 Found 173 hits with Last Name = 'matsumoto' and Initial = 'i'
Found 173 hits with Last Name = 'matsumoto' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM21392
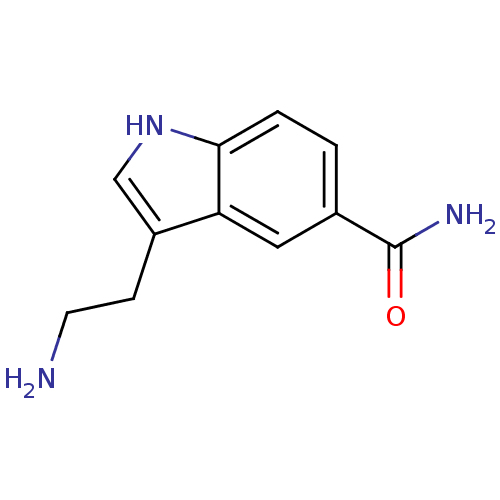
(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM82087
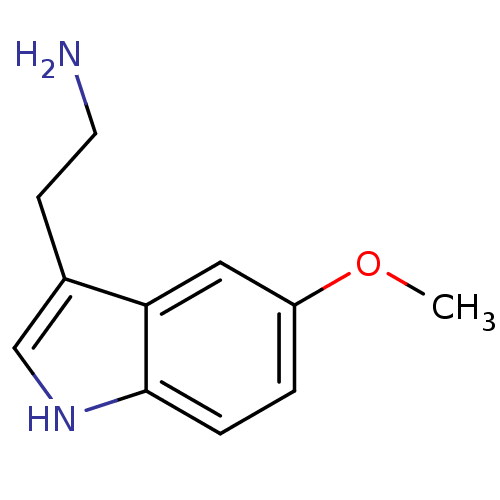
(2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...)Show InChI InChI=1S/C11H14N2O/c1-14-9-2-3-11-10(6-9)8(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM81498
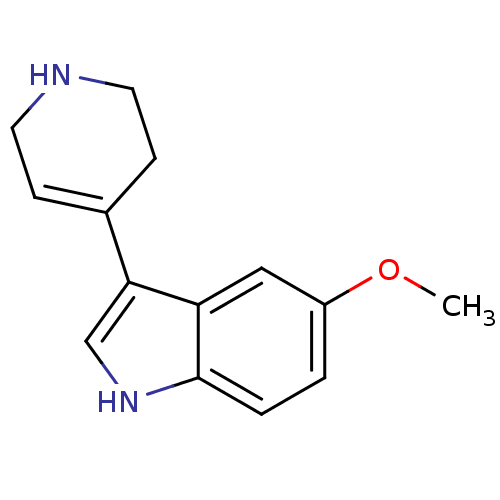
(5-Methoxy-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-i...)Show InChI InChI=1S/C14H16N2O/c1-17-11-2-3-14-12(8-11)13(9-16-14)10-4-6-15-7-5-10/h2-4,8-9,15-16H,5-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM10755
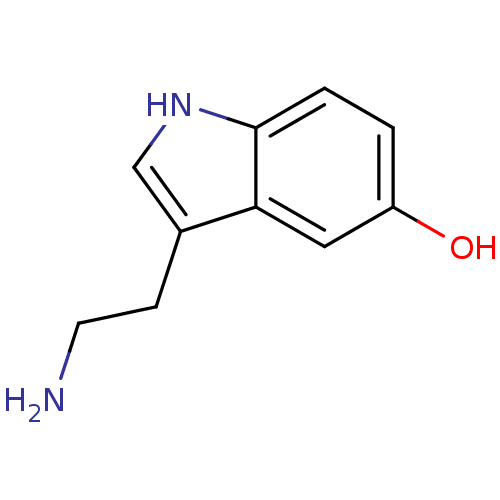
(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50019443
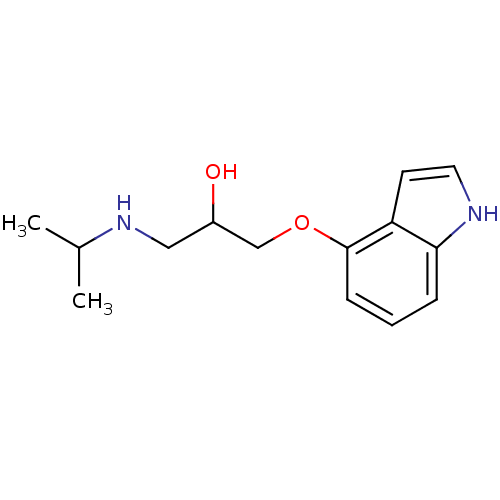
(1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...)Show InChI InChI=1S/C14H20N2O2/c1-10(2)16-8-11(17)9-18-14-5-3-4-13-12(14)6-7-15-13/h3-7,10-11,15-17H,8-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM81497
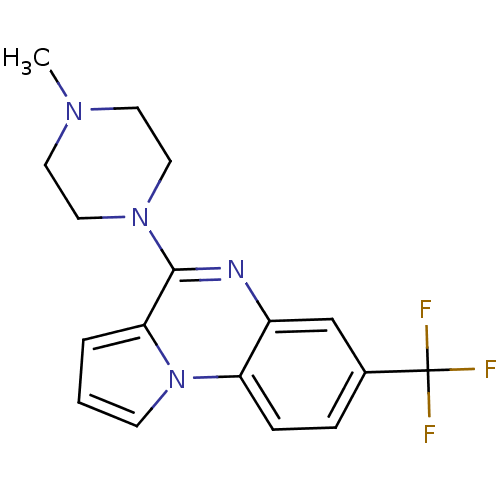
(4-(4-Methyl-piperazin-1-yl)-7-trifluoromethyl-pyrr...)Show InChI InChI=1S/C17H17F3N4/c1-22-7-9-23(10-8-22)16-15-3-2-6-24(15)14-5-4-12(17(18,19)20)11-13(14)21-16/h2-6,11H,7-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM25761
