

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50562760 (CHEMBL4750447) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50562761 (CHEMBL4782021) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50562759 (CHEMBL4783605) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50562758 (CHEMBL4755938) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27213 (4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50562757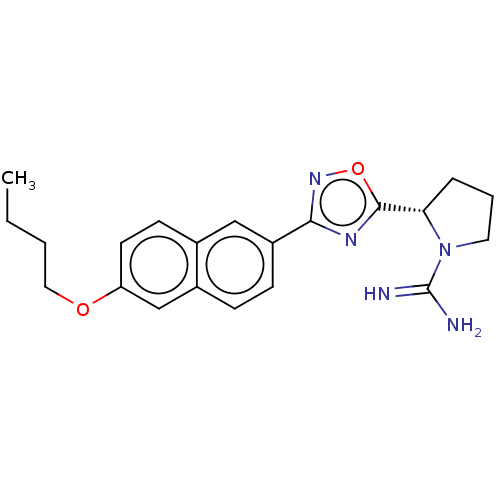 (CHEMBL3814580) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50177006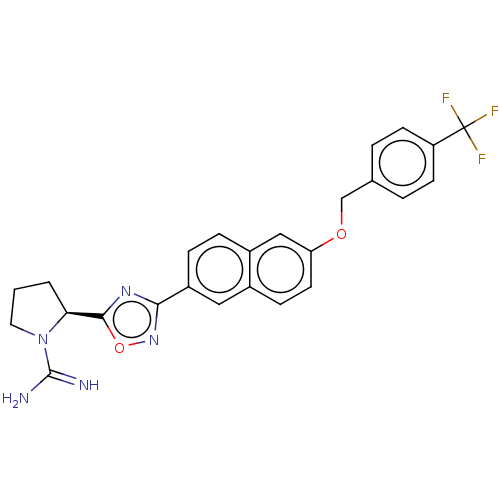 (CHEMBL3814849) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive-inhibition of recombinant human C-terminal His6-tagged Sphk1 expressed in baculovirus infected Sf21 insect cells using varying levels of ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146147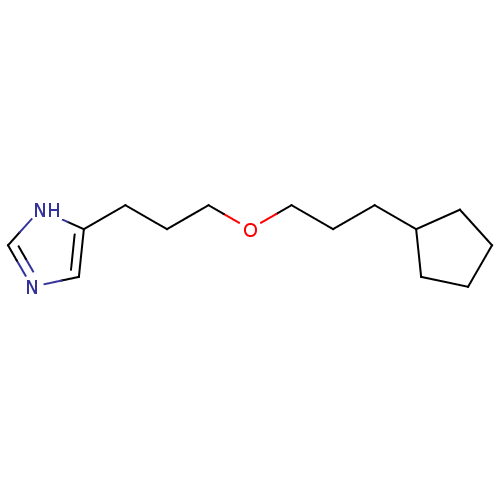 (4-[3-(3-Cyclopentyl-propoxy)-propyl]-1H-imidazole;...