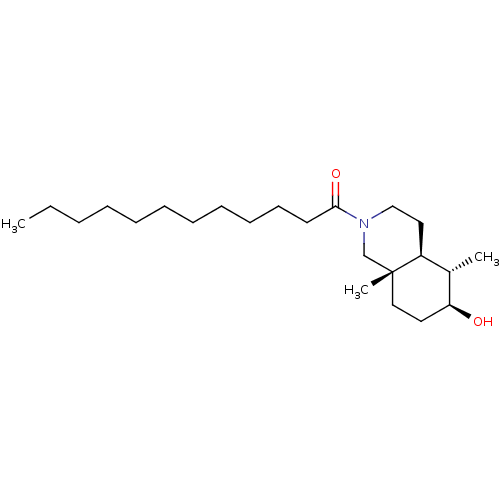
(1-((4aS,5S,6S,8aS)-6-Hydroxy-5,8a-dimethyl-octahyd...)Show SMILES CCCCCCCCCCCC(=O)N1CC[C@H]2[C@H](C)[C@@H](O)CC[C@]2(C)C1 Show InChI InChI=1S/C23H43NO2/c1-4-5-6-7-8-9-10-11-12-13-22(26)24-17-15-20-19(2)21(25)14-16-23(20,3)18-24/h19-21,25H,4-18H2,1-3H3/t19-,20-,21-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against purified rat liver Oxidosqualene cyclase (OSC) |
Bioorg Med Chem Lett 3: 1175-1178 (1993)
Article DOI: 10.1016/S0960-894X(00)80309-1
BindingDB Entry DOI: 10.7270/Q2BK1C83 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10868
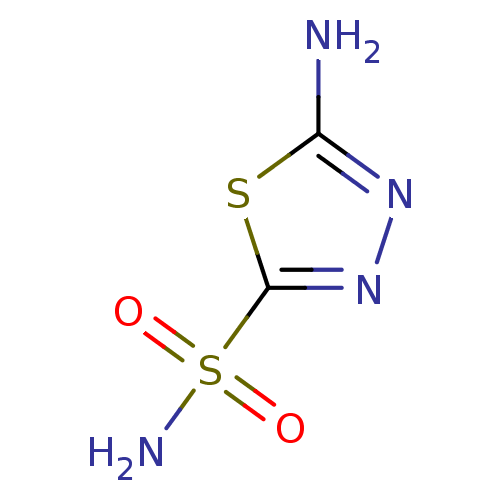
(1,3,4-Thiadiazole-2-sulfonamide, 6 | 1,3,4-thiadia...)Show InChI InChI=1S/C2H4N4O2S2/c3-1-5-6-2(9-1)10(4,7)8/h(H2,3,5)(H2,4,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10868