(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50007406
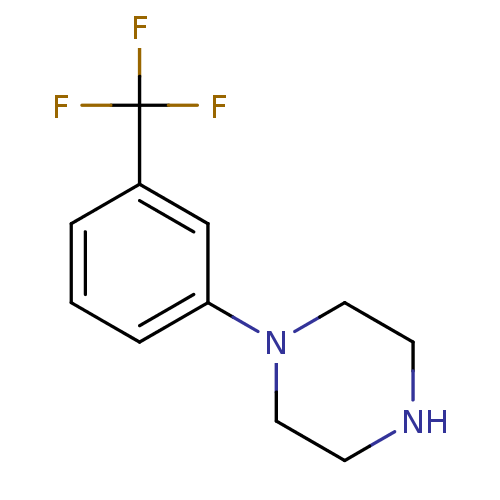
(1-(3-(trifluoromethyl)phenyl)piperazine | 1-(3-Tri...)Show InChI InChI=1S/C11H13F3N2/c12-11(13,14)9-2-1-3-10(8-9)16-6-4-15-5-7-16/h1-3,8,15H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM78940
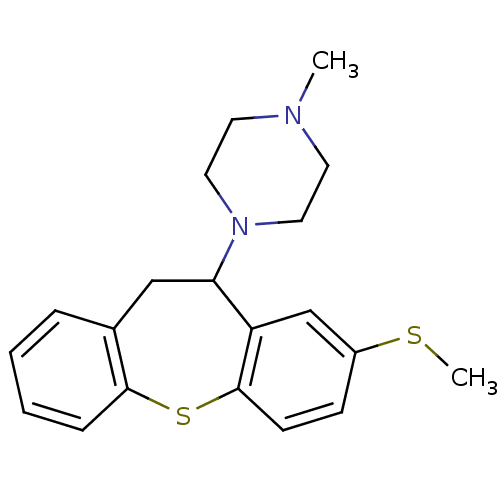
(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50001915
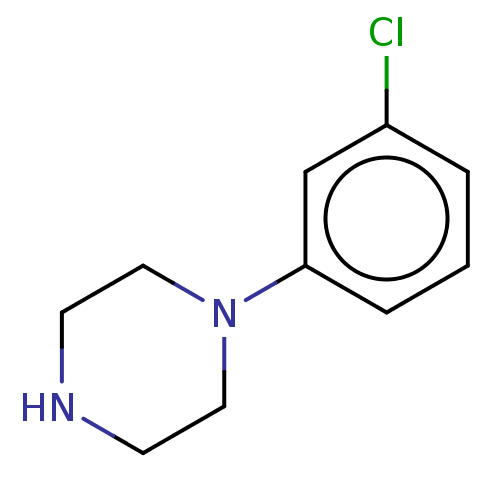
(1-(3-chlorophenyl)piperazine | CHEMBL478 | m-Chlor...)Show InChI InChI=1S/C10H13ClN2/c11-9-2-1-3-10(8-9)13-6-4-12-5-7-13/h1-3,8,12H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50014407
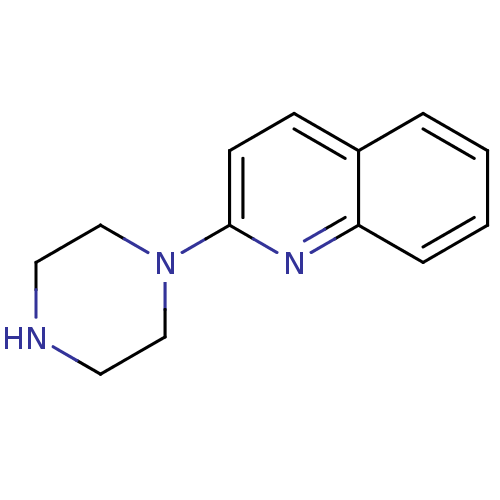
(2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...)Show InChI InChI=1S/C13H15N3/c1-2-4-12-11(3-1)5-6-13(15-12)16-9-7-14-8-10-16/h1-6,14H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50031942
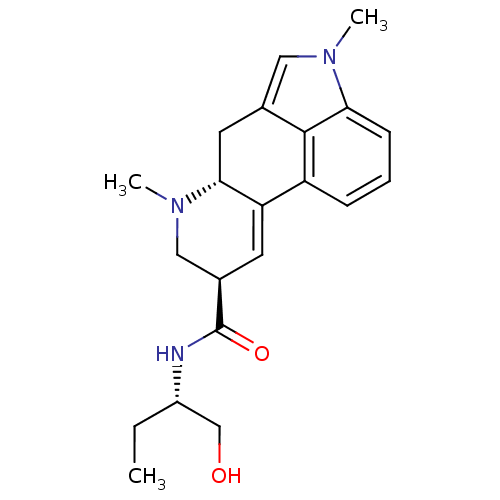
((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3cn(C)c4cccc(C2=C1)c34 |r,c:24| Show InChI InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 386 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM31046
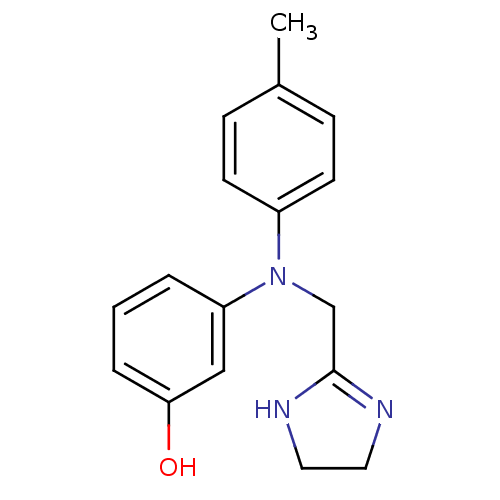
(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM35256
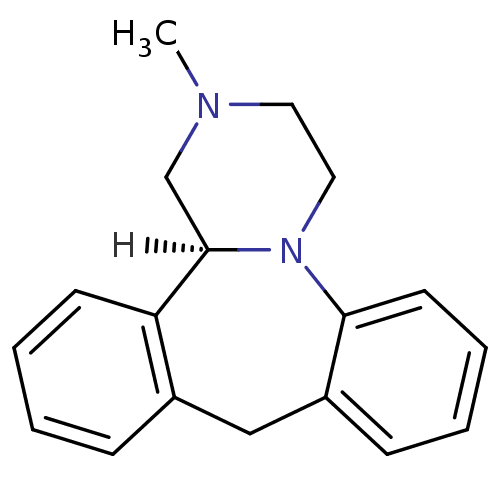
((S)-mianserin | Lerivon | MIANSERIN | MIANSERIN (+...)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50019443

