 Found 120 hits with Last Name = 'marini' and Initial = 'e'
Found 120 hits with Last Name = 'marini' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Caspase-1
(Homo sapiens (Human)) | BDBM50033886
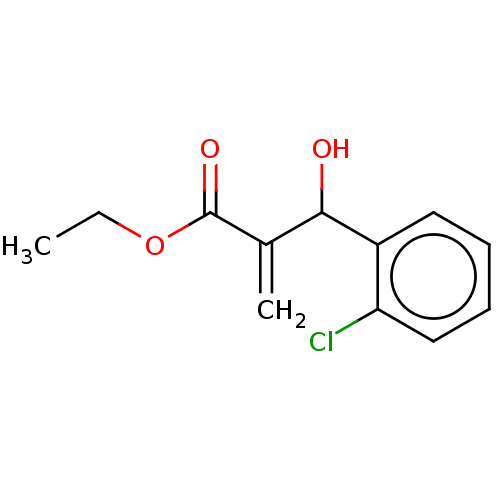
(CHEMBL2047937 | Ethyl 2-((2-Chlorophenyl)(Hydroxy)...)Show InChI InChI=1S/C12H13ClO3/c1-3-16-12(15)8(2)11(14)9-6-4-5-7-10(9)13/h4-7,11,14H,2-3H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino
Curated by ChEMBL
| Assay Description
Inhibition of caspase 1 (unknown origin) using N-acetyl-Tyr-Val-Ala-Asp-para-nitroanilide substrate assessed as rate of para-nitroanilide release by ... |
J Med Chem 57: 10366-82 (2014)
Article DOI: 10.1021/jm501072b
BindingDB Entry DOI: 10.7270/Q29P337T |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50433441
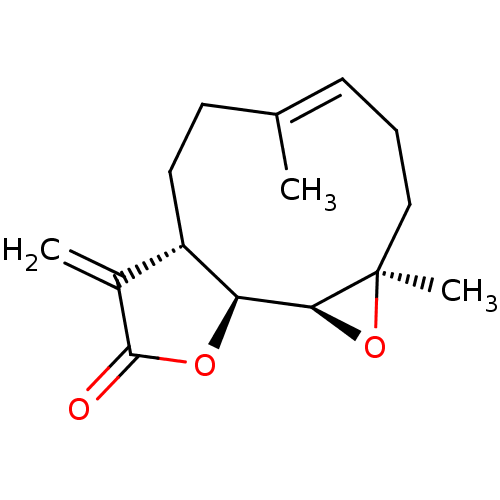
(PARTHENOLIDE)Show SMILES C\C1=C/CC[C@@]2(C)O[C@H]2[C@H]2OC(=O)C(=C)[C@@H]2CC1 |r,c:1| Show InChI InChI=1S/C15H20O3/c1-9-5-4-8-15(3)13(18-15)12-11(7-6-9)10(2)14(16)17-12/h5,11-13H,2,4,6-8H2,1,3H3/b9-5+/t11-,12-,13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino
Curated by ChEMBL
| Assay Description
Inhibition of caspase 1 (unknown origin) using N-acetyl-Tyr-Val-Ala-Asp-para-nitroanilide substrate assessed as rate of para-nitroanilide release by ... |
J Med Chem 57: 10366-82 (2014)
Article DOI: 10.1021/jm501072b
BindingDB Entry DOI: 10.7270/Q29P337T |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50131622

