 Found 155 hits with Last Name = 'maw' and Initial = 'a'
Found 155 hits with Last Name = 'maw' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human mTOR using 4EBP1 as substrate in presence of [33gammaP]-ATP after 120 mins by filter binding method |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243194
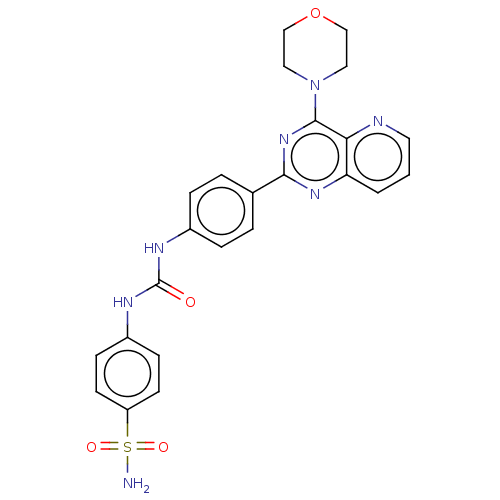
(CHEMBL4097194)Show SMILES NS(=O)(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C24H23N7O4S/c25-36(33,34)19-9-7-18(8-10-19)28-24(32)27-17-5-3-16(4-6-17)22-29-20-2-1-11-26-21(20)23(30-22)31-12-14-35-15-13-31/h1-11H,12-15H2,(H2,25,33,34)(H2,27,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243144
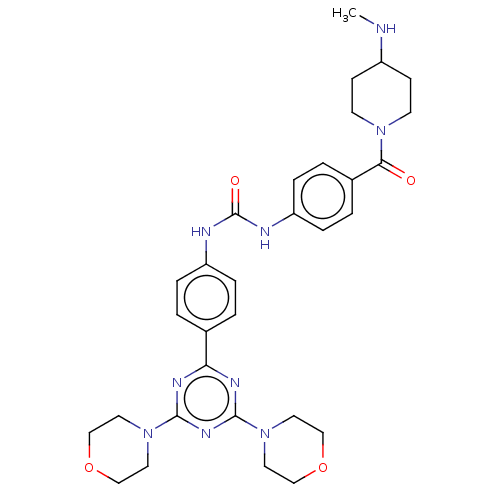
(CHEMBL4065928)Show SMILES CNC1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C31H39N9O4/c1-32-24-10-12-38(13-11-24)28(41)23-4-8-26(9-5-23)34-31(42)33-25-6-2-22(3-7-25)27-35-29(39-14-18-43-19-15-39)37-30(36-27)40-16-20-44-21-17-40/h2-9,24,32H,10-21H2,1H3,(H2,33,34,42) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243193
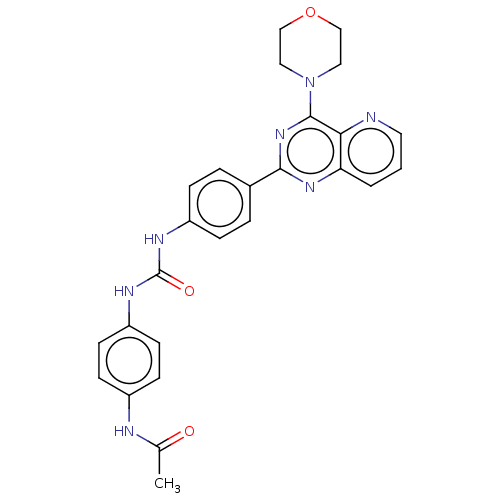
(CHEMBL4060651)Show SMILES CC(=O)Nc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C26H25N7O3/c1-17(34)28-19-8-10-21(11-9-19)30-26(35)29-20-6-4-18(5-7-20)24-31-22-3-2-12-27-23(22)25(32-24)33-13-15-36-16-14-33/h2-12H,13-16H2,1H3,(H,28,34)(H2,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243339
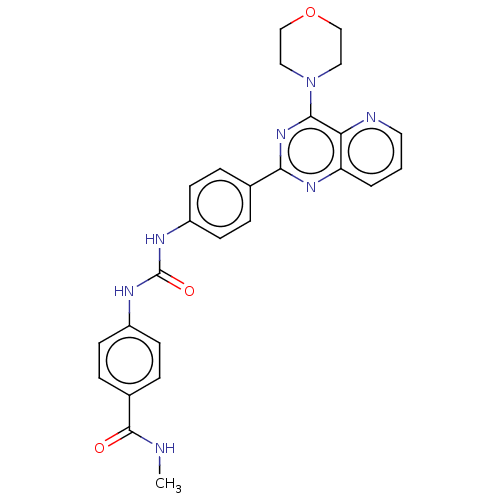
(CHEMBL4089494)Show SMILES CNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C26H25N7O3/c1-27-25(34)18-6-10-20(11-7-18)30-26(35)29-19-8-4-17(5-9-19)23-31-21-3-2-12-28-22(21)24(32-23)33-13-15-36-16-14-33/h2-12H,13-16H2,1H3,(H,27,34)(H2,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243292
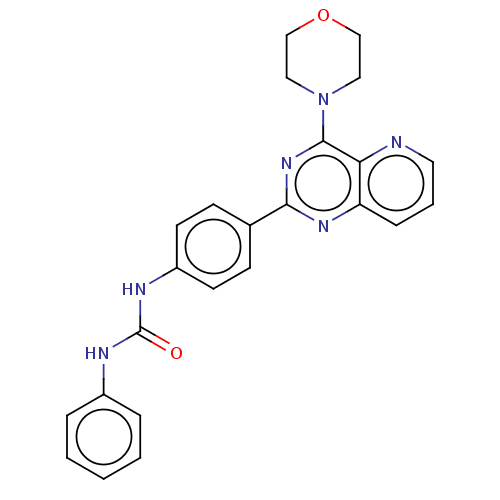
(CHEMBL4092587)Show SMILES O=C(Nc1ccccc1)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2ncccc2n1 Show InChI InChI=1S/C24H22N6O2/c31-24(26-18-5-2-1-3-6-18)27-19-10-8-17(9-11-19)22-28-20-7-4-12-25-21(20)23(29-22)30-13-15-32-16-14-30/h1-12H,13-16H2,(H2,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243304

(CHEMBL4104902)Show SMILES NC(=N)NS(=O)(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C25H25N9O4S/c26-24(27)33-39(36,37)19-9-7-18(8-10-19)30-25(35)29-17-5-3-16(4-6-17)22-31-20-2-1-11-28-21(20)23(32-22)34-12-14-38-15-13-34/h1-11H,12-15H2,(H4,26,27,33)(H2,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50561775

