

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343634 (4-((E)-4-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343641 (CHEMBL1774964 | cis-4-((E)-4-((S)-4-methyl-1-oxo-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343632 (4-((E)-4-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343644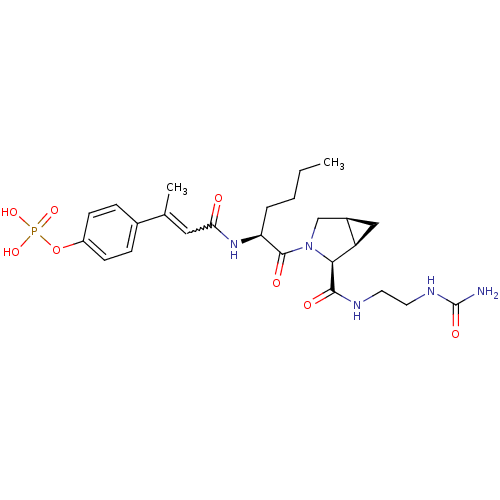 (CHEMBL1774967 | cis-4-((E)-4-oxo-4-((S)-1-oxo-1-((...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343633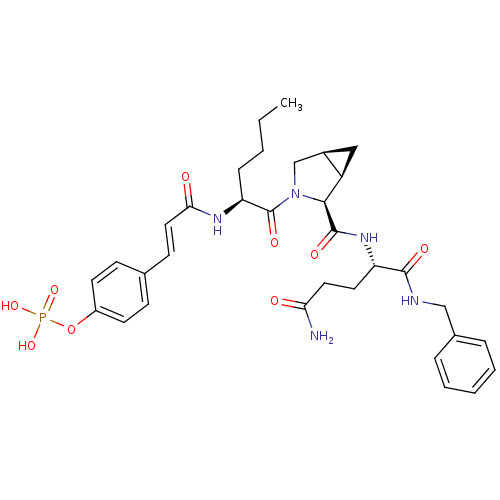 (4-((E)-3-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343635 (4-((E)-4-((3S,6S)-6-((S)-5-amino-1-(benzylamino)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343642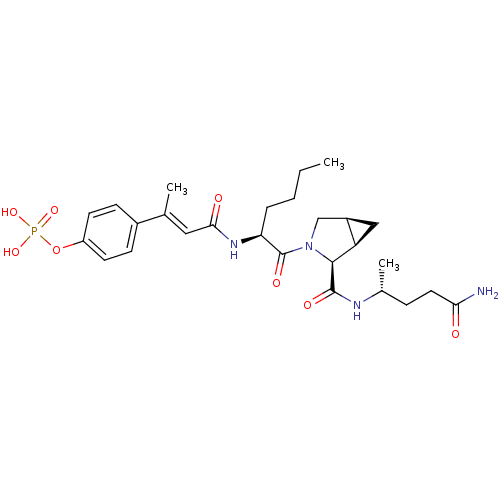 (CHEMBL1774965 | cis-4-((E)-4-((S)-1-((1R,2S,5S)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343631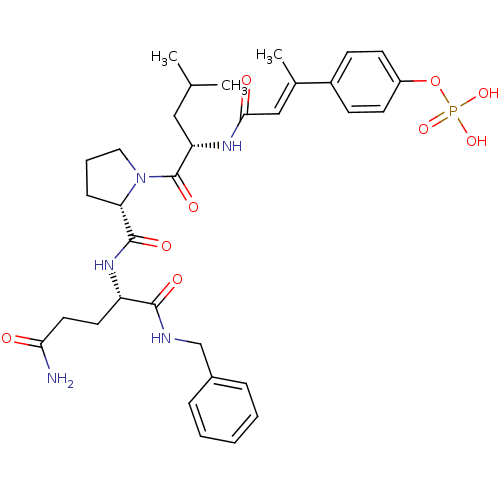 (4-((E)-4-((S)-1-((S)-2-((S)-5-amino-1-(benzylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343639 (CHEMBL1774962 | cis-4-((E)-4-((S)-1-((1R,2S,5S)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343638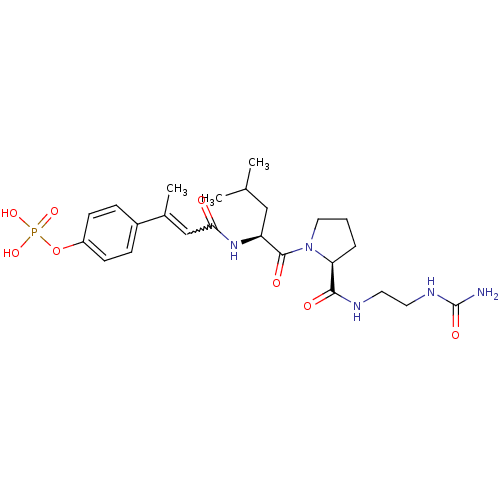 (4-((E)-4-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343645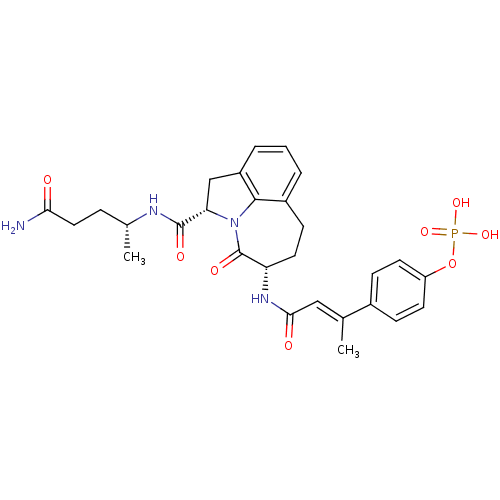 (4-((E)-4-((3S,6S)-6-((R)-5-amino-5-oxopentan-2-ylc...