(4-Chloro-5-(3-fluoro-4-methoxy-phenyl)-1-(4-methan...)Show SMILES COc1ccc(cc1F)-c1c(Cl)ncn1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C17H14ClFN2O3S/c1-24-15-8-3-11(9-14(15)19)16-17(18)20-10-21(16)12-4-6-13(7-5-12)25(2,22)23/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Torino
Curated by ChEMBL
| Assay Description
Activity of COX2 in human heparinized blood assessed as inhibition of LPS-induced PGE2 production after 24 hrs |
J Med Chem 50: 1449-57 (2007)
Article DOI: 10.1021/jm0607247
BindingDB Entry DOI: 10.7270/Q2Q23ZW6 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Antagonistic activity against TP-receptor by inhibition of U 46619-induced contraction of isolated guinea pig trachea |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Turin
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 assessed as reduction in PGF2alpha production by ELISA |
ACS Med Chem Lett 10: 437-443 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00484
BindingDB Entry DOI: 10.7270/Q2HT2SPS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50131593
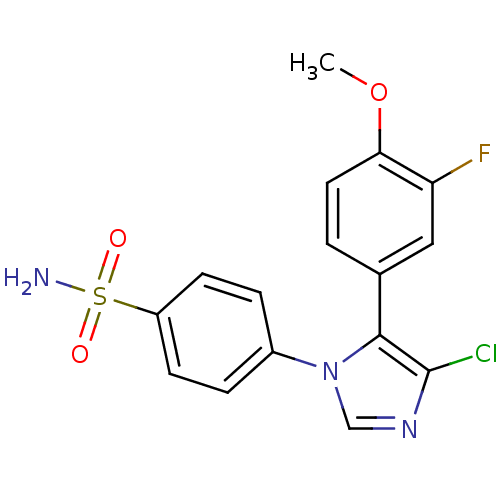
(4-[4-Chloro-5-(3-fluoro-4-methoxy-phenyl)-imidazol...)Show SMILES COc1ccc(cc1F)-c1c(Cl)ncn1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H13ClFN3O3S/c1-24-14-7-2-10(8-13(14)18)15-16(17)20-9-21(15)11-3-5-12(6-4-11)25(19,22)23/h2-9H,1H3,(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Torino
Curated by ChEMBL
| Assay Description
Activity of COX2 in human heparinized blood assessed as inhibition of LPS-induced PGE2 production after 24 hrs |
J Med Chem 50: 1449-57 (2007)
Article DOI: 10.1021/jm0607247
BindingDB Entry DOI: 10.7270/Q2Q23ZW6 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50462392
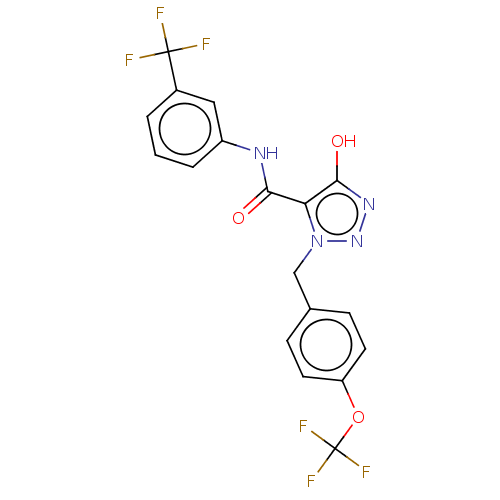
(CHEMBL4248154)Show SMILES Oc1nnn(Cc2ccc(OC(F)(F)F)cc2)c1C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C18H12F6N4O3/c19-17(20,21)11-2-1-3-12(8-11)25-15(29)14-16(30)26-27-28(14)9-10-4-6-13(7-5-10)31-18(22,23)24/h1-8,30H,9H2,(H,25,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50462395
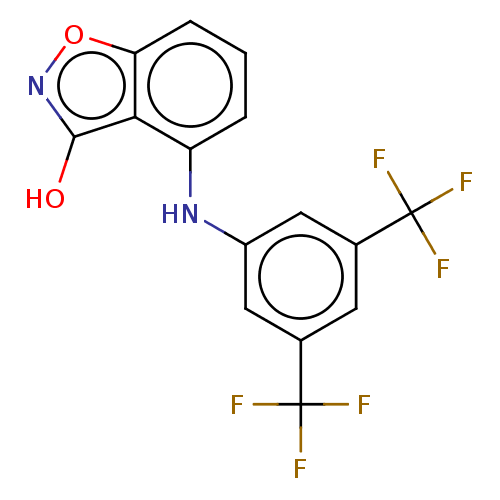
(CHEMBL4238437)Show SMILES Oc1noc2cccc(Nc3cc(cc(c3)C(F)(F)F)C(F)(F)F)c12 Show InChI InChI=1S/C15H8F6N2O2/c16-14(17,18)7-4-8(15(19,20)21)6-9(5-7)22-10-2-1-3-11-12(10)13(24)23-25-11/h1-6,22H,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50509974