(CHEMBL4792696)Show SMILES CC(=O)Nc1ccc(NC(=O)Nc2nc(N3CCOCC3)c3ccccc3n2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate incubated for 10 mins followed by ATP addition and measured after 30 mins in pres... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00289
BindingDB Entry DOI: 10.7270/Q2DF6VXJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50243145
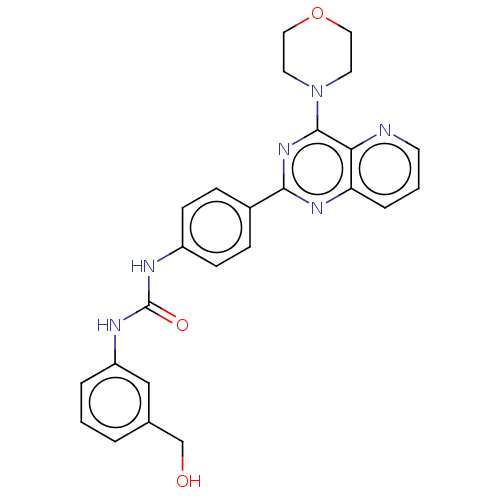
(CHEMBL3109141)Show SMILES OCc1cccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)c1 Show InChI InChI=1S/C25H24N6O3/c32-16-17-3-1-4-20(15-17)28-25(33)27-19-8-6-18(7-9-19)23-29-21-5-2-10-26-22(21)24(30-23)31-11-13-34-14-12-31/h1-10,15,32H,11-14,16H2,(H2,27,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human mTOR using 4EBP1 as substrate in presence of [33gammaP]-ATP after 120 mins by filter binding method |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50561773
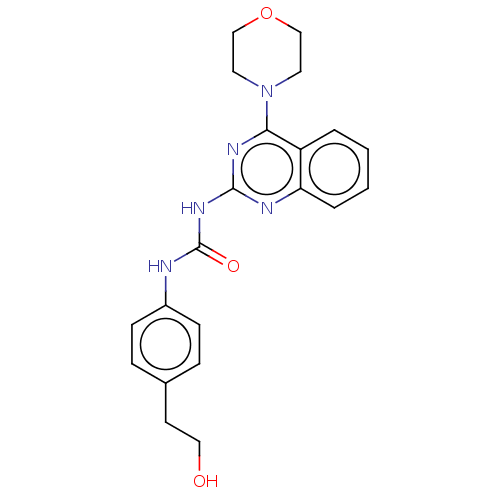
(CHEMBL4751330)Show SMILES OCCc1ccc(NC(=O)Nc2nc(N3CCOCC3)c3ccccc3n2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate incubated for 10 mins followed by ATP addition and measured after 30 mins in pres... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00289
BindingDB Entry DOI: 10.7270/Q2DF6VXJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50243144

(CHEMBL4065928)Show SMILES CNC1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C31H39N9O4/c1-32-24-10-12-38(13-11-24)28(41)23-4-8-26(9-5-23)34-31(42)33-25-6-2-22(3-7-25)27-35-29(39-14-18-43-19-15-39)37-30(36-27)40-16-20-44-21-17-40/h2-9,24,32H,10-21H2,1H3,(H2,33,34,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human mTOR using 4EBP1 as substrate in presence of [33gammaP]-ATP after 120 mins by filter binding method |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243176
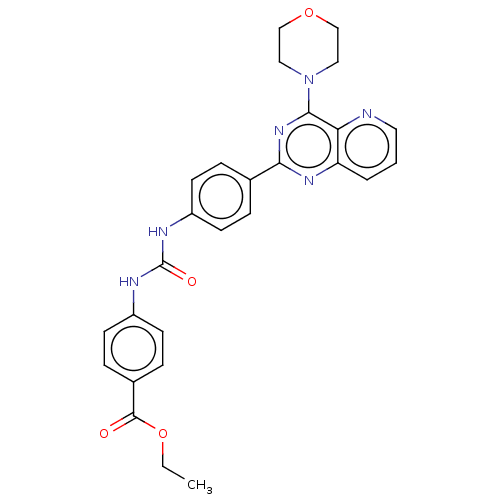
(CHEMBL4081617)Show SMILES CCOC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C27H26N6O4/c1-2-37-26(34)19-7-11-21(12-8-19)30-27(35)29-20-9-5-18(6-10-20)24-31-22-4-3-13-28-23(22)25(32-24)33-14-16-36-17-15-33/h3-13H,2,14-17H2,1H3,(H2,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243146
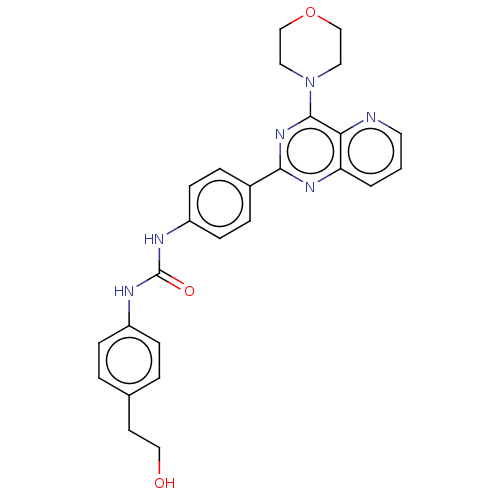
(CHEMBL4102783)Show SMILES OCCc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C26H26N6O3/c33-15-11-18-3-7-20(8-4-18)28-26(34)29-21-9-5-19(6-10-21)24-30-22-2-1-12-27-23(22)25(31-24)32-13-16-35-17-14-32/h1-10,12,33H,11,13-17H2,(H2,28,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate incubated for 10 mins followed by ATP addition and measured after 30 mins in pres... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00289