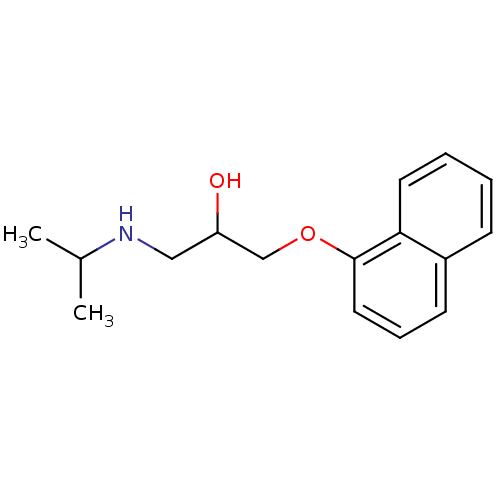
(1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...)Show InChI InChI=1S/C14H20N2O2/c1-10(2)16-8-11(17)9-18-14-5-3-4-13-12(14)6-7-15-13/h3-7,10-11,15-17H,8-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50024204
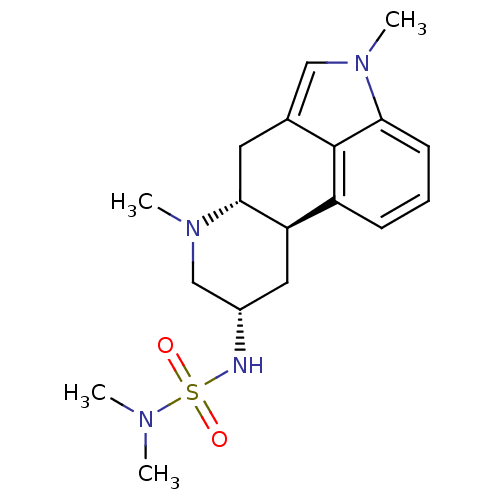
(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50016897
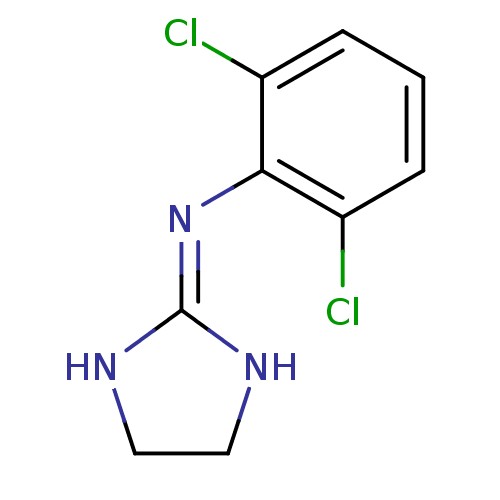
(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50241143
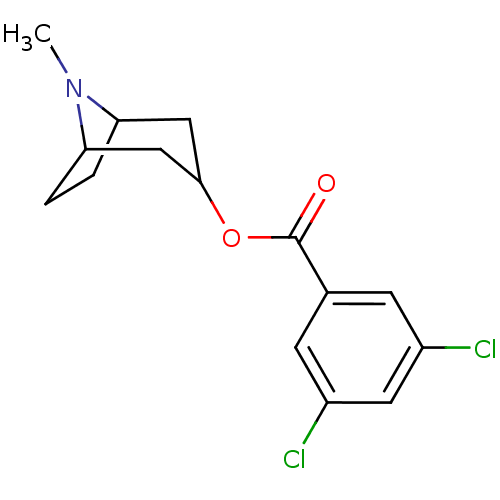
((MDL 72222)3,5-Dichloro-benzoic acid 8-methyl-8-az...)Show SMILES CN1C2CCC1CC(C2)OC(=O)c1cc(Cl)cc(Cl)c1 |THB:9:7:1:3.4| Show InChI InChI=1S/C15H17Cl2NO2/c1-18-12-2-3-13(18)8-14(7-12)20-15(19)9-4-10(16)6-11(17)5-9/h4-6,12-14H,2-3,7-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM35234