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50562757 (CHEMBL3814580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50562759 (CHEMBL4783605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50562760 (CHEMBL4750447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50562761 (CHEMBL4782021) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50562758 (CHEMBL4755938) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146145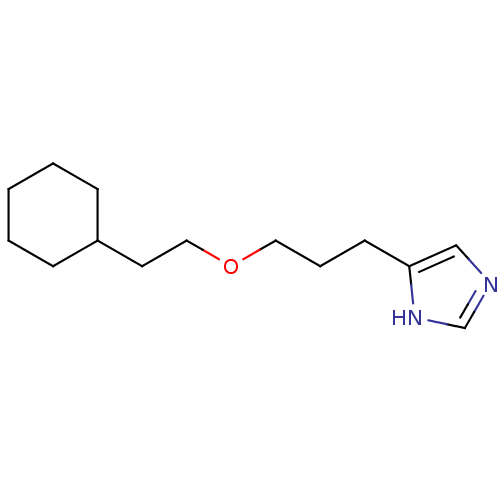 (4-[3-(2-Cyclohexyl-ethoxy)-propyl]-1H-imidazole; c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146126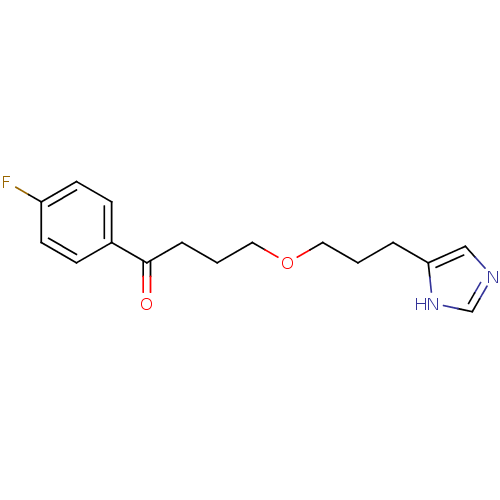 (1-(4-Fluoro-phenyl)-4-[3-(1H-imidazol-4-yl)-propox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146129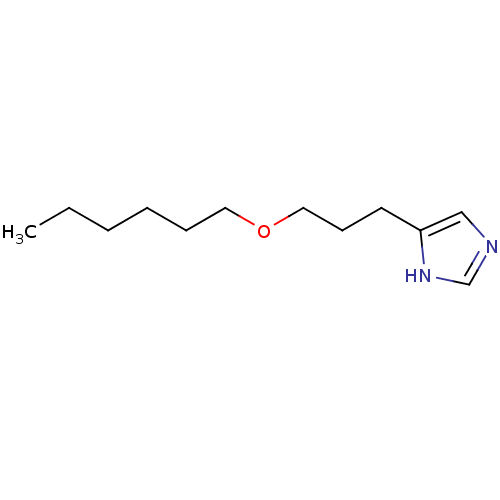 (4-(3-Hexyloxy-propyl)-1H-imidazole; compound with ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146146 (4-(3-Heptyloxy-propyl)-1H-imidazole; compound with...