BindingDB Entry DOI: 10.7270/Q2DF6VXJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50561771
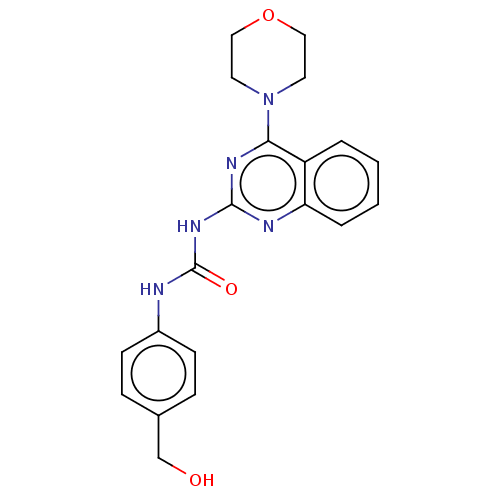
(CHEMBL4764755)Show SMILES OCc1ccc(NC(=O)Nc2nc(N3CCOCC3)c3ccccc3n2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate incubated for 10 mins followed by ATP addition and measured after 30 mins in pres... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00289
BindingDB Entry DOI: 10.7270/Q2DF6VXJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243195

(CHEMBL4079178)Show SMILES CS(=O)(=O)Nc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C25H25N7O4S/c1-37(34,35)31-20-10-8-19(9-11-20)28-25(33)27-18-6-4-17(5-7-18)23-29-21-3-2-12-26-22(21)24(30-23)32-13-15-36-16-14-32/h2-12,31H,13-16H2,1H3,(H2,27,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50561776

(CHEMBL4759291)Show SMILES CNC(=O)c1ccc(NC(=O)Nc2nc(N3CCOCC3)c3ccccc3n2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate incubated for 10 mins followed by ATP addition and measured after 30 mins in pres... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00289
BindingDB Entry DOI: 10.7270/Q2DF6VXJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243313
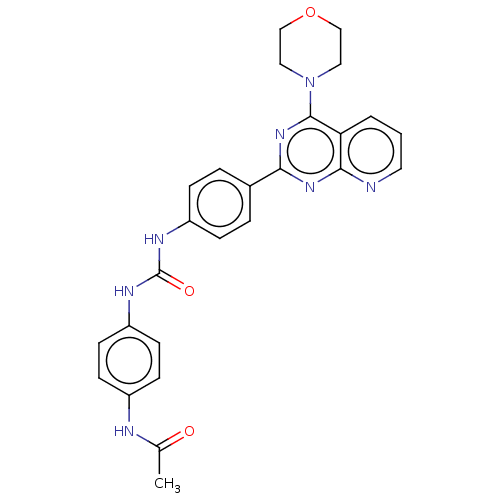
(CHEMBL4103534)Show SMILES CC(=O)Nc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3cccnc3n2)cc1 Show InChI InChI=1S/C26H25N7O3/c1-17(34)28-19-8-10-21(11-9-19)30-26(35)29-20-6-4-18(5-7-20)23-31-24-22(3-2-12-27-24)25(32-23)33-13-15-36-16-14-33/h2-12H,13-16H2,1H3,(H,28,34)(H2,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
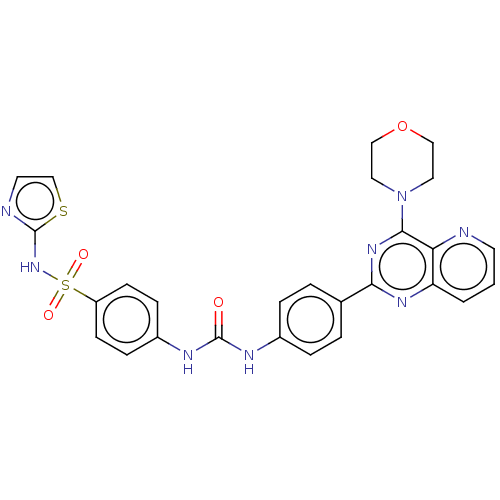
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243196
(CHEMBL4087043)Show SMILES O=C(Nc1ccc(cc1)-c1nc(N2CCOCC2)c2ncccc2n1)Nc1ccc(cc1)S(=O)(=O)Nc1nccs1 Show InChI InChI=1S/C27H24N8O4S2/c36-26(31-20-7-9-21(10-8-20)41(37,38)34-27-29-12-17-40-27)30-19-5-3-18(4-6-19)24-32-22-2-1-11-28-23(22)25(33-24)35-13-15-39-16-14-35/h1-12,17H,13-16H2,(H,29,34)(H2,30,31,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50429701
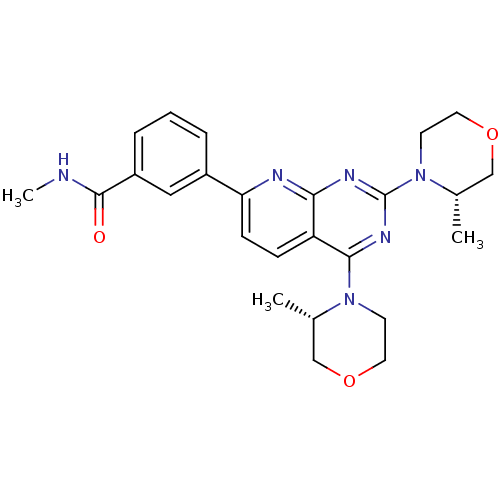
(AZD-2014 | CHEMBL2336325 | US9102670, 1ap)Show SMILES CNC(=O)c1cccc(c1)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H30N6O3/c1-16-14-33-11-9-30(16)23-20-7-8-21(18-5-4-6-19(13-18)24(32)26-3)27-22(20)28-25(29-23)31-10-12-34-15-17(31)2/h4-8,13,16-17H,9-12,14-15H2,1-3H3,(H,26,32)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human mTOR using 4EBP1 as substrate in presence of [33gammaP]-ATP after 120 mins by filter binding method |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243293