(CHEMBL4434843)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(Cc3nonc3O)c2c1 Show InChI InChI=1S/C20H16ClN3O4/c1-11-15(10-17-19(25)23-28-22-17)16-9-14(27-2)7-8-18(16)24(11)20(26)12-3-5-13(21)6-4-12/h3-9H,10H2,1-2H3,(H,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Turin
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged recombinant human AKR1C3 His5Gln mutant expressed in Escherichia coli BL21 (DE) Codon Plus RP cells assessed as r... |
ACS Med Chem Lett 10: 437-443 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00484
BindingDB Entry DOI: 10.7270/Q2HT2SPS |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50462394

(CHEMBL4249650)Show InChI InChI=1S/C13H9F5N2O2S/c14-23(15,16,17,18)9-4-1-3-8(7-9)19-10-5-2-6-11-12(10)13(21)20-22-11/h1-7,19H,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50277998
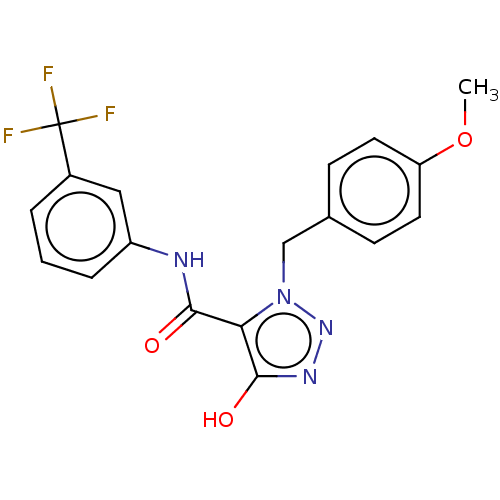
(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50277998

(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) using S-tetralol as substrate in presence of NADP+ by fluorimtery |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50464816

(CHEMBL4285001)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)OCc2ccc(OCCOc3no[n+]([O-])c3S(=O)(=O)c3ccccc3)nc2)cc1 Show InChI InChI=1S/C31H28N6O9S/c32-25-8-4-5-9-26(25)35-28(38)23-13-10-21(11-14-23)18-34-31(39)45-20-22-12-15-27(33-19-22)43-16-17-44-29-30(37(40)46-36-29)47(41,42)24-6-2-1-3-7-24/h1-15,19H,16-18,20,32H2,(H,34,39)(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC2 (unknown origin) expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetri... |
Eur J Med Chem 144: 612-625 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.047
BindingDB Entry DOI: 10.7270/Q2KP84T7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50464815
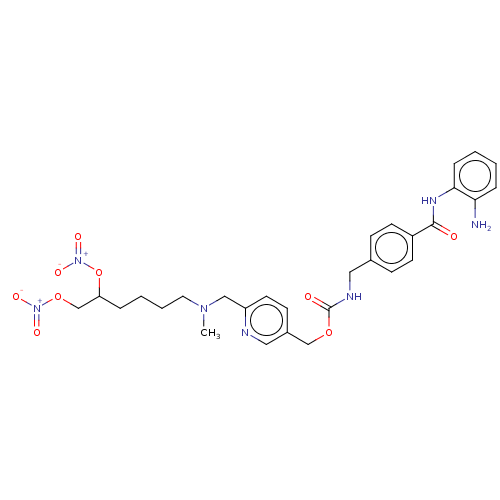
(CHEMBL4281580)Show SMILES Cl.Cl.Cl.CN(CCCCC(CO[N+]([O-])=O)O[N+]([O-])=O)Cc1ccc(COC(=O)NCc2ccc(cc2)C(=O)Nc2ccccc2N)cn1 Show InChI InChI=1S/C29H35N7O9.3ClH/c1-34(15-5-4-6-25(45-36(41)42)20-44-35(39)40)18-24-14-11-22(17-31-24)19-43-29(38)32-16-21-9-12-23(13-10-21)28(37)33-27-8-3-2-7-26(27)30;;;/h2-3,7-14,17,25H,4-6,15-16,18-20,30H2,1H3,(H,32,38)(H,33,37);3*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC2 (unknown origin) expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetri... |
Eur J Med Chem 144: 612-625 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.047
BindingDB Entry DOI: 10.7270/Q2KP84T7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50462391
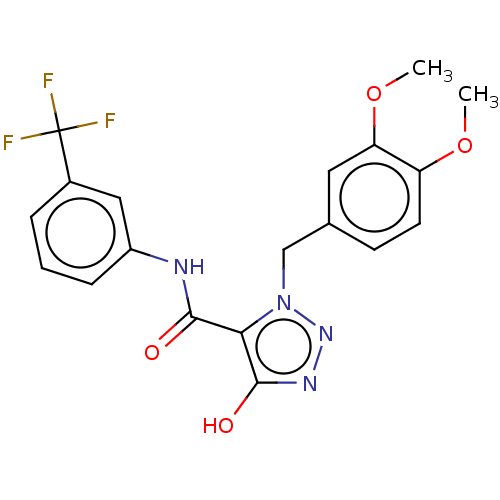
(CHEMBL4238142)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1OC Show InChI InChI=1S/C19H17F3N4O4/c1-29-14-7-6-11(8-15(14)30-2)10-26-16(18(28)24-25-26)17(27)23-13-5-3-4-12(9-13)19(20,21)22/h3-9,28H,10H2,1-2H3,(H,23,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM17636