(1,3,4-Thiadiazole-2-sulfonamide, 6 | 1,3,4-thiadia...)Show InChI InChI=1S/C2H4N4O2S2/c3-1-5-6-2(9-1)10(4,7)8/h(H2,3,5)(H2,4,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165742
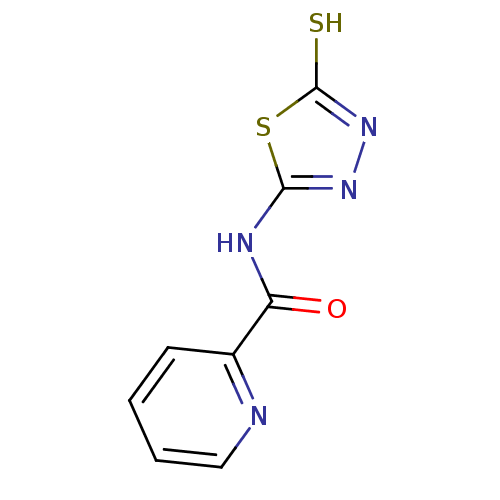
(CHEMBL194016 | Pyridine-2-carboxylic acid (5-merca...)Show InChI InChI=1S/C8H6N4OS2/c13-6(5-3-1-2-4-9-5)10-7-11-12-8(14)15-7/h1-4H,(H,12,14)(H,10,11,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50282635
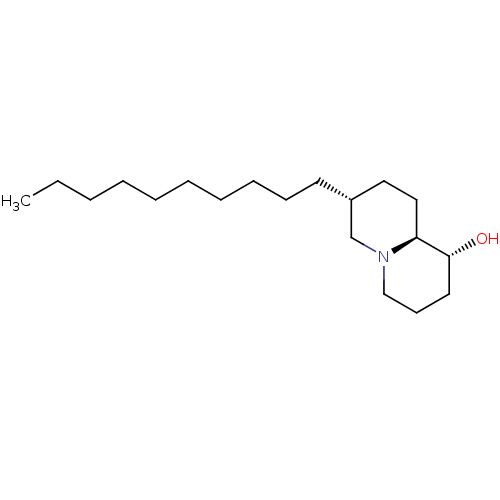
((1R,7R,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...)Show InChI InChI=1S/C19H37NO/c1-2-3-4-5-6-7-8-9-11-17-13-14-18-19(21)12-10-15-20(18)16-17/h17-19,21H,2-16H2,1H3/t17-,18+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki |
Bioorg Med Chem Lett 4: 1317-1318 (1994)
Article DOI: 10.1016/S0960-894X(01)80352-8
BindingDB Entry DOI: 10.7270/Q23T9H5P |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50282634
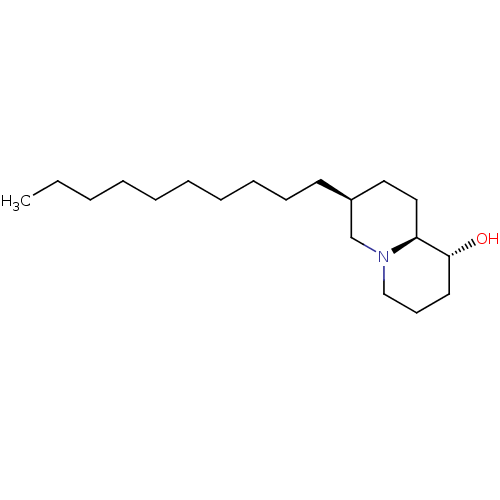
((1R,7S,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...)Show InChI InChI=1S/C19H37NO/c1-2-3-4-5-6-7-8-9-11-17-13-14-18-19(21)12-10-15-20(18)16-17/h17-19,21H,2-16H2,1H3/t17-,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki |
Bioorg Med Chem Lett 4: 1317-1318 (1994)
Article DOI: 10.1016/S0960-894X(01)80352-8
BindingDB Entry DOI: 10.7270/Q23T9H5P |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165739
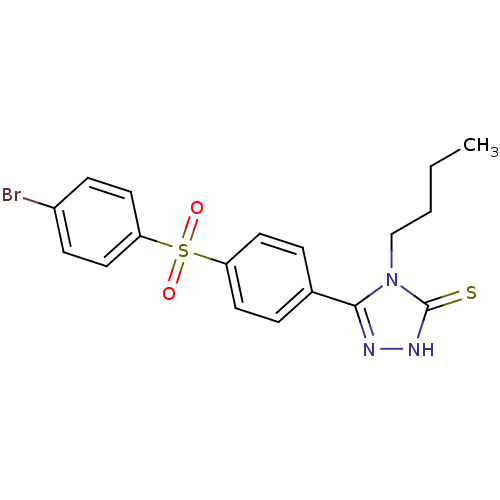
(5-[4-(4-Bromo-benzenesulfonyl)-phenyl]-4-butyl-4H-...)Show SMILES CCCCn1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C18H18BrN3O2S2/c1-2-3-12-22-17(20-21-18(22)25)13-4-8-15(9-5-13)26(23,24)16-10-6-14(19)7-11-16/h4-11H,2-3,12H2,1H3,(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165736