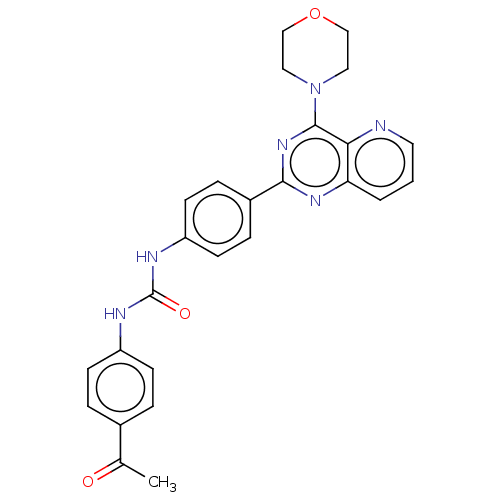
(CHEMBL4088235)Show SMILES CC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C26H24N6O3/c1-17(33)18-4-8-20(9-5-18)28-26(34)29-21-10-6-19(7-11-21)24-30-22-3-2-12-27-23(22)25(31-24)32-13-15-35-16-14-32/h2-12H,13-16H2,1H3,(H2,28,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243172
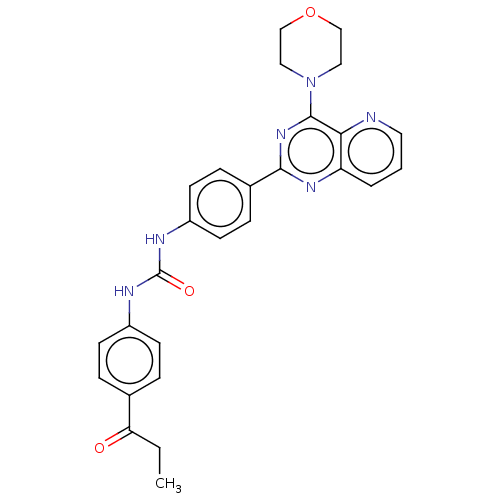
(CHEMBL4059989)Show SMILES CCC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C27H26N6O3/c1-2-23(34)18-5-9-20(10-6-18)29-27(35)30-21-11-7-19(8-12-21)25-31-22-4-3-13-28-24(22)26(32-25)33-14-16-36-17-15-33/h3-13H,2,14-17H2,1H3,(H2,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50561777
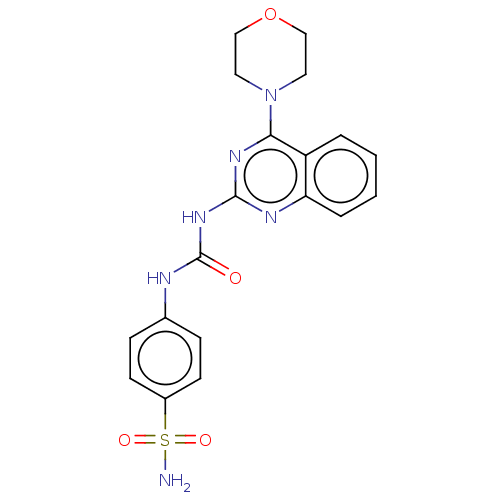
(CHEMBL4758082)Show SMILES NS(=O)(=O)c1ccc(NC(=O)Nc2nc(N3CCOCC3)c3ccccc3n2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate incubated for 10 mins followed by ATP addition and measured after 30 mins in pres... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00289
BindingDB Entry DOI: 10.7270/Q2DF6VXJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243230
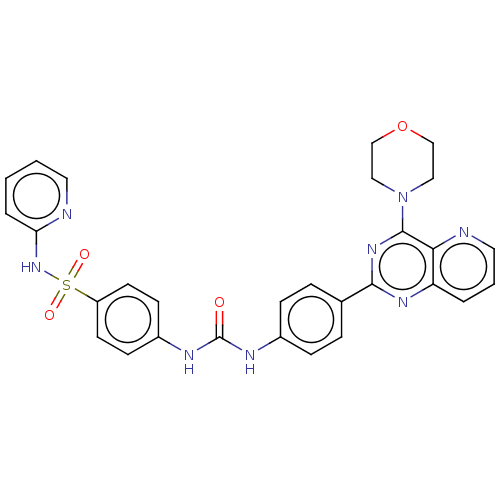
(CHEMBL4092840)Show SMILES O=C(Nc1ccc(cc1)-c1nc(N2CCOCC2)c2ncccc2n1)Nc1ccc(cc1)S(=O)(=O)Nc1ccccn1 Show InChI InChI=1S/C29H26N8O4S/c38-29(33-22-10-12-23(13-11-22)42(39,40)36-25-5-1-2-14-30-25)32-21-8-6-20(7-9-21)27-34-24-4-3-15-31-26(24)28(35-27)37-16-18-41-19-17-37/h1-15H,16-19H2,(H,30,36)(H2,32,33,38) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50243304

(CHEMBL4104902)Show SMILES NC(=N)NS(=O)(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C25H25N9O4S/c26-24(27)33-39(36,37)19-9-7-18(8-10-19)30-25(35)29-17-5-3-16(4-6-17)22-31-20-2-1-11-28-21(20)23(32-22)34-12-14-38-15-13-34/h1-11H,12-15H2,(H4,26,27,33)(H2,29,30,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human mTOR using 4EBP1 as substrate in presence of [33gammaP]-ATP after 120 mins by filter binding method |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243251
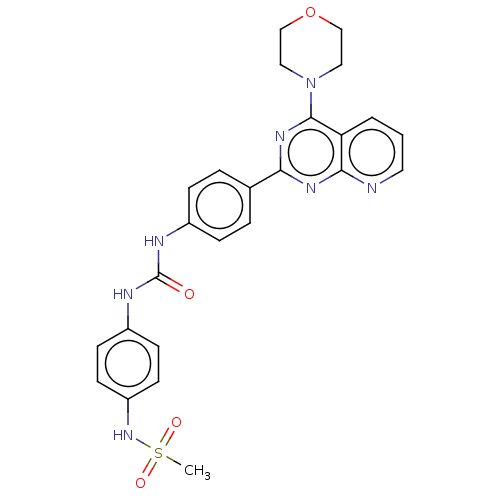
(CHEMBL4088056)Show SMILES CS(=O)(=O)Nc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3cccnc3n2)cc1 Show InChI InChI=1S/C25H25N7O4S/c1-37(34,35)31-20-10-8-19(9-11-20)28-25(33)27-18-6-4-17(5-7-18)22-29-23-21(3-2-12-26-23)24(30-22)32-13-15-36-16-14-32/h2-12,31H,13-16H2,1H3,(H2,27,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243248
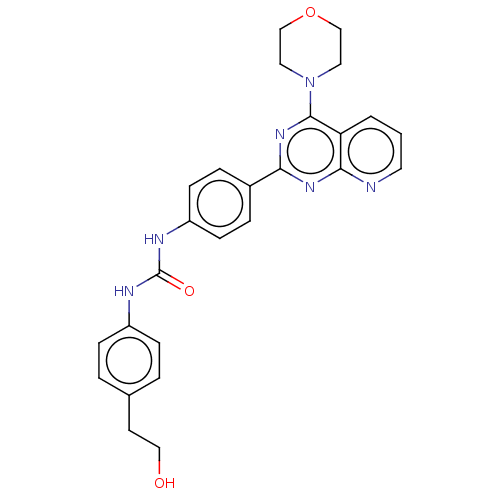