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146138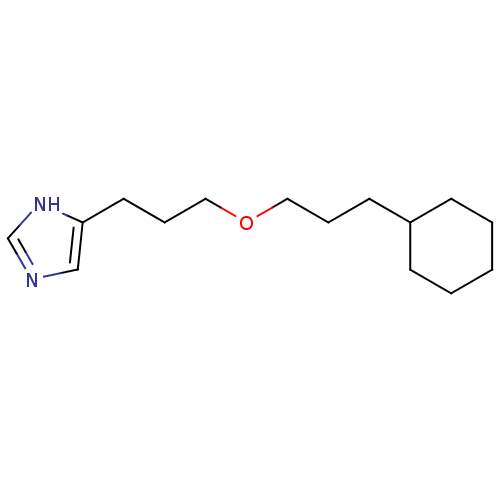 (4-[3-(3-Cyclohexyl-propoxy)-propyl]-1H-imidazole; ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50177006 (CHEMBL3814849) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146139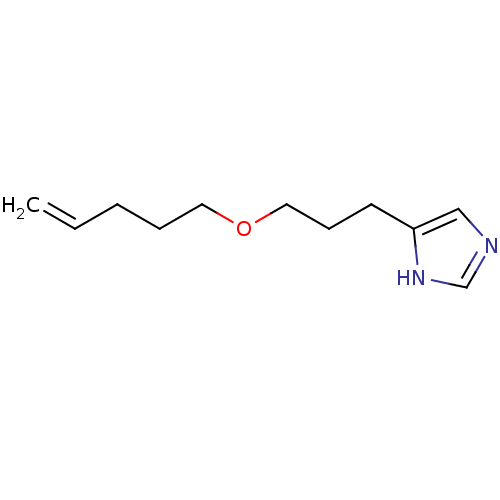 (4-(3-Pent-4-enyloxy-propyl)-1H-imidazole; oxalic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146135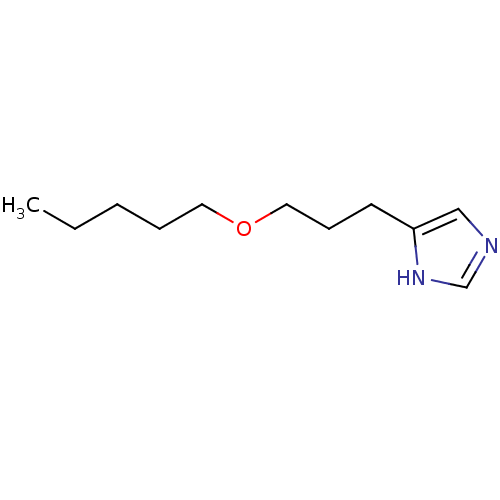 (4-(3-Pentyloxy-propyl)-1H-imidazole; compound with...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146136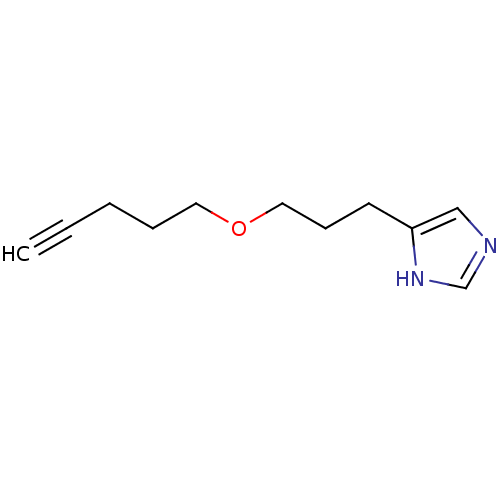 (4-(3-Pent-4-ynyloxy-propyl)-1H-imidazole; compound...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146141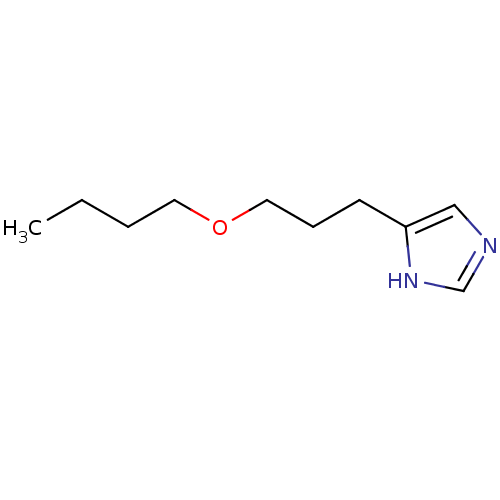 (4-(3-Butoxy-propyl)-1H-imidazole; compound with (Z...