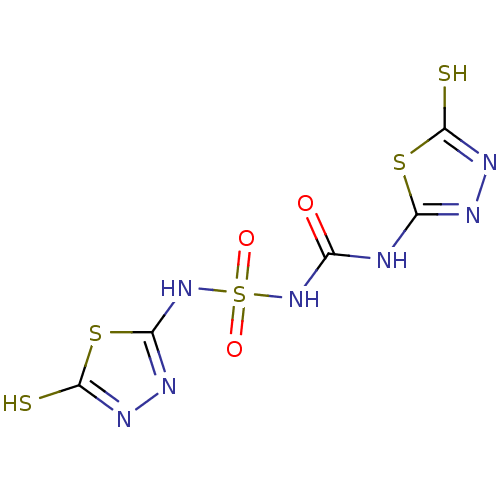
(1-(5-sulfanyl-1,3,4-thiadiazol-2-yl)-3-[(5-sulfany...)Show InChI InChI=1S/C5H5N7O3S5/c13-1(6-2-7-9-4(16)18-2)11-20(14,15)12-3-8-10-5(17)19-3/h(H,8,12)(H,9,16)(H,10,17)(H2,6,7,11,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165745
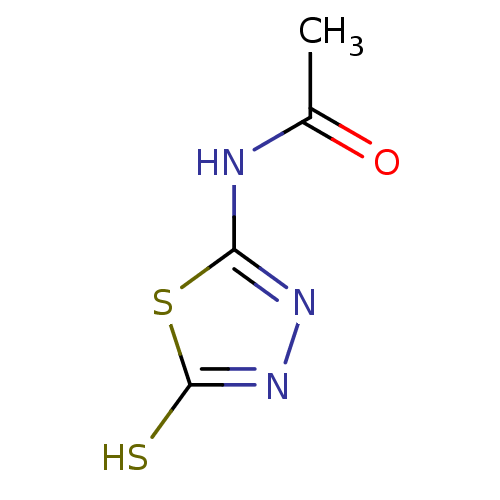
(CHEMBL382111 | N-(5-Mercapto-[1,3,4]thiadiazol-2-y...)Show InChI InChI=1S/C4H5N3OS2/c1-2(8)5-3-6-7-4(9)10-3/h1H3,(H,7,9)(H,5,6,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165740
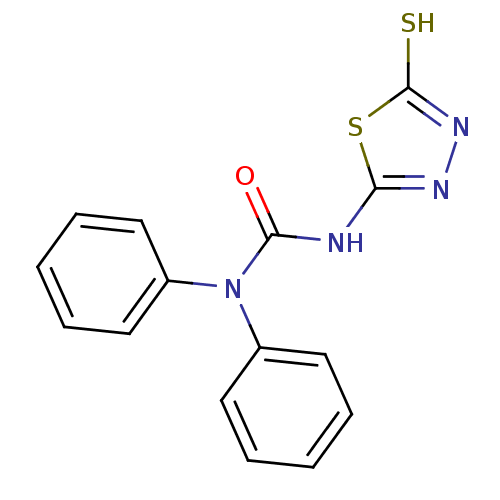
(3-(5-Mercapto-[1,3,4]thiadiazol-2-yl)-1,1-diphenyl...)Show InChI InChI=1S/C15H12N4OS2/c20-14(16-13-17-18-15(21)22-13)19(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H,18,21)(H,16,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165743
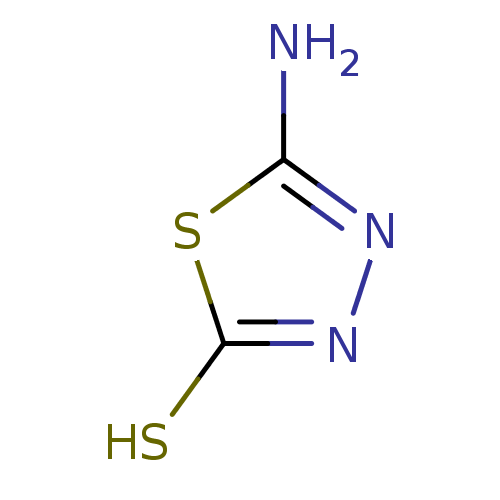
(5-Amino-[1,3,4]thiadiazole-2-thiol | CHEMBL372507)Show InChI InChI=1S/C2H3N3S2/c3-1-4-5-2(6)7-1/h(H2,3,4)(H,5,6) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165732
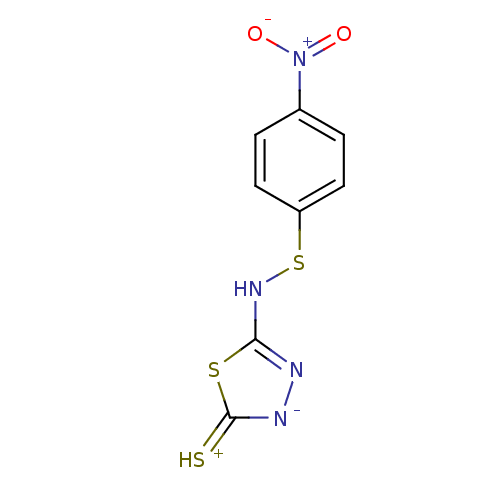
(5-(4-Nitro-phenylsulfanylamino)-[1,3,4]thiadiazole...)Show InChI InChI=1S/C8H6N4O2S3/c13-12(14)5-1-3-6(4-2-5)17-11-7-9-10-8(15)16-7/h1-4H,(H2,9,10,11,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165748
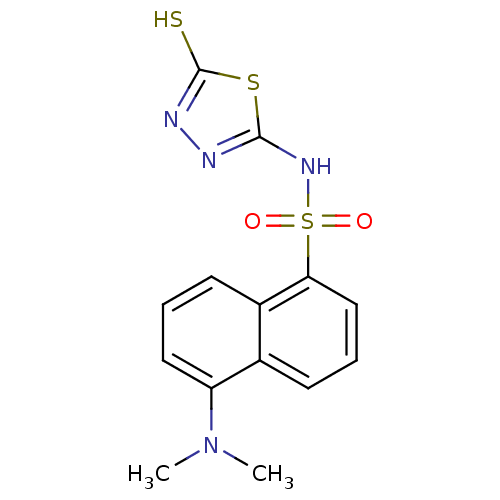
(5-Dimethylamino-naphthalene-1-sulfonic acid (5-mer...)Show InChI InChI=1S/C14H14N4O2S3/c1-18(2)11-7-3-6-10-9(11)5-4-8-12(10)23(19,20)17-13-15-16-14(21)22-13/h3-8H,1-2H3,(H,15,17)(H,16,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165748