(2-{[3-(trifluoromethyl)phenyl]amino}benzoic acid |...)Show InChI InChI=1S/C14H10F3NO2/c15-14(16,17)9-4-3-5-10(8-9)18-12-7-2-1-6-11(12)13(19)20/h1-8,18H,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM17636

(2-{[3-(trifluoromethyl)phenyl]amino}benzoic acid |...)Show InChI InChI=1S/C14H10F3NO2/c15-14(16,17)9-4-3-5-10(8-9)18-12-7-2-1-6-11(12)13(19)20/h1-8,18H,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50278013

(CHEMBL4166887)Show SMILES Oc1nnn(Cc2ccccc2)c1C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C17H13F3N4O2/c18-17(19,20)12-7-4-8-13(9-12)21-15(25)14-16(26)22-23-24(14)10-11-5-2-1-3-6-11/h1-9,26H,10H2,(H,21,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Thromboxane (TXA2) receptor antagonist activity using human platelet |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM17636

(2-{[3-(trifluoromethyl)phenyl]amino}benzoic acid |...)Show InChI InChI=1S/C14H10F3NO2/c15-14(16,17)9-4-3-5-10(8-9)18-12-7-2-1-6-11(12)13(19)20/h1-8,18H,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Antagonistic activity against TP-receptor by inhibition of U 46619-induced contraction of isolated guinea pig trachea |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM17636

(2-{[3-(trifluoromethyl)phenyl]amino}benzoic acid |...)Show InChI InChI=1S/C14H10F3NO2/c15-14(16,17)9-4-3-5-10(8-9)18-12-7-2-1-6-11(12)13(19)20/h1-8,18H,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C2 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Thromboxane (TXA2) receptor antagonist activity using human platelet |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50462397
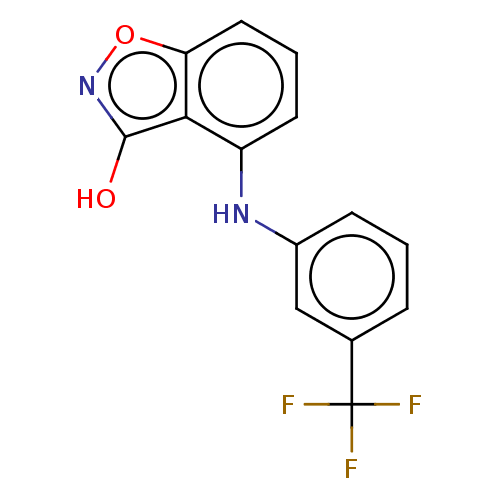
(CHEMBL4245231)Show InChI InChI=1S/C14H9F3N2O2/c15-14(16,17)8-3-1-4-9(7-8)18-10-5-2-6-11-12(10)13(20)19-21-11/h1-7,18H,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Antagonistic activity against TP-receptor by inhibition of U 46619-induced contraction of isolated guinea pig trachea |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Turin
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 assessed as reduction in PGF2alpha production by ELISA |
ACS Med Chem Lett 10: 437-443 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00484
BindingDB Entry DOI: 10.7270/Q2HT2SPS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC2 (unknown origin) expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetri... |
Eur J Med Chem 144: 612-625 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.047
BindingDB Entry DOI: 10.7270/Q2KP84T7 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50095990