(DL-[7-3H]norepinephrine | NOREPINEPHRINE | Noradre...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM21395
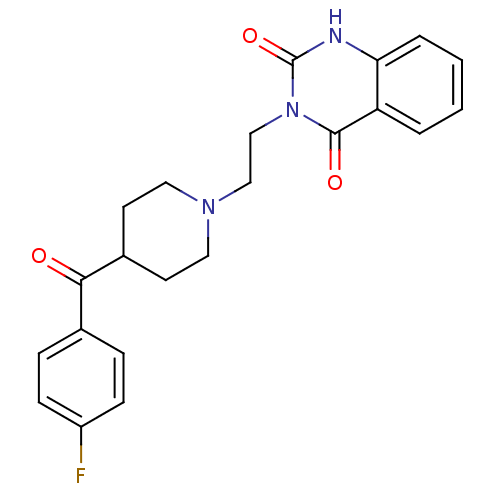
(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50007871
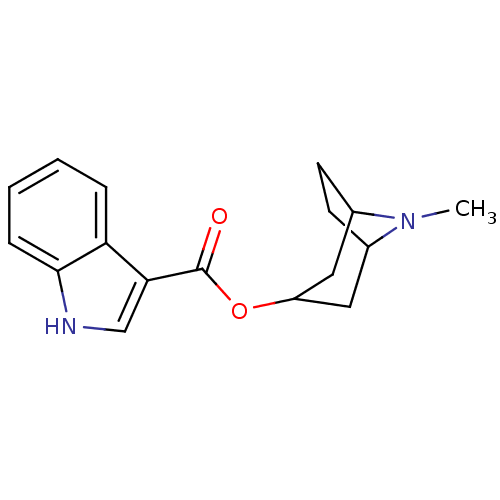
(CHEMBL8197 | ICS 205-930 | TROPISETRON)Show SMILES CN1C2CCC1CC(C2)OC(=O)c1c[nH]c2ccccc12 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 614-26 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1J3M |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451372
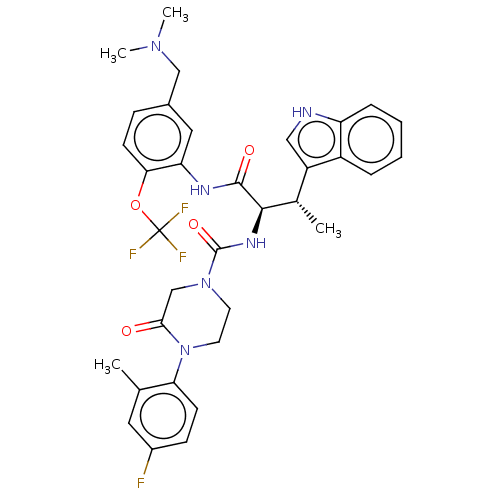
(CHEMBL4217405)Show SMILES C[C@H]([C@@H](NC(=O)N1CCN(C(=O)C1)c1ccc(F)cc1C)C(=O)Nc1cc(CN(C)C)ccc1OC(F)(F)F)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C34H36F4N6O4/c1-20-15-23(35)10-11-28(20)44-14-13-43(19-30(44)45)33(47)41-31(21(2)25-17-39-26-8-6-5-7-24(25)26)32(46)40-27-16-22(18-42(3)4)9-12-29(27)48-34(36,37)38/h5-12,15-17,21,31,39H,13-14,18-19H2,1-4H3,(H,40,46)(H,41,47)/t21-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451373