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343643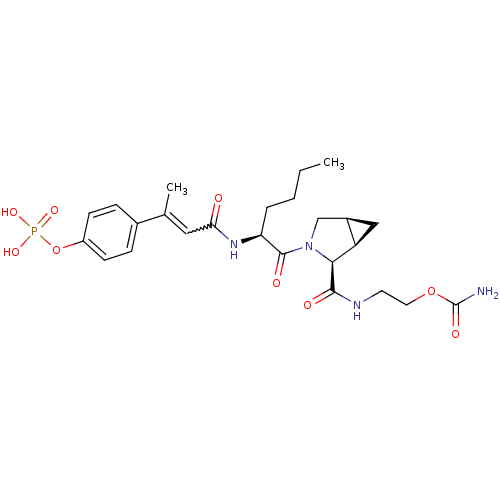 (CHEMBL1774966 | cis-2-((1R,2S,5S)-3-((S)-2-((E)-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343636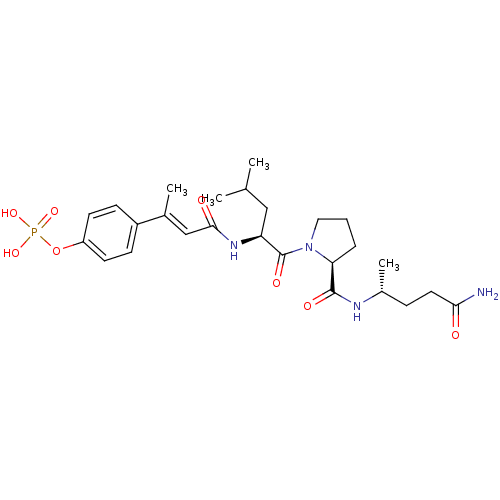 (4-((E)-4-((S)-1-((S)-2-((R)-5-amino-5-oxopentan-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343646 (2-((3S,6S)-4-oxo-3-((E)-3-(4-(phosphonooxy)phenyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343640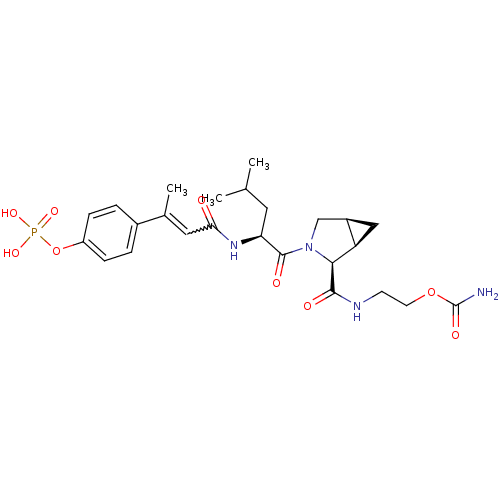 (CHEMBL1774963 | cis-2-((1R,2S,5S)-3-((S)-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343637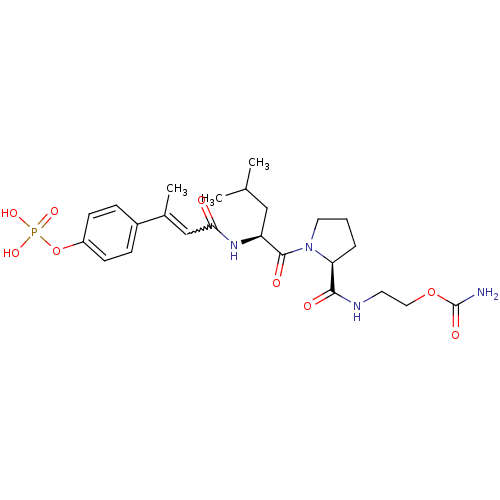 (2-((S)-1-((S)-4-methyl-2-((E)-3-(4-(phosphonooxy)p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343647 (4-((E)-4-oxo-4-((3S,6S)-4-oxo-6-(2-ureidoethylcarb...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 386 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of HDAC4 (unknown origin) | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM117170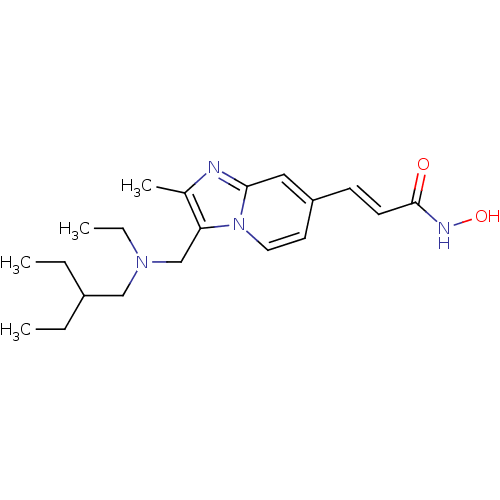 (US8648092, 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged HDAC1 expressed in baculovirus infected Sf9 insect cells Fluor-de-lys as substrate measured after 2 hrs by... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM117141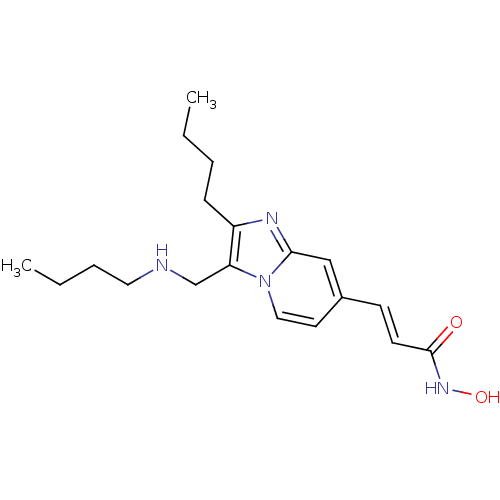 (US8648092, 72) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged HDAC1 expressed in baculovirus infected Sf9 insect cells Fluor-de-lys as substrate measured after 2 hrs by... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM117146 (US8648092, 77) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged HDAC1 expressed in baculovirus infected Sf9 insect cells Fluor-de-lys as substrate measured after 2 hrs by... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50531385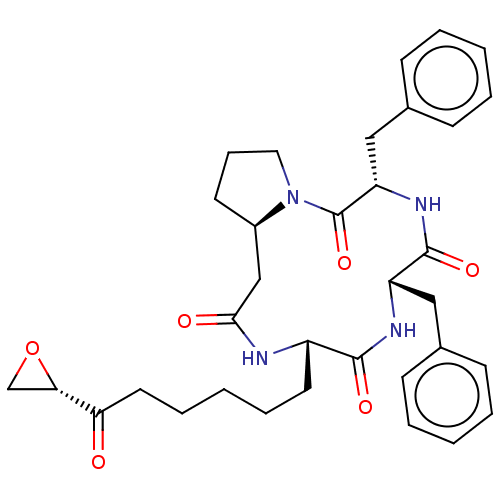 (CHEMBL4568666) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 expressed in NIH/3T3 cells using [3H]acetyl histone as substrate measured after 15 mins by liquid scintillation... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50531393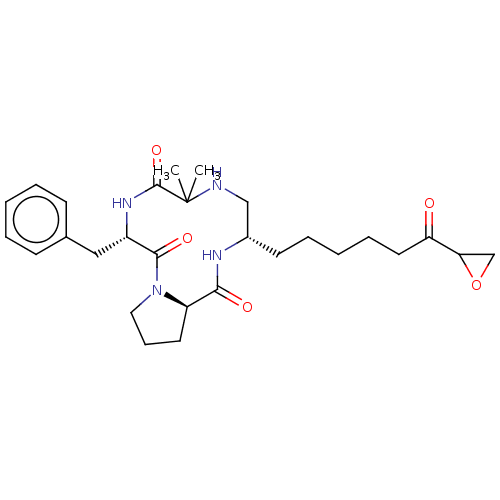 (CHEMBL4438063) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM506627 (US11046649, Ex. 