(CHEMBL3593282)Show SMILES [I-].CCCOc1ccc2S\C(=C/C=C/c3sc4ccccc4[n+]3CC)N(CC)c2c1 Show InChI InChI=1S/C18H20FN5O5/c19-10-3-1-9(2-4-10)5-6-28-18-22-15(20)12-16(23-18)24(8-21-12)17-14(27)13(26)11(7-25)29-17/h1-4,8,11,13-14,17,25-27H,5-7H2,(H2,20,22,23)/t11-,13+,14+,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino
Curated by ChEMBL
| Assay Description
Inhibition of Amyloid beta (1 to 42) (unknown origin) aggregation assessed as amount of fibrils formation after 2 hrs by thioflavin T based spectrofl... |
Bioorg Med Chem 23: 4688-98 (2015)
Article DOI: 10.1016/j.bmc.2015.05.050
BindingDB Entry DOI: 10.7270/Q29888RC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50509973
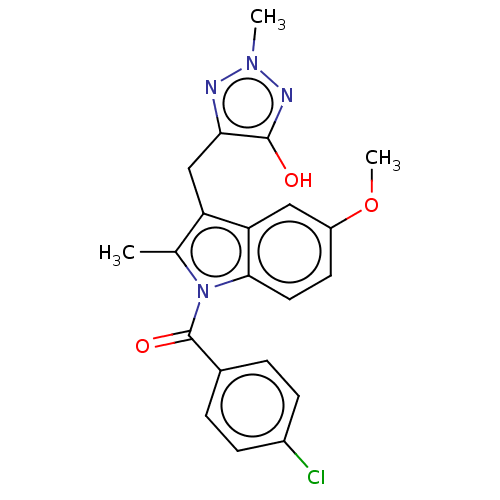
(CHEMBL4590005)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(Cc3nn(C)nc3O)c2c1 Show InChI InChI=1S/C21H19ClN4O3/c1-12-16(11-18-20(27)24-25(2)23-18)17-10-15(29-3)8-9-19(17)26(12)21(28)13-4-6-14(22)7-5-13/h4-10H,11H2,1-3H3,(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Turin
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged recombinant human AKR1C3 His5Gln mutant expressed in Escherichia coli BL21 (DE) Codon Plus RP cells assessed as r... |
ACS Med Chem Lett 10: 437-443 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00484
BindingDB Entry DOI: 10.7270/Q2HT2SPS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50464816

(CHEMBL4285001)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)OCc2ccc(OCCOc3no[n+]([O-])c3S(=O)(=O)c3ccccc3)nc2)cc1 Show InChI InChI=1S/C31H28N6O9S/c32-25-8-4-5-9-26(25)35-28(38)23-13-10-21(11-14-23)18-34-31(39)45-20-22-12-15-27(33-19-22)43-16-17-44-29-30(37(40)46-36-29)47(41,42)24-6-2-1-3-7-24/h1-15,19H,16-18,20,32H2,(H,34,39)(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged HDAC1 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... |
Eur J Med Chem 144: 612-625 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.047
BindingDB Entry DOI: 10.7270/Q2KP84T7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50464815

(CHEMBL4281580)Show SMILES Cl.Cl.Cl.CN(CCCCC(CO[N+]([O-])=O)O[N+]([O-])=O)Cc1ccc(COC(=O)NCc2ccc(cc2)C(=O)Nc2ccccc2N)cn1 Show InChI InChI=1S/C29H35N7O9.3ClH/c1-34(15-5-4-6-25(45-36(41)42)20-44-35(39)40)18-24-14-11-22(17-31-24)19-43-29(38)32-16-21-9-12-23(13-10-21)28(37)33-27-8-3-2-7-26(27)30;;;/h2-3,7-14,17,25H,4-6,15-16,18-20,30H2,1H3,(H,32,38)(H,33,37);3*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged HDAC1 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... |
Eur J Med Chem 144: 612-625 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.047
BindingDB Entry DOI: 10.7270/Q2KP84T7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged HDAC1 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... |
Eur J Med Chem 144: 612-625 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.047
BindingDB Entry DOI: 10.7270/Q2KP84T7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50464816