(CHEMBL4206924)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCC(CC1)c1ccccc1)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C35H43N5O3/c1-5-43-32-16-15-25(23-39(3)4)21-31(32)37-34(41)33(24(2)29-22-36-30-14-10-9-13-28(29)30)38-35(42)40-19-17-27(18-20-40)26-11-7-6-8-12-26/h6-16,21-22,24,27,33,36H,5,17-20,23H2,1-4H3,(H,37,41)(H,38,42)/t24-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451394

(CHEMBL4205606)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCN(CC1)c1ccc(F)cc1)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C34H41FN6O3/c1-5-44-31-15-10-24(22-39(3)4)20-30(31)37-33(42)32(23(2)28-21-36-29-9-7-6-8-27(28)29)38-34(43)41-18-16-40(17-19-41)26-13-11-25(35)12-14-26/h6-15,20-21,23,32,36H,5,16-19,22H2,1-4H3,(H,37,42)(H,38,43)/t23-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451392

(CHEMBL4214725)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCN(C(=O)C1)c1ccc(F)cc1)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C34H39FN6O4/c1-5-45-30-15-10-23(20-39(3)4)18-29(30)37-33(43)32(22(2)27-19-36-28-9-7-6-8-26(27)28)38-34(44)40-16-17-41(31(42)21-40)25-13-11-24(35)12-14-25/h6-15,18-19,22,32,36H,5,16-17,20-21H2,1-4H3,(H,37,43)(H,38,44)/t22-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451391
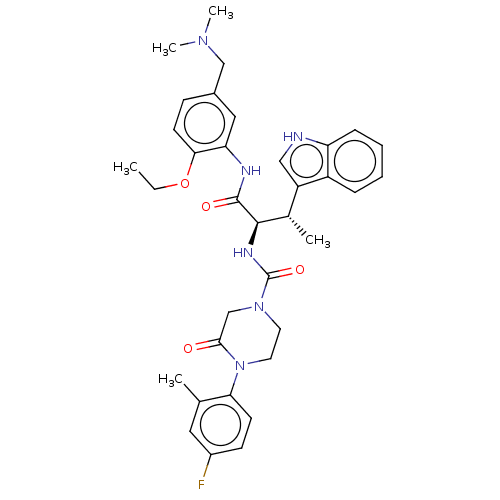
(CHEMBL4212088)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCN(C(=O)C1)c1ccc(F)cc1C)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C35H41FN6O4/c1-6-46-31-14-11-24(20-40(4)5)18-29(31)38-34(44)33(23(3)27-19-37-28-10-8-7-9-26(27)28)39-35(45)41-15-16-42(32(43)21-41)30-13-12-25(36)17-22(30)2/h7-14,17-19,23,33,37H,6,15-16,20-21H2,1-5H3,(H,38,44)(H,39,45)/t23-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451384