(CHEMBL4102715)Show SMILES OCCc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3cccnc3n2)cc1 Show InChI InChI=1S/C26H26N6O3/c33-15-11-18-3-7-20(8-4-18)28-26(34)29-21-9-5-19(6-10-21)23-30-24-22(2-1-12-27-24)25(31-23)32-13-16-35-17-14-32/h1-10,12,33H,11,13-17H2,(H2,28,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Rattus norvegicus (rat)) | BDBM2714
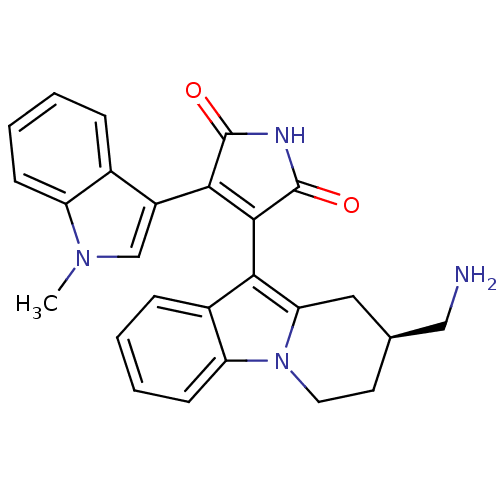
(3-[(2S)-2-(aminomethyl)-1H,2H,3H,4H-pyrido[1,2-a]i...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2c3C[C@@H](CN)CCn3c3ccccc23)c2ccccc12 |r,t:4| Show InChI InChI=1S/C26H24N4O2/c1-29-14-18(16-6-2-4-8-19(16)29)23-24(26(32)28-25(23)31)22-17-7-3-5-9-20(17)30-11-10-15(13-27)12-21(22)30/h2-9,14-15H,10-13,27H2,1H3,(H,28,31,32)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited
| Assay Description
The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... |
J Med Chem 36: 21-9 (1993)
Article DOI: 10.1021/jm00053a003
BindingDB Entry DOI: 10.7270/Q29P2ZTD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436459
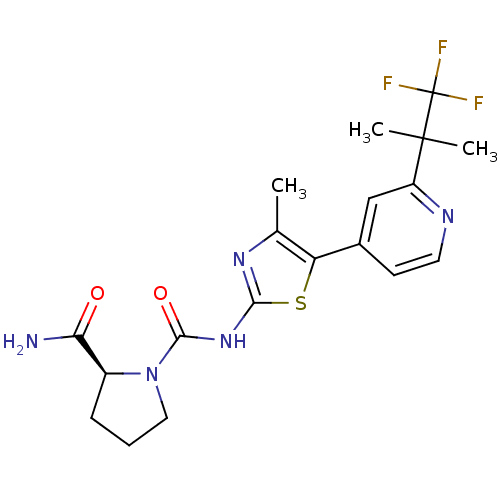
(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00289
BindingDB Entry DOI: 10.7270/Q2DF6VXJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50243143
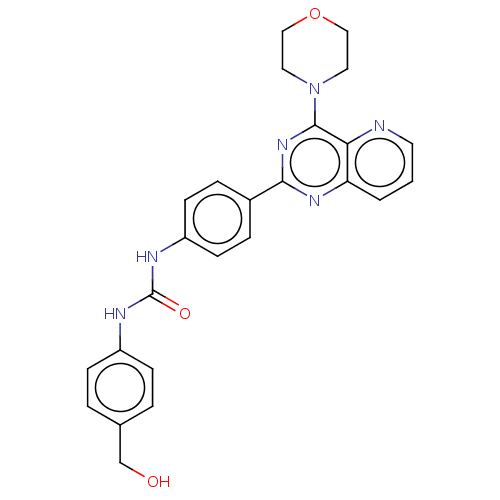
(CHEMBL3109142)Show SMILES OCc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C25H24N6O3/c32-16-17-3-7-19(8-4-17)27-25(33)28-20-9-5-18(6-10-20)23-29-21-2-1-11-26-22(21)24(30-23)31-12-14-34-15-13-31/h1-11,32H,12-16H2,(H2,27,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human mTOR using 4EBP1 as substrate in presence of [33gammaP]-ATP after 120 mins by filter binding method |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243296
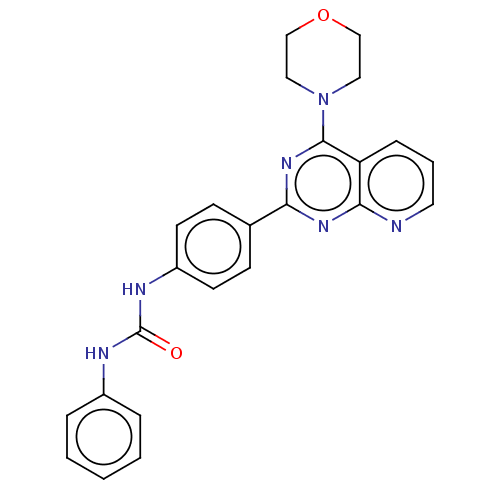
(CHEMBL4065878)Show SMILES O=C(Nc1ccccc1)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cccnc2n1 Show InChI InChI=1S/C24H22N6O2/c31-24(26-18-5-2-1-3-6-18)27-19-10-8-17(9-11-19)21-28-22-20(7-4-12-25-22)23(29-21)30-13-15-32-16-14-30/h1-12H,13-16H2,(H2,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243249
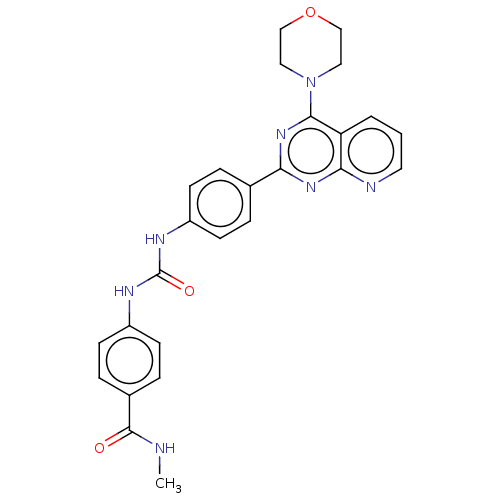
(CHEMBL4079272)Show SMILES CNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3cccnc3n2)cc1 Show InChI InChI=1S/C26H25N7O3/c1-27-25(34)18-6-10-20(11-7-18)30-26(35)29-19-8-4-17(5-9-19)22-31-23-21(3-2-12-28-23)24(32-22)33-13-15-36-16-14-33/h2-12H,13-16H2,1H3,(H,27,34)(H2,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243246
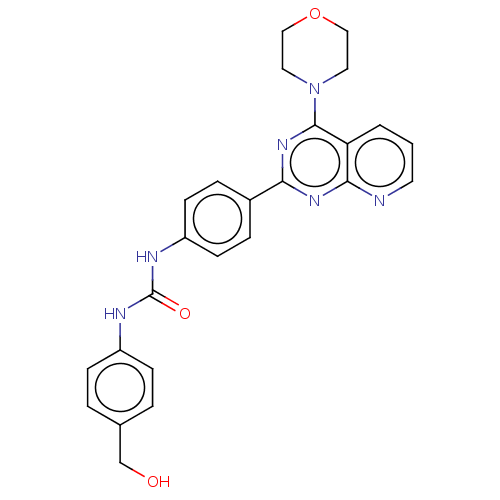
(CHEMBL4064842)Show SMILES OCc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3cccnc3n2)cc1 Show InChI InChI=1S/C25H24N6O3/c32-16-17-3-7-19(8-4-17)27-25(33)28-20-9-5-18(6-10-20)22-29-23-21(2-1-11-26-23)24(30-22)31-12-14-34-15-13-31/h1-11,32H,12-16H2,(H2,27,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243314