(5-Dimethylamino-naphthalene-1-sulfonic acid (5-mer...)Show InChI InChI=1S/C14H14N4O2S3/c1-18(2)11-7-3-6-10-9(11)5-4-8-12(10)23(19,20)17-13-15-16-14(21)22-13/h3-8H,1-2H3,(H,15,17)(H,16,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165736

(1-(5-sulfanyl-1,3,4-thiadiazol-2-yl)-3-[(5-sulfany...)Show InChI InChI=1S/C5H5N7O3S5/c13-1(6-2-7-9-4(16)18-2)11-20(14,15)12-3-8-10-5(17)19-3/h(H,8,12)(H,9,16)(H,10,17)(H2,6,7,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165732

(5-(4-Nitro-phenylsulfanylamino)-[1,3,4]thiadiazole...)Show InChI InChI=1S/C8H6N4O2S3/c13-12(14)5-1-3-6(4-2-5)17-11-7-9-10-8(15)16-7/h1-4H,(H2,9,10,11,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165735

(2-(5-Mercapto-[1,3,4]thiadiazol-2-ylcarbamoyl)-nic...)Show InChI InChI=1S/C9H6N4O3S2/c14-6(11-8-12-13-9(17)18-8)5-4(7(15)16)2-1-3-10-5/h1-3H,(H,13,17)(H,15,16)(H,11,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165741
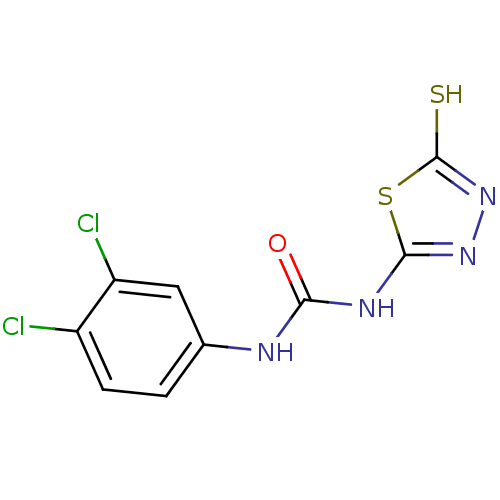
(1-(3,4-Dichloro-phenyl)-3-(5-mercapto-[1,3,4]thiad...)Show InChI InChI=1S/C9H6Cl2N4OS2/c10-5-2-1-4(3-6(5)11)12-7(16)13-8-14-15-9(17)18-8/h1-3H,(H,15,17)(H2,12,13,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10868