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00679 BindingDB Entry DOI: 10.7270/Q2RJ4P9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50531382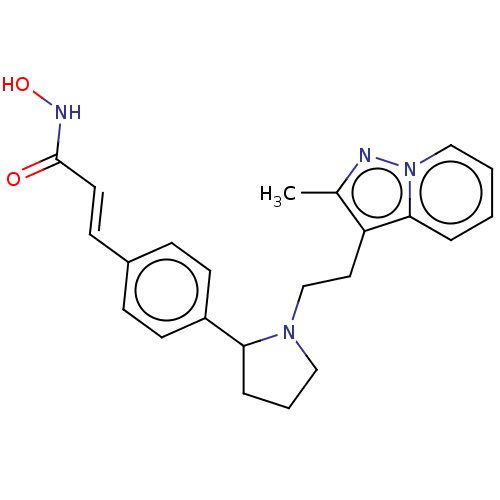 (CHEMBL4455057) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged HDAC1 (unknown origin) expressed in insect cells using poly (Glu, Tyr) 4:1 as substrate measured after 15 mins in presence o... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50578624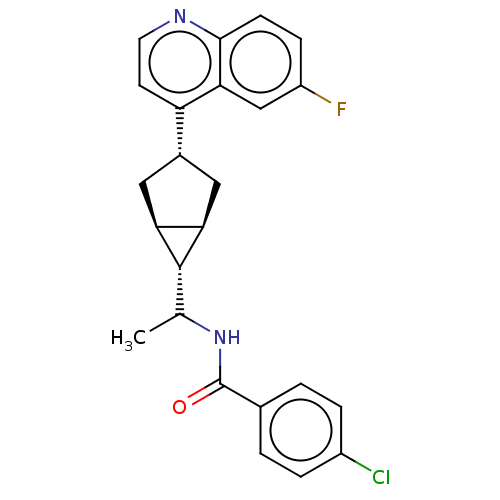 (CHEMBL4856710) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00679 BindingDB Entry DOI: 10.7270/Q2RJ4P9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50578623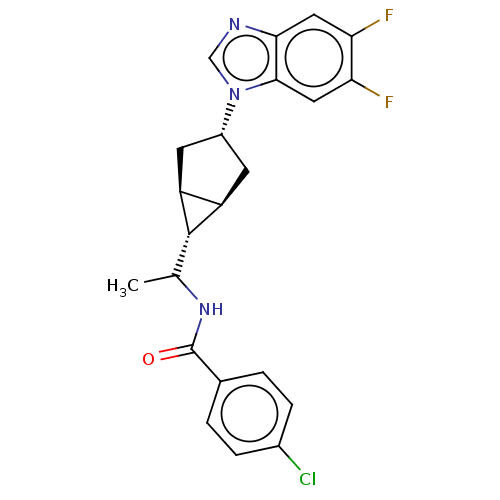 (CHEMBL4858888) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00679 BindingDB Entry DOI: 10.7270/Q2RJ4P9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50531391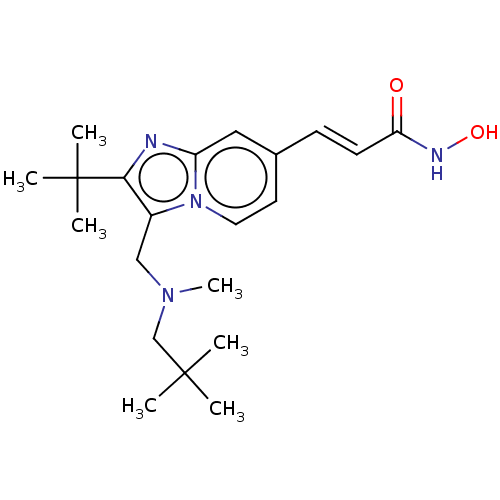 (CHEMBL4472220) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged HDAC1 expressed in baculovirus infected Sf9 insect cells Fluor-de-lys as substrate measured after 2 hrs by... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50531357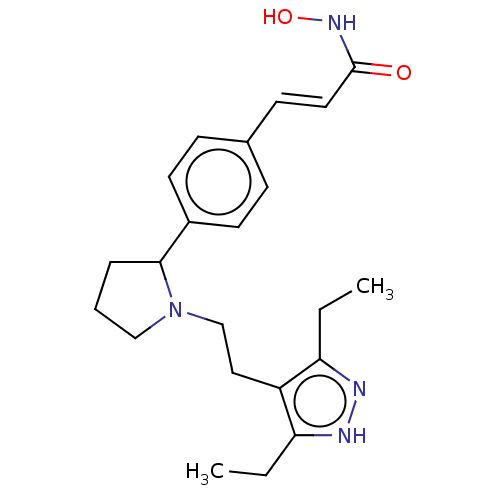 (CHEMBL4543166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged HDAC1 (unknown origin) expressed in insect cells using poly (Glu, Tyr) 4:1 as substrate measured after 15 mins in presence o... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50531378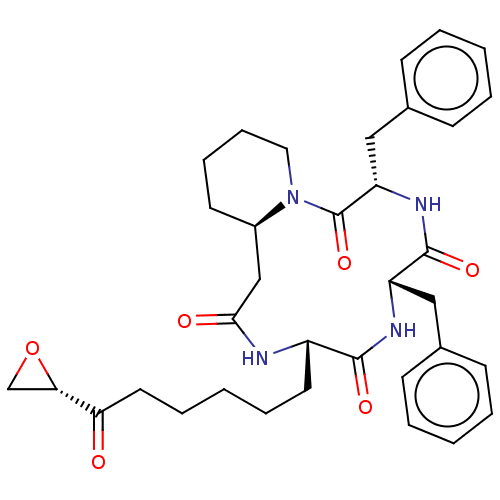 (CHEMBL4571133) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 expressed in NIH/3T3 cells using [3H]acetyl histone as substrate measured after 15 mins by liquid scintillation... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50531384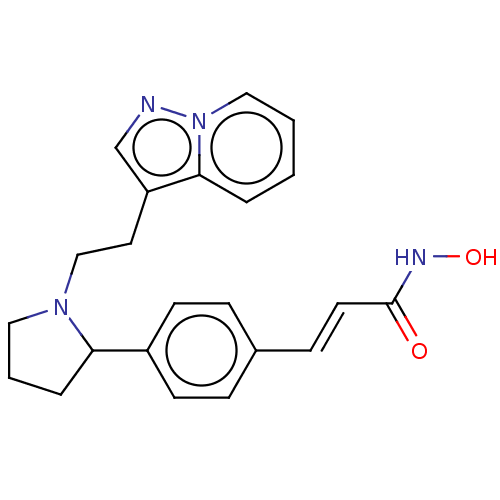 (CHEMBL4574244) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged HDAC1 (unknown origin) expressed in insect cells using poly (Glu, Tyr) 4:1 as substrate measured after 15 mins in presence o... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50578677 (CHEMBL4849690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00679 BindingDB Entry DOI: 10.7270/Q2RJ4P9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511394 (3-(4,4- difluorocyclohexyl)-1-(7- fluoro-3-(2-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50531374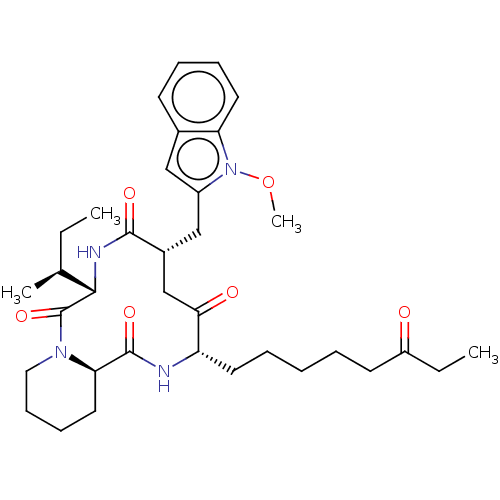 (CHEMBL4467135) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of HDAC (unknown origin) | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511390 (1-(7-fluoro-3-(2- methylthiazol-5- yl)isoquinolin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511377 (3-(4,4- difluorocyclohexyl)-N- methyl-1-(2-(1-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50578635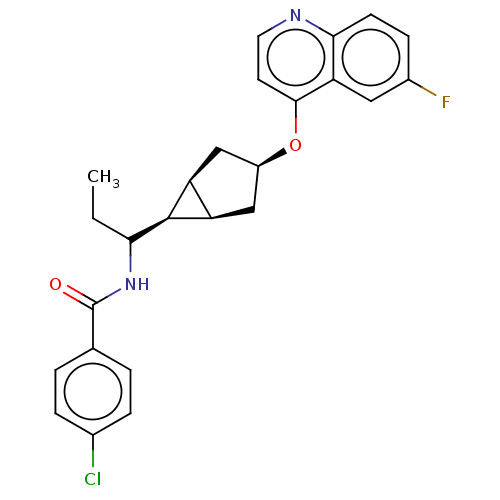 (CHEMBL4875089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00679 BindingDB Entry DOI: 10.7270/Q2RJ4P9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511316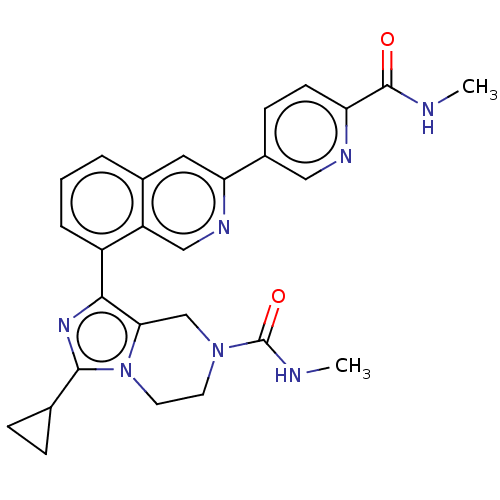 (3-Cyclopropyl-N-methyl-1-(3-(6-(methylcarbamoyl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511322 (3-Cyclopropyl-N-methyl-1-(3-(2-methylthiazol-5-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50578623 (CHEMBL4858888) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in mouse Panc02 cells assessed as reduction in NFK level incubated for 48 hrs by RFMS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00679 BindingDB Entry DOI: 10.7270/Q2RJ4P9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50531398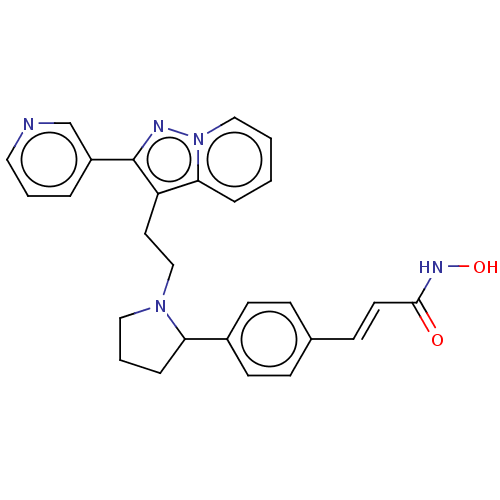 (CHEMBL4454463) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged HDAC1 (unknown origin) expressed in insect cells using poly (Glu, Tyr) 4:1 as substrate measured after 15 mins in presence o... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein [1081-1197] (Homo sapiens (Human)) | BDBM511356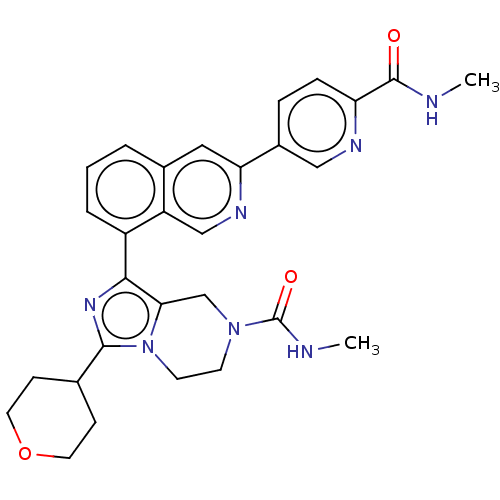 (N-methyl-1-(3-(6- (methylcarbamoyl)pyridin- 3-yl)i...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM GST-CBP(1081-1197) and 20 nM biotin-H4(1-21) Ac-K5/8/12/16 (AnaSpec. 64989) were incubated with varying concentrations of CBP inhibitors in 15 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531392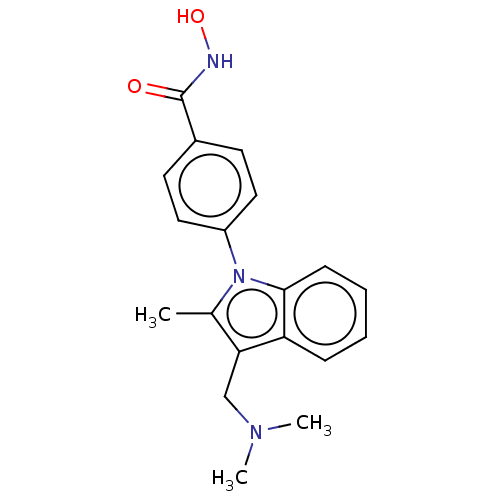 (CHEMBL4441675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant full-length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected Sf9 insect cells using fluorescent-labelled... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50578639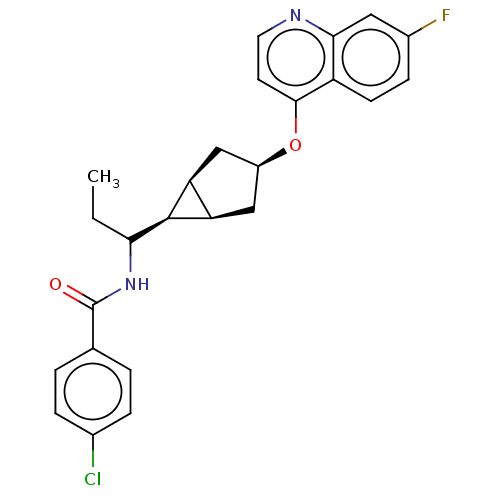 (CHEMBL4868112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00679 BindingDB Entry DOI: 10.7270/Q2RJ4P9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50531386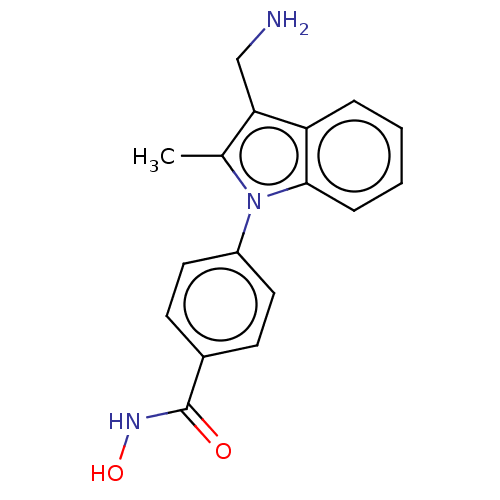 (CHEMBL4562547) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant full-length human N-terminal GST-tagged HDAC6 expressed in baculovirus infected Sf9 insect cells using fluorescent-labelled... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50578625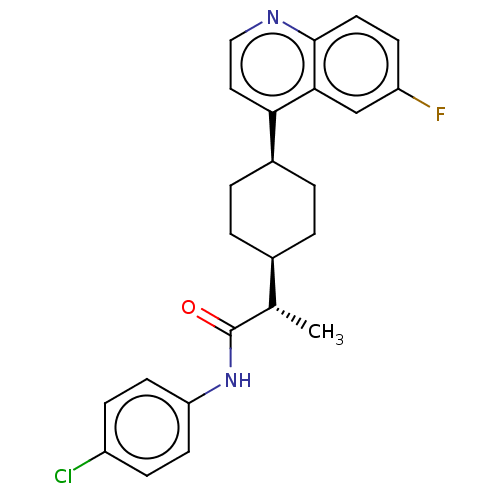 (CHEMBL4855340) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in mouse Panc02 cells assessed as reduction in NFK level incubated for 48 hrs by RFMS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00679 BindingDB Entry DOI: 10.7270/Q2RJ4P9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant full-length human HDAC6 using RHKK(Ac) as substrate measured after 2 hrs by fluorescence assay | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50578633 (CHEMBL4872522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00679 BindingDB Entry DOI: 10.7270/Q2RJ4P9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50578622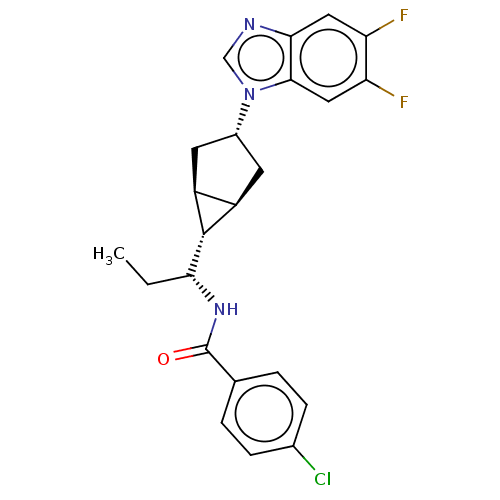 (CHEMBL4861332) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00679 BindingDB Entry DOI: 10.7270/Q2RJ4P9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1009 total ) | Next | Last >> |