

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451372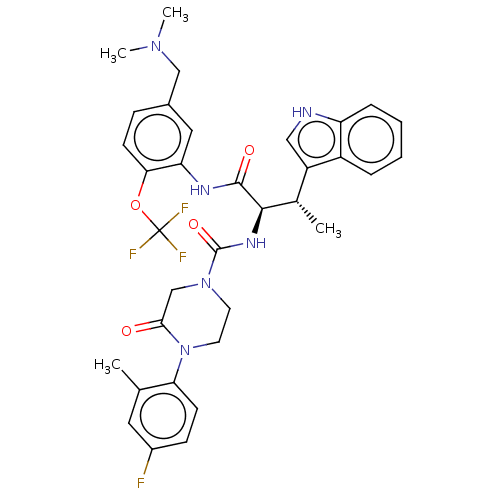 (CHEMBL4217405) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451373 (CHEMBL4206924) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451394 (CHEMBL4205606) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451392 (CHEMBL4214725) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451391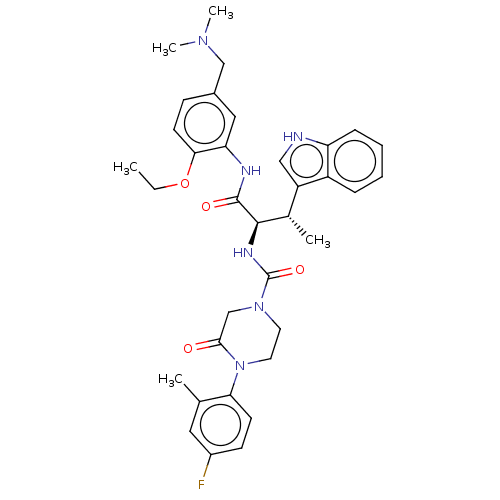 (CHEMBL4212088) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451384 (CHEMBL4210064) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451385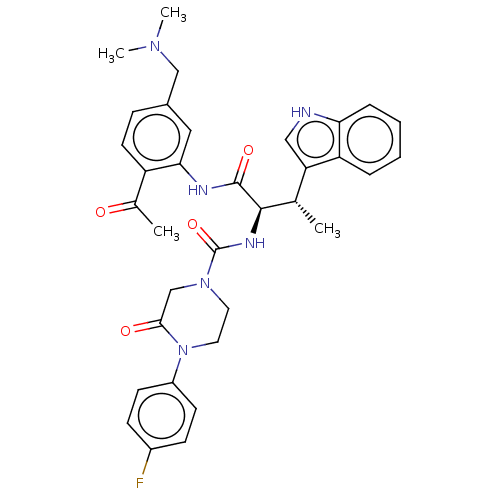 (CHEMBL4205969) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451382 (CHEMBL4205696) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451399 (CHEMBL4208528) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451395 (CHEMBL4211422) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451390 (CHEMBL4210627) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451388 (CHEMBL4215053) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451396 (CHEMBL4216387) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451386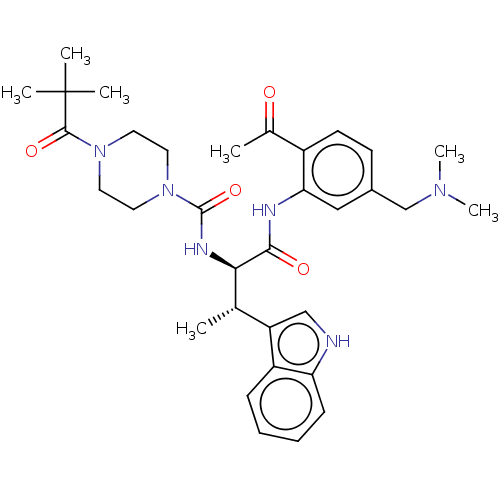 (CHEMBL4203793) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451397 (CHEMBL4208009) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451387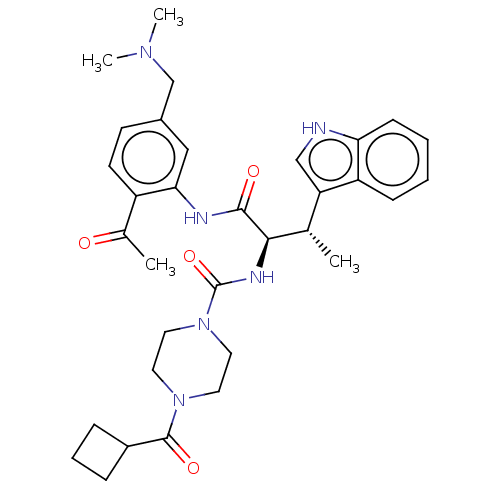 (CHEMBL4203387) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451398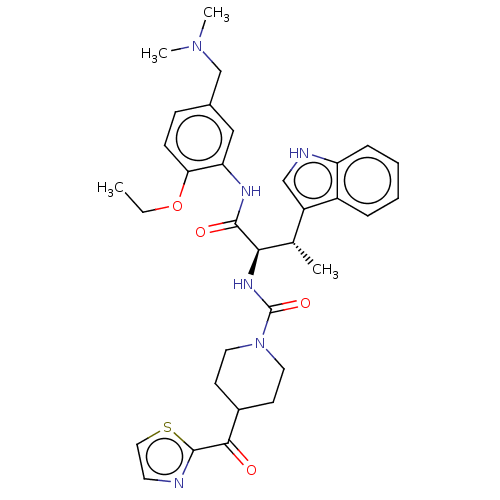 (CHEMBL4203652) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451389 (CHEMBL4215052) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451393 (CHEMBL4202861) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451370 (CHEMBL4204193) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451369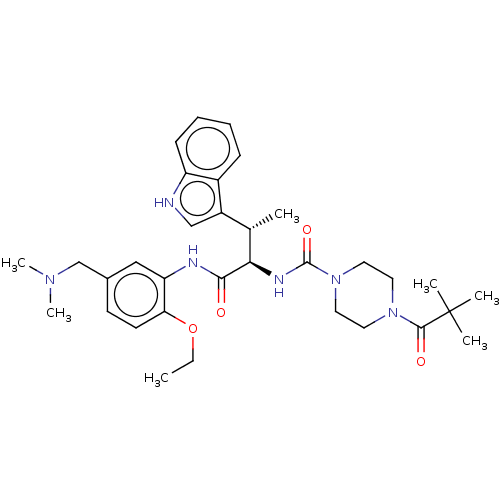 (CHEMBL4207735) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451371 (CHEMBL4212910) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451383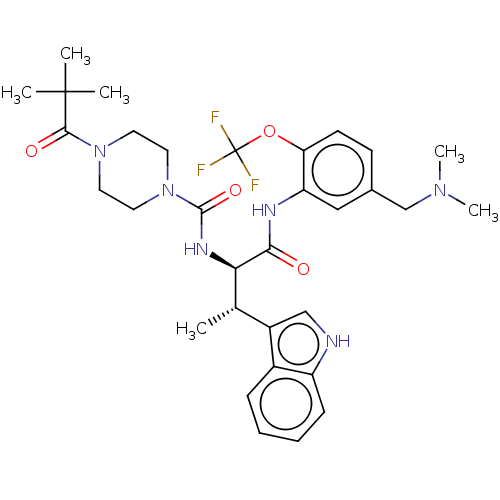 (CHEMBL4205170) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50282224 (CHEMBL4172856) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase obtained from human | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50282208 (CHEMBL4177314) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Folyl-polyglutamate synthase obtained from porcine | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50451372 (CHEMBL4217405) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR4 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50451373 (CHEMBL4206924) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR5 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50451372 (CHEMBL4217405) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR3 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50282210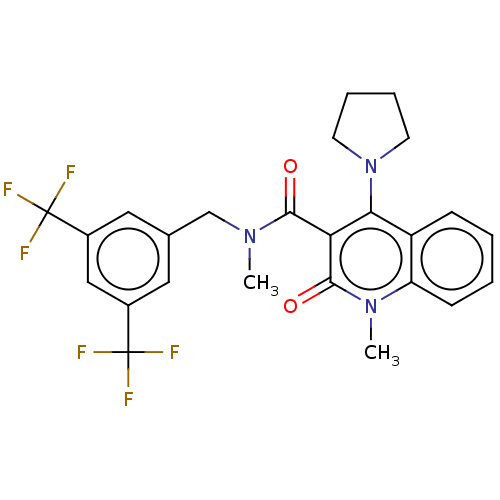 (CHEMBL4162182) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SMS2 expressed in HEK293T cells using C2-ceramide as substrate preincubated for 60 mins followed by substrate addition measured a... | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50282210 (CHEMBL4162182) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of thymidylate synthase obtained from human | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50282210 (CHEMBL4162182) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SMS2 | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50282224 (CHEMBL4172856) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of [3H]- glycine binding to NMDA receptor from rat cortical membranes. | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50451372 (CHEMBL4217405) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR5 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50282210 (CHEMBL4162182) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of glycinamide ribonucleotide transformylase obtained from porcine (GAR Tfase) | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50451372 (CHEMBL4217405) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR1 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50282208 (CHEMBL4177314) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of full length C-terminal FLAG tagged human SMS1 expressed in HEK293 cell membranes using DMPC-d72 and C17-ceramide as substrate preincuba... | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50282209 (CHEMBL1161894) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of full length C-terminal FLAG tagged human SMS2 expressed in HEK293 cell membranes using DMPC-d72 and C17-ceramide as substrate preincuba... | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50282209 (CHEMBL1161894) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human Dihydrofolate reductase | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50282210 (CHEMBL4162182) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SMS1 | Eur J Med Chem 136: 283-293 (2017) Article DOI: 10.1016/j.ejmech.2017.04.067 BindingDB Entry DOI: 10.7270/Q29G5QB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50196417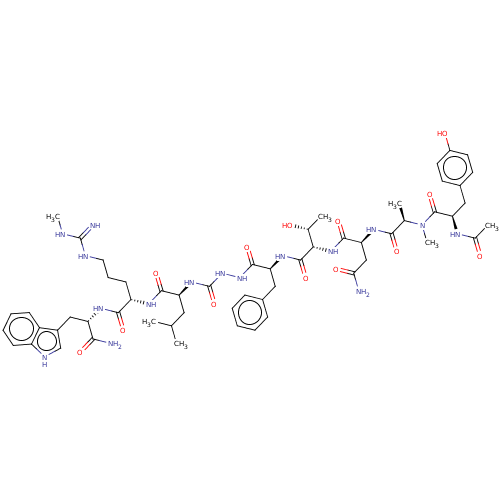 (CHEMBL3977235) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Agonist activity at human KISS1R expressed in CHO/dhfr cells assessed as increase in intracellular Ca2+ levels measured for 180 secs by Fluo 3-AM dye... | J Med Chem 59: 8804-8811 (2016) Article DOI: 10.1021/acs.jmedchem.6b00379 BindingDB Entry DOI: 10.7270/Q2000425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50196418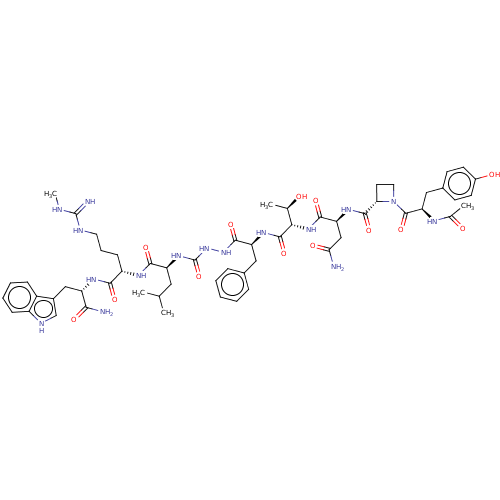 (CHEMBL3967058) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Agonist activity at human KISS1R expressed in CHO/dhfr cells assessed as increase in intracellular Ca2+ levels measured for 180 secs by Fluo 3-AM dye... | J Med Chem 59: 8804-8811 (2016) Article DOI: 10.1021/acs.jmedchem.6b00379 BindingDB Entry DOI: 10.7270/Q2000425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50196419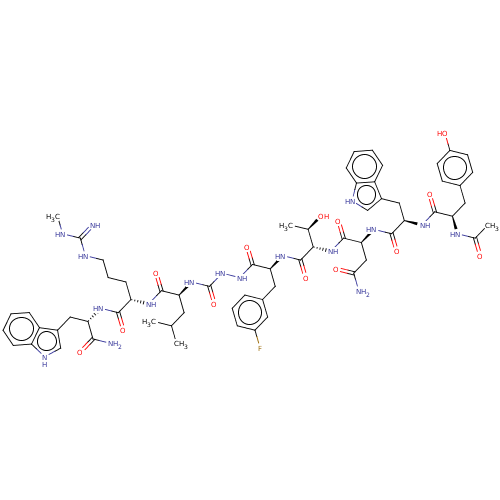 (CHEMBL3930297) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Agonist activity at human KISS1R expressed in CHO/dhfr cells assessed as increase in intracellular Ca2+ levels measured for 180 secs by Fluo 3-AM dye... | J Med Chem 59: 8804-8811 (2016) Article DOI: 10.1021/acs.jmedchem.6b00379 BindingDB Entry DOI: 10.7270/Q2000425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50196420 (CHEMBL3924151) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Agonist activity at human KISS1R expressed in CHO/dhfr cells assessed as increase in intracellular Ca2+ levels measured for 180 secs by Fluo 3-AM dye... | J Med Chem 59: 8804-8811 (2016) Article DOI: 10.1021/acs.jmedchem.6b00379 BindingDB Entry DOI: 10.7270/Q2000425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Rattus norvegicus) | BDBM50196421 (CHEMBL3985112) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Agonist activity at rat KISS1R expressed in CHO/dhfr cells assessed as increase in intracellular Ca2+ levels measured for 180 secs by Fluo 3-AM dye b... | J Med Chem 59: 8804-8811 (2016) Article DOI: 10.1021/acs.jmedchem.6b00379 BindingDB Entry DOI: 10.7270/Q2000425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50196422 (CHEMBL3967782) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Agonist