(1,3,4-Thiadiazole-2-sulfonamide, 6 | 1,3,4-thiadia...)Show InChI InChI=1S/C2H4N4O2S2/c3-1-5-6-2(9-1)10(4,7)8/h(H2,3,5)(H2,4,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165745

(CHEMBL382111 | N-(5-Mercapto-[1,3,4]thiadiazol-2-y...)Show InChI InChI=1S/C4H5N3OS2/c1-2(8)5-3-6-7-4(9)10-3/h1H3,(H,7,9)(H,5,6,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165738
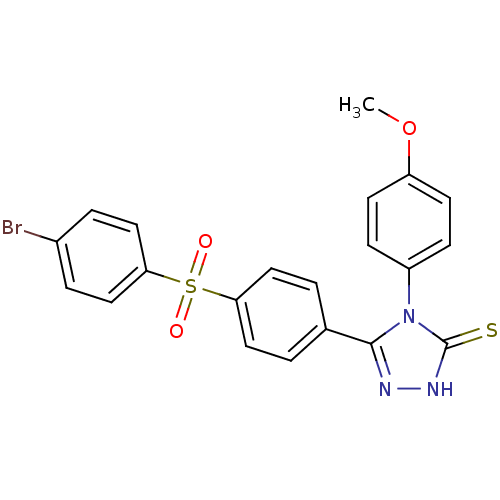
(5-[4-(4-Bromo-benzenesulfonyl)-phenyl]-4-(4-methox...)Show SMILES COc1ccc(cc1)-n1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C21H16BrN3O3S2/c1-28-17-8-6-16(7-9-17)25-20(23-24-21(25)29)14-2-10-18(11-3-14)30(26,27)19-12-4-15(22)5-13-19/h2-13H,1H3,(H,24,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165735

(2-(5-Mercapto-[1,3,4]thiadiazol-2-ylcarbamoyl)-nic...)Show InChI InChI=1S/C9H6N4O3S2/c14-6(11-8-12-13-9(17)18-8)5-4(7(15)16)2-1-3-10-5/h1-3H,(H,13,17)(H,15,16)(H,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165741

(1-(3,4-Dichloro-phenyl)-3-(5-mercapto-[1,3,4]thiad...)Show InChI InChI=1S/C9H6Cl2N4OS2/c10-5-2-1-4(3-6(5)11)12-7(16)13-8-14-15-9(17)18-8/h1-3H,(H,15,17)(H2,12,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165743

(5-Amino-[1,3,4]thiadiazole-2-thiol | CHEMBL372507)Show InChI InChI=1S/C2H3N3S2/c3-1-4-5-2(6)7-1/h(H2,3,4)(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165740

(3-(5-Mercapto-[1,3,4]thiadiazol-2-yl)-1,1-diphenyl...)Show InChI InChI=1S/C15H12N4OS2/c20-14(16-13-17-18-15(21)22-13)19(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H,18,21)(H,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165743

(5-Amino-[1,3,4]thiadiazole-2-thiol | CHEMBL372507)Show InChI InChI=1S/C2H3N3S2/c3-1-4-5-2(6)7-1/h(H2,3,4)(H,5,6) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165745

(CHEMBL382111 | N-(5-Mercapto-[1,3,4]thiadiazol-2-y...)Show InChI InChI=1S/C4H5N3OS2/c1-2(8)5-3-6-7-4(9)10-3/h1H3,(H,7,9)(H,5,6,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165744
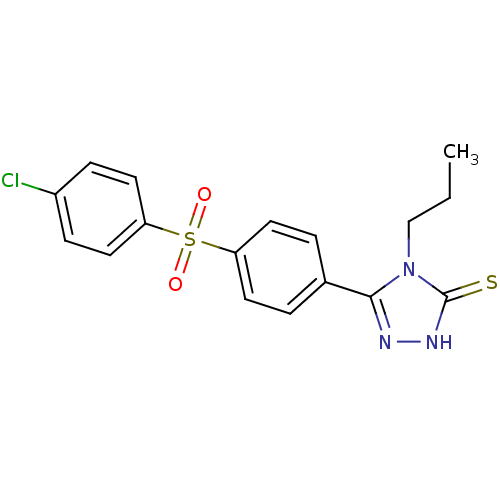
(3-(4-(4-chlorophenylsulfonyl)phenyl)-4-propyl-1H-1...)Show SMILES CCCn1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C17H16ClN3O2S2/c1-2-11-21-16(19-20-17(21)24)12-3-7-14(8-4-12)25(22,23)15-9-5-13(18)6-10-15/h3-10H,2,11H2,1H3,(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165739