(CHEMBL4285001)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)OCc2ccc(OCCOc3no[n+]([O-])c3S(=O)(=O)c3ccccc3)nc2)cc1 Show InChI InChI=1S/C31H28N6O9S/c32-25-8-4-5-9-26(25)35-28(38)23-13-10-21(11-14-23)18-34-31(39)45-20-22-12-15-27(33-19-22)43-16-17-44-29-30(37(40)46-36-29)47(41,42)24-6-2-1-3-7-24/h1-15,19H,16-18,20,32H2,(H,34,39)(H,35,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged HDAC3 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... |
Eur J Med Chem 144: 612-625 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.047
BindingDB Entry DOI: 10.7270/Q2KP84T7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50464815

(CHEMBL4281580)Show SMILES Cl.Cl.Cl.CN(CCCCC(CO[N+]([O-])=O)O[N+]([O-])=O)Cc1ccc(COC(=O)NCc2ccc(cc2)C(=O)Nc2ccccc2N)cn1 Show InChI InChI=1S/C29H35N7O9.3ClH/c1-34(15-5-4-6-25(45-36(41)42)20-44-35(39)40)18-24-14-11-22(17-31-24)19-43-29(38)32-16-21-9-12-23(13-10-21)28(37)33-27-8-3-2-7-26(27)30;;;/h2-3,7-14,17,25H,4-6,15-16,18-20,30H2,1H3,(H,32,38)(H,33,37);3*1H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged HDAC3 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... |
Eur J Med Chem 144: 612-625 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.047
BindingDB Entry DOI: 10.7270/Q2KP84T7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged HDAC3 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... |
Eur J Med Chem 144: 612-625 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.047
BindingDB Entry DOI: 10.7270/Q2KP84T7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Torino
Curated by ChEMBL
| Assay Description
Activity of COX2 in human heparinized blood assessed as inhibition of LPS-induced PGE2 production after 24 hrs |
J Med Chem 50: 1449-57 (2007)
Article DOI: 10.1021/jm0607247
BindingDB Entry DOI: 10.7270/Q2Q23ZW6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50462393
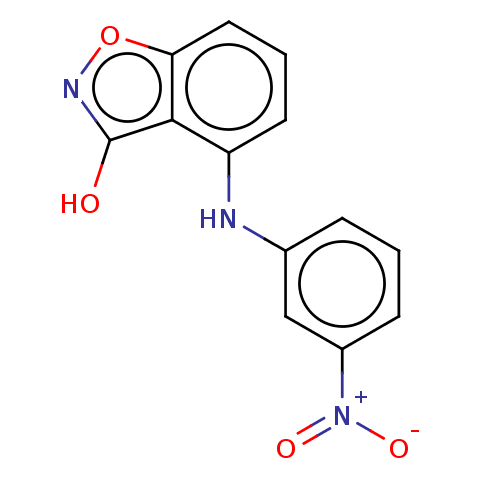
(CHEMBL4249427)Show InChI InChI=1S/C13H9N3O4/c17-13-12-10(5-2-6-11(12)20-15-13)14-8-3-1-4-9(7-8)16(18)19/h1-7,14H,(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Torino
Curated by ChEMBL
| Assay Description
Activity of COX2 in human heparinized blood assessed as inhibition of LPS-induced PGE2 production after 24 hrs |
J Med Chem 50: 1449-57 (2007)
Article DOI: 10.1021/jm0607247
BindingDB Entry DOI: 10.7270/Q2Q23ZW6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50277997