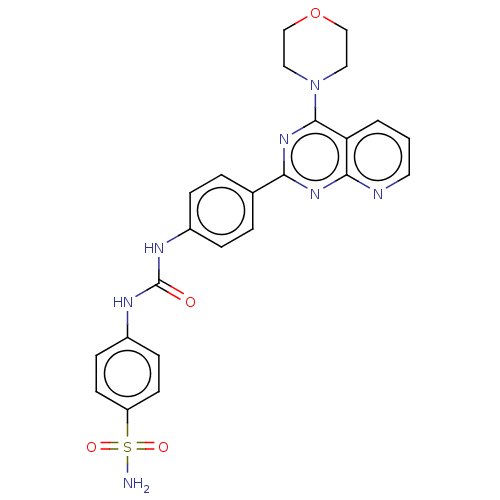
(CHEMBL4087157)Show SMILES NS(=O)(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3cccnc3n2)cc1 Show InChI InChI=1S/C24H23N7O4S/c25-36(33,34)19-9-7-18(8-10-19)28-24(32)27-17-5-3-16(4-6-17)21-29-22-20(2-1-11-26-22)23(30-21)31-12-14-35-15-13-31/h1-11H,12-15H2,(H2,25,33,34)(H2,27,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50561778
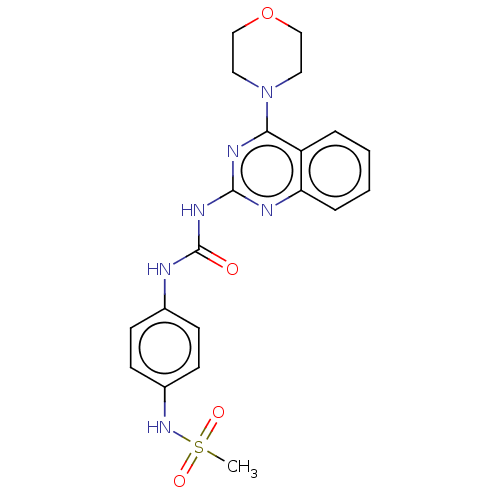
(CHEMBL4793846)Show SMILES CS(=O)(=O)Nc1ccc(NC(=O)Nc2nc(N3CCOCC3)c3ccccc3n2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate incubated for 10 mins followed by ATP addition and measured after 30 mins in pres... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00289
BindingDB Entry DOI: 10.7270/Q2DF6VXJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449890

(CHEMBL4161876)Show SMILES COc1cc(cc(OC)c1OC)C1=N\C(=C/c2cc(Br)cc(Br)c2OC(C)=O)C(=O)NN1c1ncc(s1)-c1ccccc1 |t:13| Show InChI InChI=1S/C30H24Br2N4O6S/c1-16(37)42-26-18(10-20(31)14-21(26)32)11-22-29(38)35-36(30-33-15-25(43-30)17-8-6-5-7-9-17)28(34-22)19-12-23(39-2)27(41-4)24(13-19)40-3/h5-15H,1-4H3,(H,35,38)/b22-11- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50561772
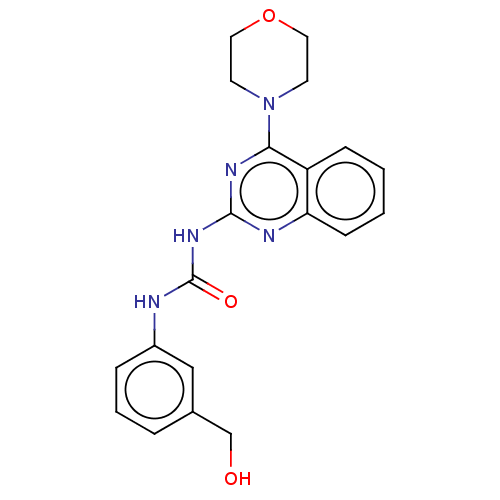
(CHEMBL4748452)Show SMILES OCc1cccc(NC(=O)Nc2nc(N3CCOCC3)c3ccccc3n2)c1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate incubated for 10 mins followed by ATP addition and measured after 30 mins in pres... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00289
BindingDB Entry DOI: 10.7270/Q2DF6VXJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Rattus norvegicus (rat)) | BDBM2700

(3-[2-(aminomethyl)-1H,2H,3H,4H-pyrido[1,2-a]indol-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2c3CC(CN)CCn3c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C26H24N4O2/c1-29-14-18(16-6-2-4-8-19(16)29)23-24(26(32)28-25(23)31)22-17-7-3-5-9-20(17)30-11-10-15(13-27)12-21(22)30/h2-9,14-15H,10-13,27H2,1H3,(H,28,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited
| Assay Description
The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... |
J Med Chem 36: 21-9 (1993)
Article DOI: 10.1021/jm00053a003
BindingDB Entry DOI: 10.7270/Q29P2ZTD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243247
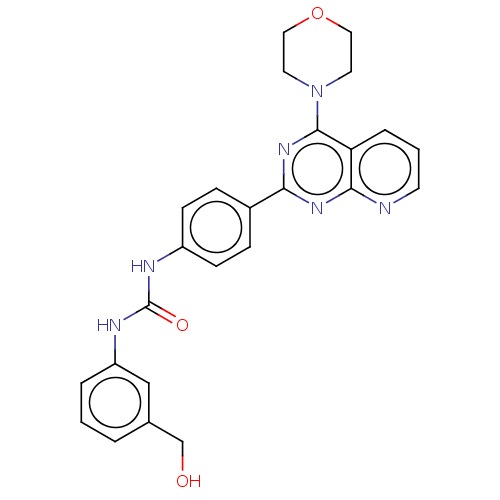