(5-[4-(4-Bromo-benzenesulfonyl)-phenyl]-4-butyl-4H-...)Show SMILES CCCCn1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C18H18BrN3O2S2/c1-2-3-12-22-17(20-21-18(22)25)13-4-8-15(9-5-13)26(23,24)16-10-6-14(19)7-11-16/h4-11H,2-3,12H2,1H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165738

(5-[4-(4-Bromo-benzenesulfonyl)-phenyl]-4-(4-methox...)Show SMILES COc1ccc(cc1)-n1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C21H16BrN3O3S2/c1-28-17-8-6-16(7-9-17)25-20(23-24-21(25)29)14-2-10-18(11-3-14)30(26,27)19-12-4-15(22)5-13-19/h2-13H,1H3,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165742

(CHEMBL194016 | Pyridine-2-carboxylic acid (5-merca...)Show InChI InChI=1S/C8H6N4OS2/c13-6(5-3-1-2-4-9-5)10-7-11-12-8(14)15-7/h1-4H,(H,12,14)(H,10,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165739

(5-[4-(4-Bromo-benzenesulfonyl)-phenyl]-4-butyl-4H-...)Show SMILES CCCCn1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C18H18BrN3O2S2/c1-2-3-12-22-17(20-21-18(22)25)13-4-8-15(9-5-13)26(23,24)16-10-6-14(19)7-11-16/h4-11H,2-3,12H2,1H3,(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165730
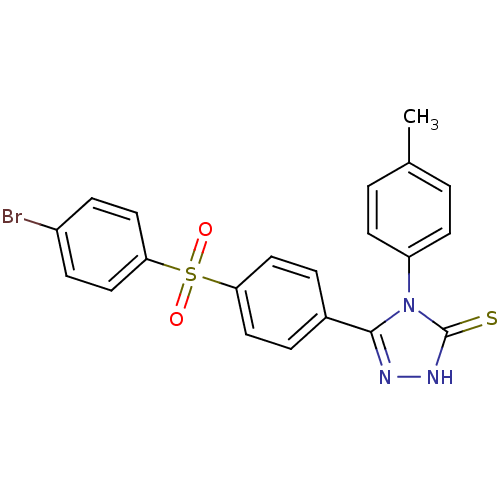
(5-[4-(4-Bromo-benzenesulfonyl)-phenyl]-4-p-tolyl-4...)Show SMILES Cc1ccc(cc1)-n1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C21H16BrN3O2S2/c1-14-2-8-17(9-3-14)25-20(23-24-21(25)28)15-4-10-18(11-5-15)29(26,27)19-12-6-16(22)7-13-19/h2-13H,1H3,(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165737
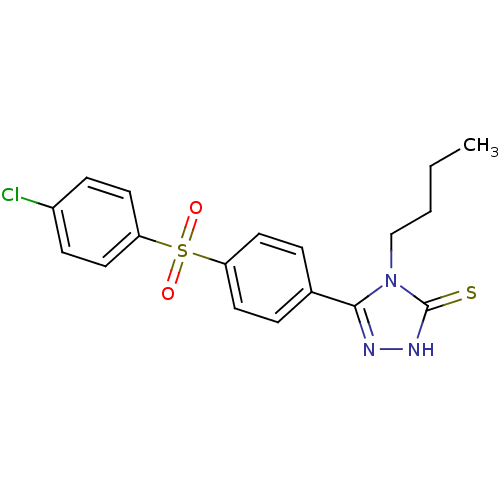
(4-Butyl-5-[4-(4-chloro-benzenesulfonyl)-phenyl]-4H...)Show SMILES CCCCn1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C18H18ClN3O2S2/c1-2-3-12-22-17(20-21-18(22)25)13-4-8-15(9-5-13)26(23,24)16-10-6-14(19)7-11-16/h4-11H,2-3,12H2,1H3,(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
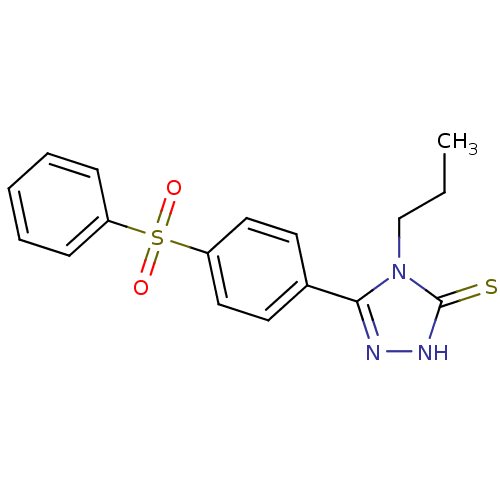
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165734
(3-(4-(phenylsulfonyl)phenyl)-4-propyl-1H-1,2,4-tri...)Show SMILES CCCn1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C17H17N3O2S2/c1-2-12-20-16(18-19-17(20)23)13-8-10-15(11-9-13)24(21,22)14-6-4-3-5-7-14/h3-11H,2,12H2,1H3,(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165733
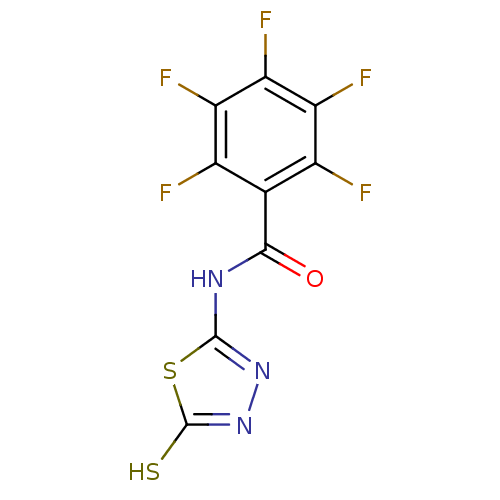
(2,3,4,5,6-Pentafluoro-N-(5-mercapto-[1,3,4]thiadia...)Show InChI InChI=1S/C9H2F5N3OS2/c10-2-1(3(11)5(13)6(14)4(2)12)7(18)15-8-16-17-9(19)20-8/h(H,17,19)(H,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165738