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146137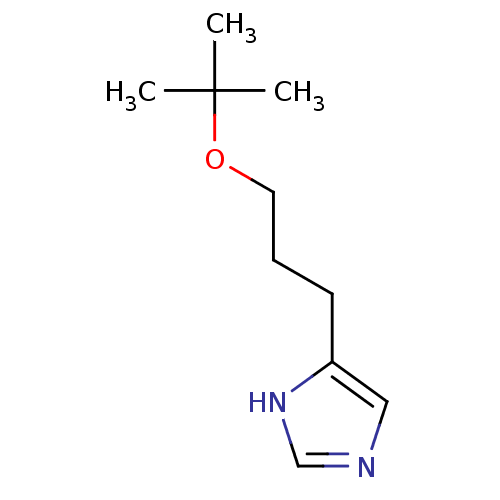 (4-(3-tert-Butoxy-propyl)-1H-imidazole; compound wi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146150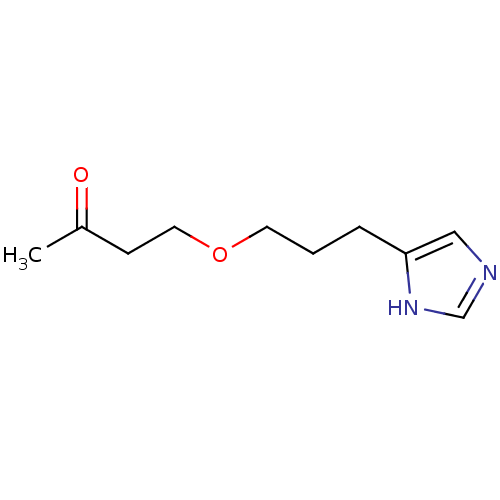 (4-[3-(1H-Imidazol-4-yl)-propoxy]-butan-2-one; comp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146142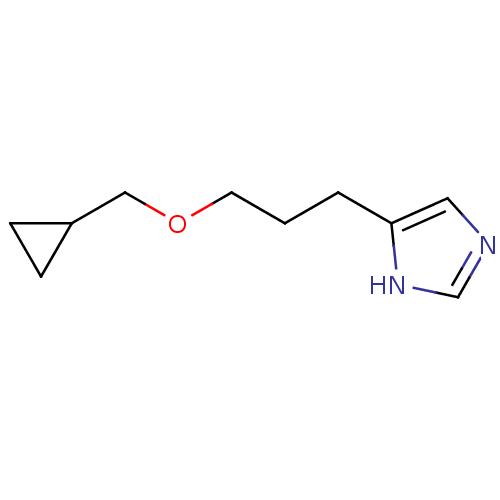 (4-(3-Cyclopropylmethoxy-propyl)-1H-imidazole; comp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146130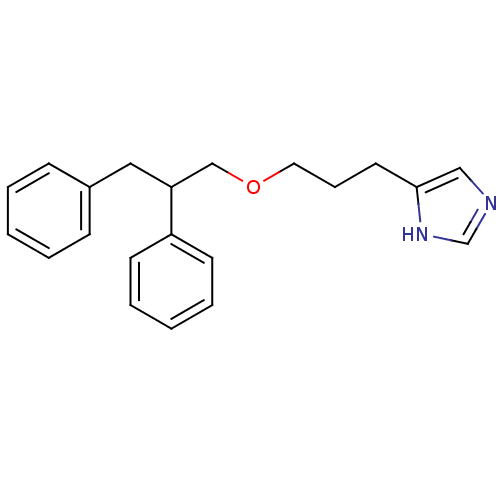 (4-[3-(2,3-Diphenyl-propoxy)-propyl]-1H-imidazole; ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146128 (4-(3-Prop-2-ynyloxy-propyl)-1H-imidazole; oxalic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146127 (4-(3-Allyloxy-propyl)-1H-imidazole; oxalic acid | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146149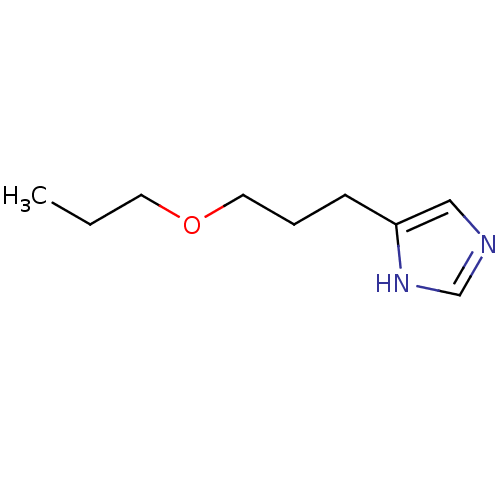 (4-(3-Propoxy-propyl)-1H-imidazole; oxalic acid | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146132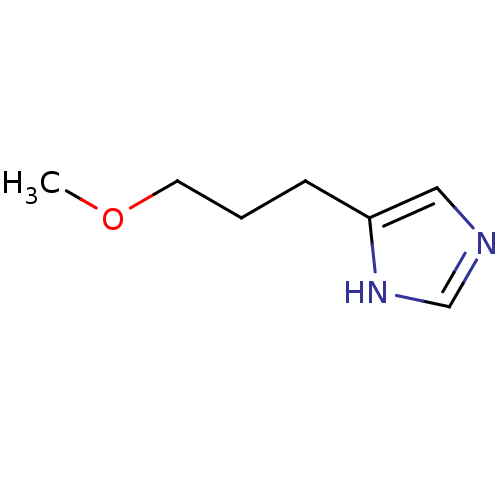 (4-(3-Methoxy-propyl)-1H-imidazole; oxalic acid | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146134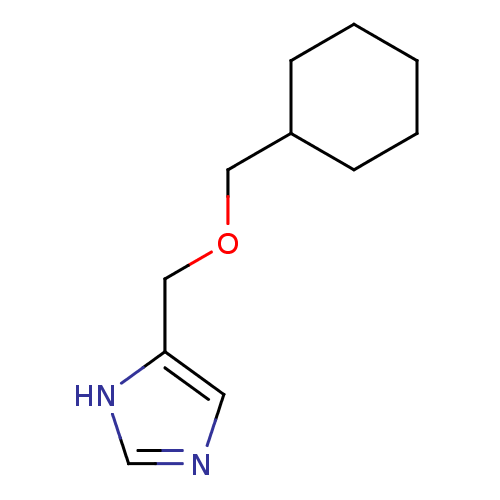 (4-Cyclohexylmethoxymethyl-1H-imidazole; compound w...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146140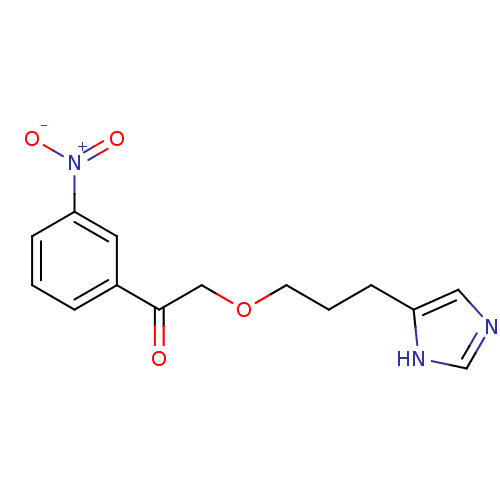 (2-[3-(1H-Imidazol-4-yl)-propoxy]-1-(3-nitro-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146143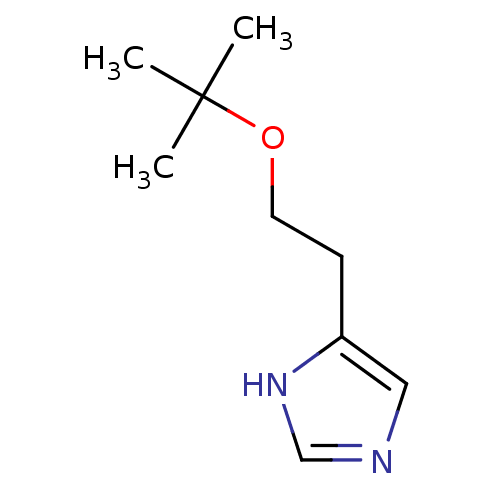 (4-(2-tert-Butoxy-ethyl)-1H-imidazole; oxalic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146144 (4-(3-Trityloxy-propyl)-1H-imidazole | CHEMBL91576) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146125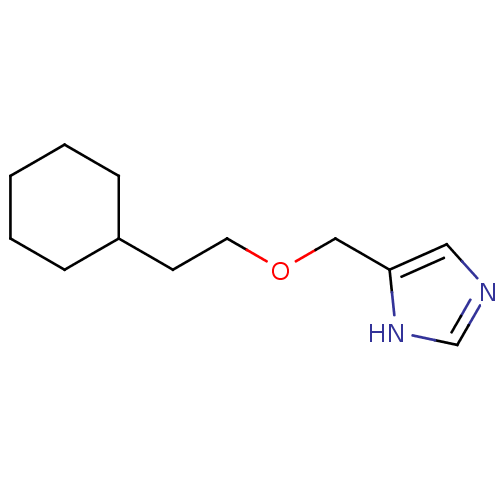 (4-(2-Cyclohexyl-ethoxymethyl)-1H-imidazole; compou...