(CHEMBL4210064)Show SMILES C[C@H]([C@@H](NC(=O)N1CCN(C(=O)C1)c1ccc(F)cc1C)C(=O)Nc1cc(CN(C)C)ccc1C(C)=O)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C35H39FN6O4/c1-21-16-25(36)11-13-31(21)42-15-14-41(20-32(42)44)35(46)39-33(22(2)28-18-37-29-9-7-6-8-27(28)29)34(45)38-30-17-24(19-40(4)5)10-12-26(30)23(3)43/h6-13,16-18,22,33,37H,14-15,19-20H2,1-5H3,(H,38,45)(H,39,46)/t22-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451385
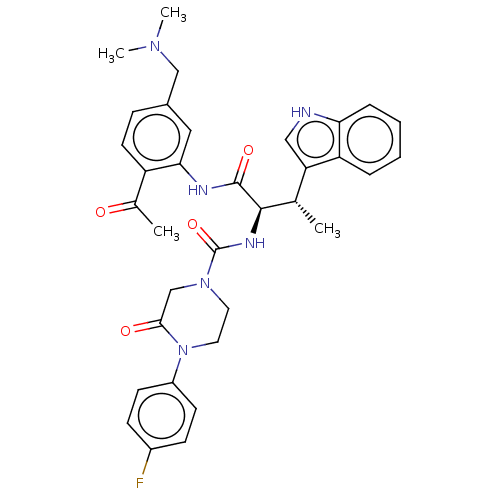
(CHEMBL4205969)Show SMILES C[C@H]([C@@H](NC(=O)N1CCN(C(=O)C1)c1ccc(F)cc1)C(=O)Nc1cc(CN(C)C)ccc1C(C)=O)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C34H37FN6O4/c1-21(28-18-36-29-8-6-5-7-27(28)29)32(33(44)37-30-17-23(19-39(3)4)9-14-26(30)22(2)42)38-34(45)40-15-16-41(31(43)20-40)25-12-10-24(35)11-13-25/h5-14,17-18,21,32,36H,15-16,19-20H2,1-4H3,(H,37,44)(H,38,45)/t21-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451382

(CHEMBL4205696)Show SMILES C[C@H]([C@@H](NC(=O)N1CCN(C(=O)C1)c1ccc(F)cc1)C(=O)Nc1cc(CN(C)C)ccc1OC(F)(F)F)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C33H34F4N6O4/c1-20(25-17-38-26-7-5-4-6-24(25)26)30(31(45)39-27-16-21(18-41(2)3)8-13-28(27)47-33(35,36)37)40-32(46)42-14-15-43(29(44)19-42)23-11-9-22(34)10-12-23/h4-13,16-17,20,30,38H,14-15,18-19H2,1-3H3,(H,39,45)(H,40,46)/t20-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451399

(CHEMBL4208528)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCC(CC1)C(=O)c1cccs1)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C34H41N5O4S/c1-5-43-29-13-12-23(21-38(3)4)19-28(29)36-33(41)31(22(2)26-20-35-27-10-7-6-9-25(26)27)37-34(42)39-16-14-24(15-17-39)32(40)30-11-8-18-44-30/h6-13,18-20,22,24,31,35H,5,14-17,21H2,1-4H3,(H,36,41)(H,37,42)/t22-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451395

(CHEMBL4211422)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCC(CC1)C(=O)C1CCC1)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C34H45N5O4/c1-5-43-30-14-13-23(21-38(3)4)19-29(30)36-33(41)31(22(2)27-20-35-28-12-7-6-11-26(27)28)37-34(42)39-17-15-25(16-18-39)32(40)24-9-8-10-24/h6-7,11-14,19-20,22,24-25,31,35H,5,8-10,15-18,21H2,1-4H3,(H,36,41)(H,37,42)/t22-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451390

(CHEMBL4210627)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)C(=O)c1cccs1)C(=O)Nc1cc(CN(C)C)ccc1C(C)=O)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C34H39N5O4S/c1-21(27-19-35-28-9-6-5-8-26(27)28)31(33(42)36-29-18-23(20-38(3)4)11-12-25(29)22(2)40)37-34(43)39-15-13-24(14-16-39)32(41)30-10-7-17-44-30/h5-12,17-19,21,24,31,35H,13-16,20H2,1-4H3,(H,36,42)(H,37,43)/t21-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451388