(5-[4-(4-Bromo-benzenesulfonyl)-phenyl]-4-(4-methox...)Show SMILES COc1ccc(cc1)-n1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C21H16BrN3O3S2/c1-28-17-8-6-16(7-9-17)25-20(23-24-21(25)29)14-2-10-18(11-3-14)30(26,27)19-12-4-15(22)5-13-19/h2-13H,1H3,(H,24,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165737

(4-Butyl-5-[4-(4-chloro-benzenesulfonyl)-phenyl]-4H...)Show SMILES CCCCn1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C18H18ClN3O2S2/c1-2-3-12-22-17(20-21-18(22)25)13-4-8-15(9-5-13)26(23,24)16-10-6-14(19)7-11-16/h4-11H,2-3,12H2,1H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
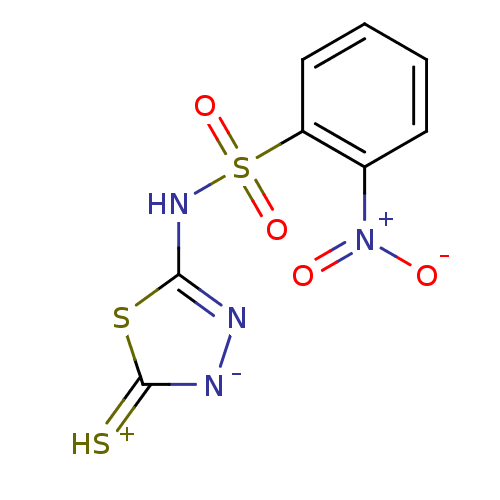
(Homo sapiens (Human)) | BDBM50165729
(CHEMBL383159 | N-(5-Mercapto-[1,3,4]thiadiazol-2-y...)Show SMILES [O-][N+](=O)c1ccccc1S(=O)(=O)Nc1n[n-]c(=[SH+])s1 Show InChI InChI=1S/C8H6N4O4S3/c13-12(14)5-3-1-2-4-6(5)19(15,16)11-7-9-10-8(17)18-7/h1-4H,(H2,9,10,11,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165735