(CHEMBL4092544)Show SMILES OCc1cccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3cccnc3n2)c1 Show InChI InChI=1S/C25H24N6O3/c32-16-17-3-1-4-20(15-17)28-25(33)27-19-8-6-18(7-9-19)22-29-23-21(5-2-10-26-23)24(30-22)31-11-13-34-14-12-31/h1-10,15,32H,11-14,16H2,(H2,27,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50243253
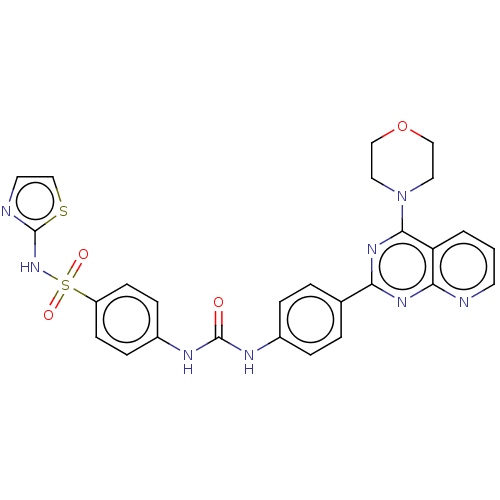
(CHEMBL4095781)Show SMILES O=C(Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cccnc2n1)Nc1ccc(cc1)S(=O)(=O)Nc1nccs1 Show InChI InChI=1S/C27H24N8O4S2/c36-26(31-20-7-9-21(10-8-20)41(37,38)34-27-29-12-17-40-27)30-19-5-3-18(4-6-19)23-32-24-22(2-1-11-28-24)25(33-23)35-13-15-39-16-14-35/h1-12,17H,13-16H2,(H,29,34)(H2,30,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human mTOR using 4EBP1 as substrate in presence of [33gammaP]-ATP after 120 mins by filter binding method |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Rattus norvegicus (rat)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited
| Assay Description
The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... |
J Med Chem 36: 21-9 (1993)
Article DOI: 10.1021/jm00053a003
BindingDB Entry DOI: 10.7270/Q29P2ZTD |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Rattus norvegicus (rat)) | BDBM2712

(3-(1-methyl-1H-indol-3-yl)-4-{2-[(methylamino)meth...)Show SMILES CNCC1CCn2c(C1)c(C1=C(C(=O)NC1=O)c1cn(C)c3ccccc13)c1ccccc21 |t:11| Show InChI InChI=1S/C27H26N4O2/c1-28-14-16-11-12-31-21-10-6-4-8-18(21)23(22(31)13-16)25-24(26(32)29-27(25)33)19-15-30(2)20-9-5-3-7-17(19)20/h3-10,15-16,28H,11-14H2,1-2H3,(H,29,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited
| Assay Description
The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... |
J Med Chem 36: 21-9 (1993)
Article DOI: 10.1021/jm00053a003
BindingDB Entry DOI: 10.7270/Q29P2ZTD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50243254
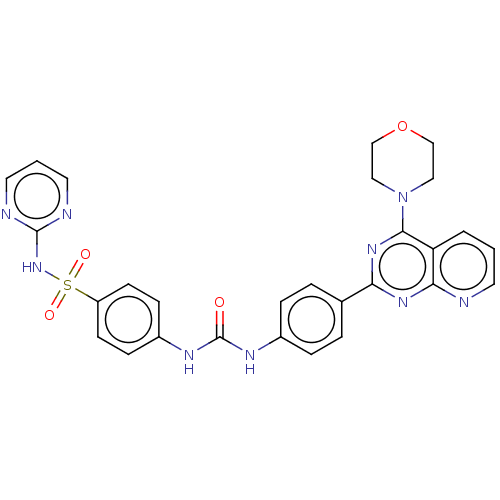
(CHEMBL4069475)Show SMILES O=C(Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cccnc2n1)Nc1ccc(cc1)S(=O)(=O)Nc1ncccn1 Show InChI InChI=1S/C28H25N9O4S/c38-28(33-21-8-10-22(11-9-21)42(39,40)36-27-30-13-2-14-31-27)32-20-6-4-19(5-7-20)24-34-25-23(3-1-12-29-25)26(35-24)37-15-17-41-18-16-37/h1-14H,15-18H2,(H,30,31,36)(H2,32,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human mTOR using 4EBP1 as substrate in presence of [33gammaP]-ATP after 120 mins by filter binding method |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Rattus norvegicus (rat)) | BDBM2715