(CHEMBL4159654)Show InChI InChI=1S/C14H13F3N4O2/c15-14(16,17)9-2-1-3-10(6-9)18-12(22)11-13(23)19-20-21(11)7-8-4-5-8/h1-3,6,8,23H,4-5,7H2,(H,18,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Thromboxane (TXA2) receptor antagonist activity using human platelet |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50095989
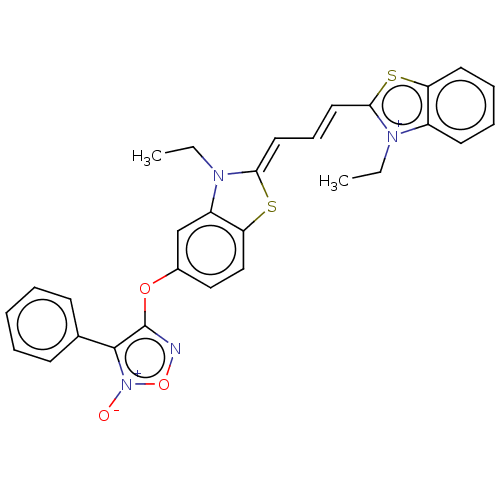
(CHEMBL3593279)Show SMILES [I-].CCN1\C(Sc2ccc(Oc3no[n+]([O-])c3-c3ccccc3)cc12)=C\C=C\c1sc2ccccc2[n+]1CC Show InChI InChI=1S/C16H25N5O5/c1-2-3-4-5-6-25-16-19-13(17)10-14(20-16)21(8-18-10)15-12(24)11(23)9(7-22)26-15/h8-9,11-12,15,22-24H,2-7H2,1H3,(H2,17,19,20)/t9-,11+,12+,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino
Curated by ChEMBL
| Assay Description
Inhibition of Amyloid beta (1 to 42) (unknown origin) aggregation assessed as amount of fibrils formation after 2 hrs by thioflavin T based spectrofl... |
Bioorg Med Chem 23: 4688-98 (2015)
Article DOI: 10.1016/j.bmc.2015.05.050
BindingDB Entry DOI: 10.7270/Q29888RC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50131593

(4-[4-Chloro-5-(3-fluoro-4-methoxy-phenyl)-imidazol...)Show SMILES COc1ccc(cc1F)-c1c(Cl)ncn1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H13ClFN3O3S/c1-24-14-7-2-10(8-13(14)18)15-16(17)20-9-21(15)11-3-5-12(6-4-11)25(19,22)23/h2-9H,1H3,(H2,19,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Torino
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as TxB2 production after 1 hr |
J Med Chem 50: 1449-57 (2007)
Article DOI: 10.1021/jm0607247
BindingDB Entry DOI: 10.7270/Q2Q23ZW6 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Antagonistic activity against TP-receptor by inhibition of U 46619-induced contraction of isolated guinea pig trachea |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM75309

((2Z)-3-ethyl-2-[(E)-3-(3-ethyl-1,3-benzothiazol-3-...)Show SMILES CCN1\C(Sc2ccccc12)=C\C=C\c1sc2ccccc2[n+]1CC Show InChI InChI=1S/C21H21N2S2/c1-3-22-16-10-5-7-12-18(16)24-20(22)14-9-15-21-23(4-2)17-11-6-8-13-19(17)25-21/h5-15H,3-4H2,1-2H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino
Curated by ChEMBL
| Assay Description
Inhibition of Amyloid beta (1 to 42) (unknown origin) aggregation assessed as amount of fibrils formation after 2 hrs by thioflavin T based spectrofl... |
Bioorg Med Chem 23: 4688-98 (2015)
Article DOI: 10.1016/j.bmc.2015.05.050
BindingDB Entry DOI: 10.7270/Q29888RC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50462396
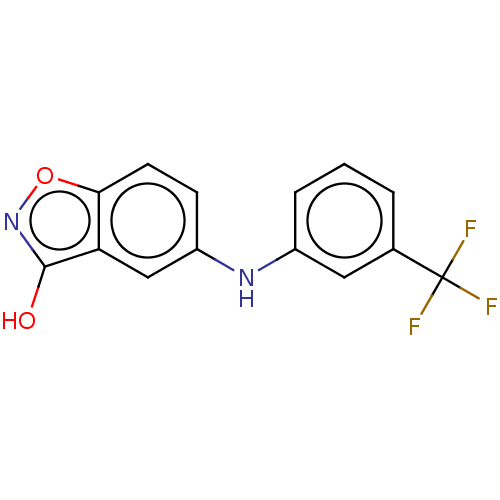
(CHEMBL4238730)Show InChI InChI=1S/C14H9F3N2O2/c15-14(16,17)8-2-1-3-9(6-8)18-10-4-5-12-11(7-10)13(20)19-21-12/h1-7,18H,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50277998

(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Thromboxane (TXA2) receptor antagonist activity using human platelet |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50462394