(2-(5-Mercapto-[1,3,4]thiadiazol-2-ylcarbamoyl)-nic...)Show InChI InChI=1S/C9H6N4O3S2/c14-6(11-8-12-13-9(17)18-8)5-4(7(15)16)2-1-3-10-5/h1-3H,(H,13,17)(H,15,16)(H,11,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165730

(5-[4-(4-Bromo-benzenesulfonyl)-phenyl]-4-p-tolyl-4...)Show SMILES Cc1ccc(cc1)-n1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C21H16BrN3O2S2/c1-14-2-8-17(9-3-14)25-20(23-24-21(25)28)15-4-10-18(11-5-15)29(26,27)19-12-6-16(22)7-13-19/h2-13H,1H3,(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50165730

(5-[4-(4-Bromo-benzenesulfonyl)-phenyl]-4-p-tolyl-4...)Show SMILES Cc1ccc(cc1)-n1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C21H16BrN3O2S2/c1-14-2-8-17(9-3-14)25-20(23-24-21(25)28)15-4-10-18(11-5-15)29(26,27)19-12-6-16(22)7-13-19/h2-13H,1H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165746
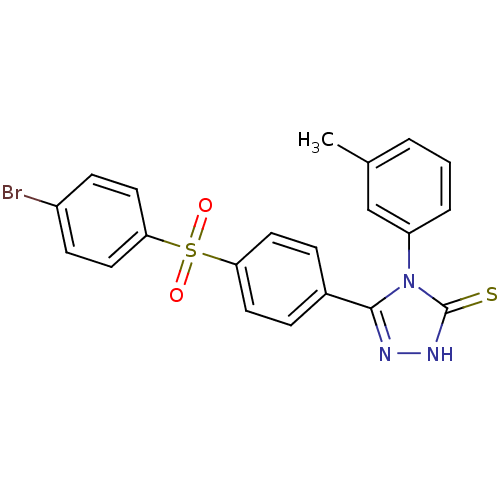
(5-[4-(4-Bromo-benzenesulfonyl)-phenyl]-4-m-tolyl-4...)Show SMILES Cc1cccc(c1)-n1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C21H16BrN3O2S2/c1-14-3-2-4-17(13-14)25-20(23-24-21(25)28)15-5-9-18(10-6-15)29(26,27)19-11-7-16(22)8-12-19/h2-13H,1H3,(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165732

(5-(4-Nitro-phenylsulfanylamino)-[1,3,4]thiadiazole...)Show InChI InChI=1S/C8H6N4O2S3/c13-12(14)5-1-3-6(4-2-5)17-11-7-9-10-8(15)16-7/h1-4H,(H2,9,10,11,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165741

(1-(3,4-Dichloro-phenyl)-3-(5-mercapto-[1,3,4]thiad...)Show InChI InChI=1S/C9H6Cl2N4OS2/c10-5-2-1-4(3-6(5)11)12-7(16)13-8-14-15-9(17)18-8/h1-3H,(H,15,17)(H2,12,13,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50165740

(3-(5-Mercapto-[1,3,4]thiadiazol-2-yl)-1,1-diphenyl...)Show InChI InChI=1S/C15H12N4OS2/c20-14(16-13-17-18-15(21)22-13)19(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H,18,21)(H,16,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50165737

(4-Butyl-5-[4-(4-chloro-benzenesulfonyl)-phenyl]-4H...)Show SMILES CCCCn1c(n[nH]c1=S)-c1ccc(cc1)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C18H18ClN3O2S2/c1-2-3-12-22-17(20-21-18(22)25)13-4-8-15(9-5-13)26(23,24)16-10-6-14(19)7-11-16/h4-11H,2-3,12H2,1H3,(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bucharest
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase I |
Bioorg Med Chem Lett 15: 2347-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.088
BindingDB Entry DOI: 10.7270/Q2348JW7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data