(3-[(2R)-2-(aminomethyl)-1H,2H,3H,4H-pyrido[1,2-a]i...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2c3C[C@H](CN)CCn3c3ccccc23)c2ccccc12 |r,t:4| Show InChI InChI=1S/C26H24N4O2/c1-29-14-18(16-6-2-4-8-19(16)29)23-24(26(32)28-25(23)31)22-17-7-3-5-9-20(17)30-11-10-15(13-27)12-21(22)30/h2-9,14-15H,10-13,27H2,1H3,(H,28,31,32)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited
| Assay Description
The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... |
J Med Chem 36: 21-9 (1993)
Article DOI: 10.1021/jm00053a003
BindingDB Entry DOI: 10.7270/Q29P2ZTD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50561769
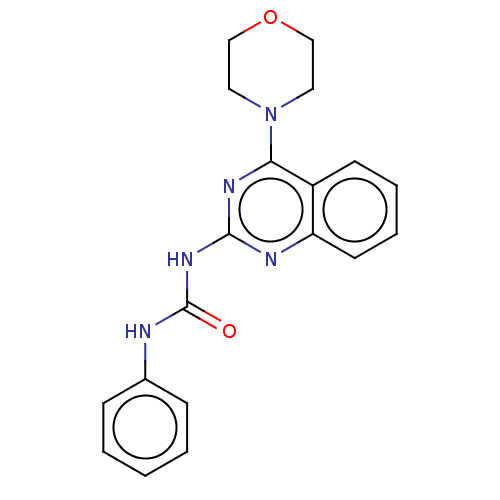
(CHEMBL4740323) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate incubated for 10 mins followed by ATP addition and measured after 30 mins in pres... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00289
BindingDB Entry DOI: 10.7270/Q2DF6VXJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449891

(CHEMBL4174756)Show SMILES COc1cc(cc(OC)c1OC)C1=N\C(=C/c2cc(Br)cc(Br)c2OC(C)=O)C(=O)NN1c1ccccc1 |t:13| Show InChI InChI=1S/C27H23Br2N3O6/c1-15(33)38-24-16(10-18(28)14-20(24)29)11-21-27(34)31-32(19-8-6-5-7-9-19)26(30-21)17-12-22(35-2)25(37-4)23(13-17)36-3/h5-14H,1-4H3,(H,31,34)/b21-11- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Rattus norvegicus (rat)) | BDBM2699
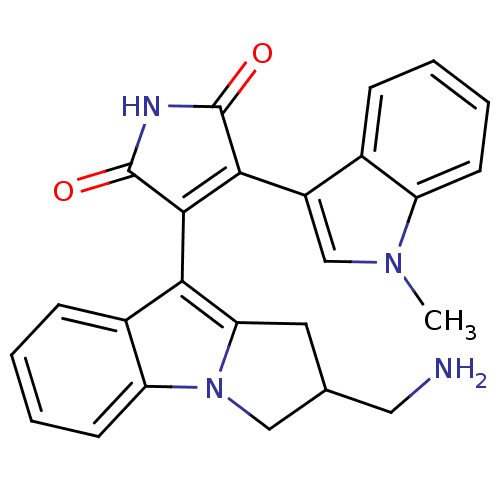
(3-[2-(aminomethyl)-1H,2H,3H-benzo[b]pyrrolizin-9-y...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2c3CC(CN)Cn3c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C25H22N4O2/c1-28-13-17(15-6-2-4-8-18(15)28)22-23(25(31)27-24(22)30)21-16-7-3-5-9-19(16)29-12-14(11-26)10-20(21)29/h2-9,13-14H,10-12,26H2,1H3,(H,27,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited
| Assay Description
The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... |
J Med Chem 36: 21-9 (1993)
Article DOI: 10.1021/jm00053a003
BindingDB Entry DOI: 10.7270/Q29P2ZTD |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Rattus norvegicus (rat)) | BDBM2702
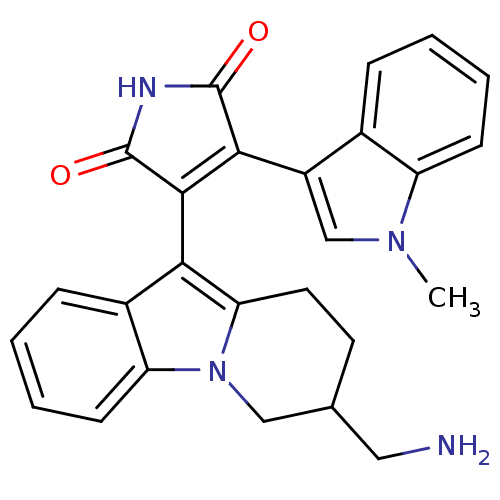
(3-[3-(aminomethyl)-1H,2H,3H,4H-pyrido[1,2-a]indol-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2c3CCC(CN)Cn3c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C26H24N4O2/c1-29-14-18(16-6-2-4-8-19(16)29)23-24(26(32)28-25(23)31)22-17-7-3-5-9-20(17)30-13-15(12-27)10-11-21(22)30/h2-9,14-15H,10-13,27H2,1H3,(H,28,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited
| Assay Description
The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... |
J Med Chem 36: 21-9 (1993)
Article DOI: 10.1021/jm00053a003
BindingDB Entry DOI: 10.7270/Q29P2ZTD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data