activity at human KISS1R expressed in CHO/dhfr cells assessed as increase in intracellular Ca2+ levels measured for 180 secs by Fluo 3-AM dye... | J Med Chem 59: 8804-8811 (2016) Article DOI: 10.1021/acs.jmedchem.6b00379 BindingDB Entry DOI: 10.7270/Q2000425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50196423 (CHEMBL3977314) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Agonist activity at human KISS1R expressed in CHO/dhfr cells assessed as increase in intracellular Ca2+ levels measured for 180 secs by Fluo 3-AM dye... | J Med Chem 59: 8804-8811 (2016) Article DOI: 10.1021/acs.jmedchem.6b00379 BindingDB Entry DOI: 10.7270/Q2000425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50196424 (CHEMBL3966187) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Agonist activity at human KISS1R expressed in CHO/dhfr cells assessed as increase in intracellular Ca2+ levels measured for 180 secs by Fluo 3-AM dye... | J Med Chem 59: 8804-8811 (2016) Article DOI: 10.1021/acs.jmedchem.6b00379 BindingDB Entry DOI: 10.7270/Q2000425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Rattus norvegicus) | BDBM50196424 (CHEMBL3966187) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Agonist activity at rat KISS1R expressed in CHO/dhfr cells assessed as increase in intracellular Ca2+ levels measured for 180 secs by Fluo 3-AM dye b... | J Med Chem 59: 8804-8811 (2016) Article DOI: 10.1021/acs.jmedchem.6b00379 BindingDB Entry DOI: 10.7270/Q2000425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Rattus norvegicus) | BDBM50196425 (CHEMBL3904889) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Agonist activity at rat KISS1R expressed in CHO/dhfr cells assessed as increase in intracellular Ca2+ levels measured for 180 secs by Fluo 3-AM dye b... | J Med Chem 59: 8804-8811 (2016) Article DOI: 10.1021/acs.jmedchem.6b00379 BindingDB Entry DOI: 10.7270/Q2000425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Rattus norvegicus) | BDBM50196426 (CHEMBL3964403) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Agonist activity at rat KISS1R expressed in CHO/dhfr cells assessed as increase in intracellular Ca2+ levels measured for 180 secs by Fluo 3-AM dye b... | J Med Chem 59: 8804-8811 (2016) Article DOI: 10.1021/acs.jmedchem.6b00379 BindingDB Entry DOI: 10.7270/Q2000425 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 147 total ) | Next | Last >> |