(CHEMBL4249650)Show InChI InChI=1S/C13H9F5N2O2S/c14-23(15,16,17,18)9-4-1-3-8(7-9)19-10-5-2-6-11-12(10)13(21)20-22-11/h1-7,19H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C2 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50462397

(CHEMBL4245231)Show InChI InChI=1S/C14H9F3N2O2/c15-14(16,17)8-3-1-4-9(7-8)18-10-5-2-6-11-12(10)13(20)19-21-11/h1-7,18H,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C2 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50462393

(CHEMBL4249427)Show InChI InChI=1S/C13H9N3O4/c17-13-12-10(5-2-6-11(12)20-15-13)14-8-3-1-4-9(7-8)16(18)19/h1-7,14H,(H,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C2 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50131622

(4-Chloro-5-(3-fluoro-4-methoxy-phenyl)-1-(4-methan...)Show SMILES COc1ccc(cc1F)-c1c(Cl)ncn1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C17H14ClFN2O3S/c1-24-15-8-3-11(9-14(15)19)16-17(18)20-10-21(16)12-4-6-13(7-5-12)25(2,22)23/h3-10H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Torino
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as TxB2 production after 1 hr |
J Med Chem 50: 1449-57 (2007)
Article DOI: 10.1021/jm0607247
BindingDB Entry DOI: 10.7270/Q2Q23ZW6 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50206929
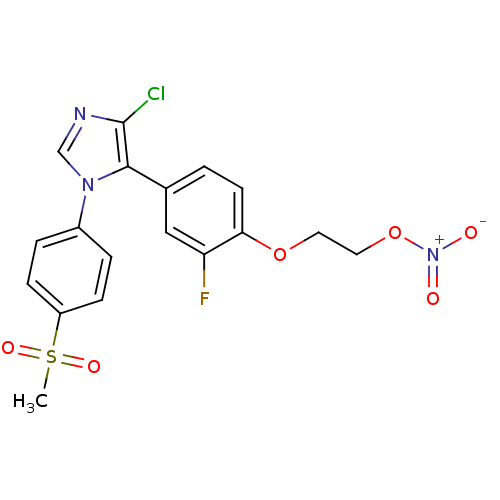
(2-{4-[4-chloro-1-(4-(methylsulfonyl)phenyl)-1H-imi...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1cnc(Cl)c1-c1ccc(OCCO[N+]([O-])=O)c(F)c1 Show InChI InChI=1S/C18H15ClFN3O6S/c1-30(26,27)14-5-3-13(4-6-14)22-11-21-18(19)17(22)12-2-7-16(15(20)10-12)28-8-9-29-23(24)25/h2-7,10-11H,8-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Torino
Curated by ChEMBL
| Assay Description
Activity of COX2 in human heparinized blood assessed as inhibition of LPS-induced PGE2 production after 24 hrs |
J Med Chem 50: 1449-57 (2007)
Article DOI: 10.1021/jm0607247
BindingDB Entry DOI: 10.7270/Q2Q23ZW6 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Turin
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged recombinant human AKR1C3 His5Gln mutant expressed in Escherichia coli BL21 (DE) Codon Plus RP cells assessed as r... |
ACS Med Chem Lett 10: 437-443 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00484
BindingDB Entry DOI: 10.7270/Q2HT2SPS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50095988

(CHEMBL3593278)Show SMILES [I-].CCN1\C(Sc2ccc(OCCCO[N+]([O-])=O)cc12)=C\C=C\c1sc2ccccc2[n+]1CC Show InChI InChI=1S/C15H23N5O5/c1-2-3-4-5-24-15-18-12(16)9-13(19-15)20(7-17-9)14-11(23)10(22)8(6-21)25-14/h7-8,10-11,14,21-23H,2-6H2,1H3,(H2,16,18,19)/t8-,10+,11+,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino
Curated by ChEMBL
| Assay Description
Inhibition of Amyloid beta (1 to 42) (unknown origin) aggregation assessed as amount of fibrils formation after 2 hrs by thioflavin T based spectrofl... |
Bioorg Med Chem 23: 4688-98 (2015)
Article DOI: 10.1016/j.bmc.2015.05.050
BindingDB Entry DOI: 10.7270/Q29888RC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data