(CHEMBL4215053)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)C(=O)C1CCC1)C(=O)Nc1cc(CN(C)C)ccc1C(C)=O)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C34H43N5O4/c1-21(28-19-35-29-11-6-5-10-27(28)29)31(33(42)36-30-18-23(20-38(3)4)12-13-26(30)22(2)40)37-34(43)39-16-14-25(15-17-39)32(41)24-8-7-9-24/h5-6,10-13,18-19,21,24-25,31,35H,7-9,14-17,20H2,1-4H3,(H,36,42)(H,37,43)/t21-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451396

(CHEMBL4216387)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCC(CC1)C(=O)CC(C)C)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C34H47N5O4/c1-7-43-31-13-12-24(21-38(5)6)19-29(31)36-33(41)32(23(4)27-20-35-28-11-9-8-10-26(27)28)37-34(42)39-16-14-25(15-17-39)30(40)18-22(2)3/h8-13,19-20,22-23,25,32,35H,7,14-18,21H2,1-6H3,(H,36,41)(H,37,42)/t23-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451386
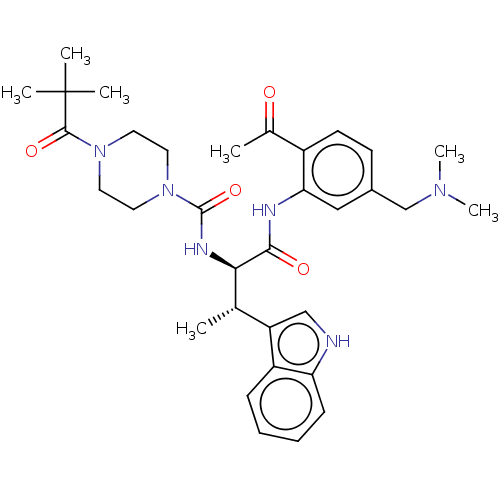
(CHEMBL4203793)Show SMILES C[C@H]([C@@H](NC(=O)N1CCN(CC1)C(=O)C(C)(C)C)C(=O)Nc1cc(CN(C)C)ccc1C(C)=O)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C33H44N6O4/c1-21(26-19-34-27-11-9-8-10-25(26)27)29(36-32(43)39-16-14-38(15-17-39)31(42)33(3,4)5)30(41)35-28-18-23(20-37(6)7)12-13-24(28)22(2)40/h8-13,18-19,21,29,34H,14-17,20H2,1-7H3,(H,35,41)(H,36,43)/t21-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451397

(CHEMBL4208009)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCC(CC1)C(=O)C(C)C)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C33H45N5O4/c1-7-42-29-13-12-23(20-37(5)6)18-28(29)35-32(40)30(22(4)26-19-34-27-11-9-8-10-25(26)27)36-33(41)38-16-14-24(15-17-38)31(39)21(2)3/h8-13,18-19,21-22,24,30,34H,7,14-17,20H2,1-6H3,(H,35,40)(H,36,41)/t22-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451387
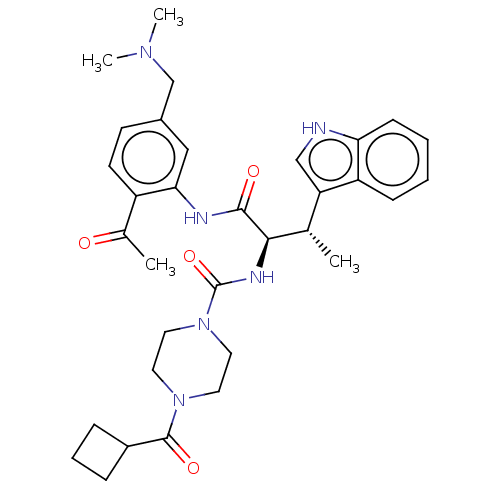
(CHEMBL4203387)Show SMILES C[C@H]([C@@H](NC(=O)N1CCN(CC1)C(=O)C1CCC1)C(=O)Nc1cc(CN(C)C)ccc1C(C)=O)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C33H42N6O4/c1-21(27-19-34-28-11-6-5-10-26(27)28)30(31(41)35-29-18-23(20-37(3)4)12-13-25(29)22(2)40)36-33(43)39-16-14-38(15-17-39)32(42)24-8-7-9-24/h5-6,10-13,18-19,21,24,30,34H,7-9,14-17,20H2,1-4H3,(H,35,41)(H,36,43)/t21-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451398
(CHEMBL4203652)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCC(CC1)C(=O)c1nccs1)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C33H40N6O4S/c1-5-43-28-11-10-22(20-38(3)4)18-27(28)36-31(41)29(21(2)25-19-35-26-9-7-6-8-24(25)26)37-33(42)39-15-12-23(13-16-39)30(40)32-34-14-17-44-32/h6-11,14,17-19,21,23,29,35H,5,12-13,15-16,20H2,1-4H3,(H,36,41)(H,37,42)/t21-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451389

(CHEMBL4215052)Show SMILES CC(C)C(=O)C1CCN(CC1)C(=O)N[C@H]([C@@H](C)c1c[nH]c2ccccc12)C(=O)Nc1cc(CN(C)C)ccc1C(C)=O |r| Show InChI InChI=1S/C33H43N5O4/c1-20(2)31(40)24-13-15-38(16-14-24)33(42)36-30(21(3)27-18-34-28-10-8-7-9-26(27)28)32(41)35-29-17-23(19-37(5)6)11-12-25(29)22(4)39/h7-12,17-18,20-21,24,30,34H,13-16,19H2,1-6H3,(H,35,41)(H,36,42)/t21-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451393

(CHEMBL4202861)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCN(CC1)C(=O)C1CCC1)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C33H44N6O4/c1-5-43-29-14-13-23(21-37(3)4)19-28(29)35-31(40)30(22(2)26-20-34-27-12-7-6-11-25(26)27)36-33(42)39-17-15-38(16-18-39)32(41)24-9-8-10-24/h6-7,11-14,19-20,22,24,30,34H,5,8-10,15-18,21H2,1-4H3,(H,35,40)(H,36,42)/t22-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451370

(CHEMBL4204193)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCC(CC1)C(=O)c1ccco1)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C34H41N5O5/c1-5-43-29-13-12-23(21-38(3)4)19-28(29)36-33(41)31(22(2)26-20-35-27-10-7-6-9-25(26)27)37-34(42)39-16-14-24(15-17-39)32(40)30-11-8-18-44-30/h6-13,18-20,22,24,31,35H,5,14-17,21H2,1-4H3,(H,36,41)(H,37,42)/t22-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451369
(CHEMBL4207735)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCN(CC1)C(=O)C(C)(C)C)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C33H46N6O4/c1-8-43-28-14-13-23(21-37(6)7)19-27(28)35-30(40)29(22(2)25-20-34-26-12-10-9-11-24(25)26)36-32(42)39-17-15-38(16-18-39)31(41)33(3,4)5/h9-14,19-20,22,29,34H,8,15-18,21H2,1-7H3,(H,35,40)(H,36,42)/t22-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451371

(CHEMBL4212910)Show SMILES CCOc1ccc(CN(C)C)cc1NC(=O)[C@H](NC(=O)N1CCC(CC1)C(=O)c1ccccn1)[C@@H](C)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C35H42N6O4/c1-5-45-31-14-13-24(22-40(3)4)20-30(31)38-34(43)32(23(2)27-21-37-28-11-7-6-10-26(27)28)39-35(44)41-18-15-25(16-19-41)33(42)29-12-8-9-17-36-29/h6-14,17,20-21,23,25,32,37H,5,15-16,18-19,22H2,1-4H3,(H,38,43)(H,39,44)/t23-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50451383
(CHEMBL4205170)Show SMILES C[C@H]([C@@H](NC(=O)N1CCN(CC1)C(=O)C(C)(C)C)C(=O)Nc1cc(CN(C)C)ccc1OC(F)(F)F)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C32H41F3N6O4/c1-20(23-18-36-24-10-8-7-9-22(23)24)27(38-30(44)41-15-13-40(14-16-41)29(43)31(2,3)4)28(42)37-25-17-21(19-39(5)6)11-12-26(25)45-32(33,34)35/h7-12,17-18,20,27,36H,13-16,19H2,1-6H3,(H,37,42)(H,38,44)/t20-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... |
Bioorg Med Chem 25: 5995-6006 (2017)
Article DOI: 10.1016/j.bmc.2017.09.031
BindingDB Entry DOI: 10.7270/Q2PR7ZK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50282224

(CHEMBL4172856)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)c1c(OCCCC2CCN(CC2)C(=O)OC2(C)CC2)c2ccccc2n(C)c1=O Show InChI InChI=1S/C34H37F6N3O5/c1-32(12-13-32)48-31(46)43-14-10-21(11-15-43)7-6-16-47-28-25-8-4-5-9-26(25)42(3)30(45)27(28)29(44)41(2)20-22-17-23(33(35,36)37)19-24(18-22)34(38,39)40/h4-5,8-9,17-19,21H,6-7,10-16,20H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of dihydrofolate reductase obtained from human |
Eur J Med Chem 136: 283-293 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.067
BindingDB Entry DOI: 10.7270/Q29G5QB9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data