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146131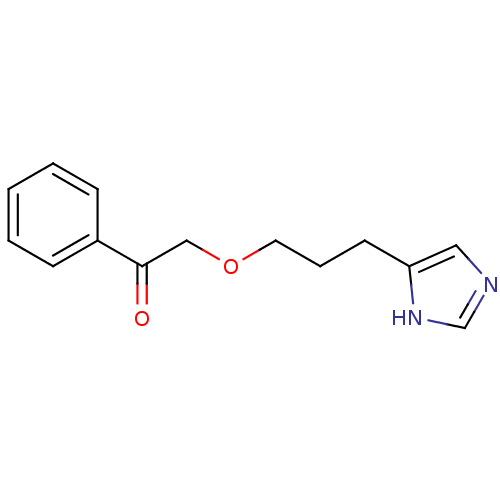 (2-[3-(1H-Imidazol-4-yl)-propoxy]-1-phenyl-ethanone...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146133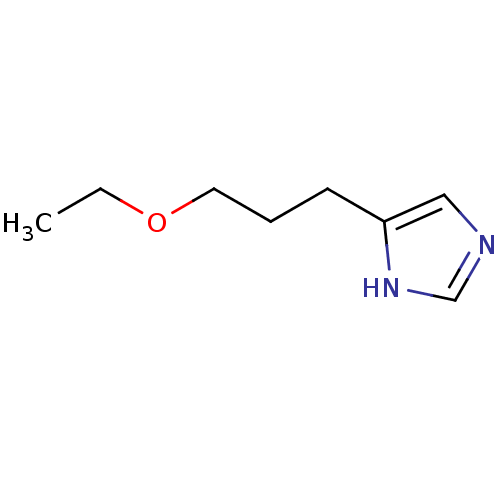 (4-(3-Ethoxy-propyl)-1H-imidazole; oxalic acid | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146148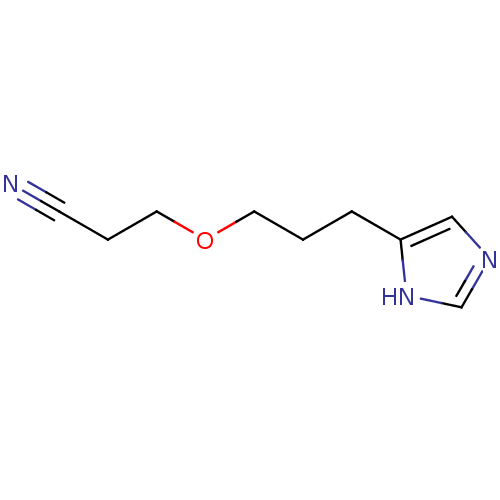 (3-[3-(1H-Imidazol-4-yl)-propoxy]-propionitrile; ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 674 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50393642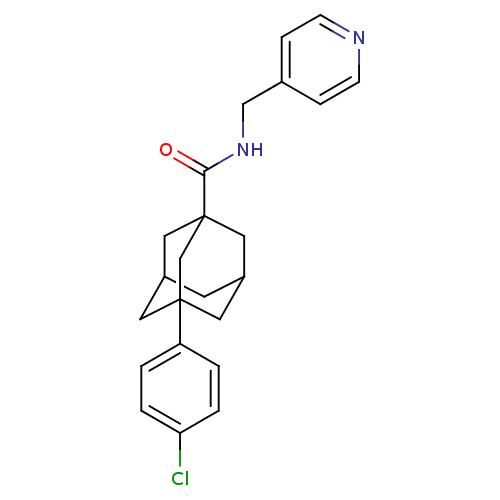 (CHEMBL2158685) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His-tagged Sphk2 (2 to 593 residues) expressed in Escherichia coli using varying level of sphingosine as s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein spinster homolog 1 (Human) | BDBM627389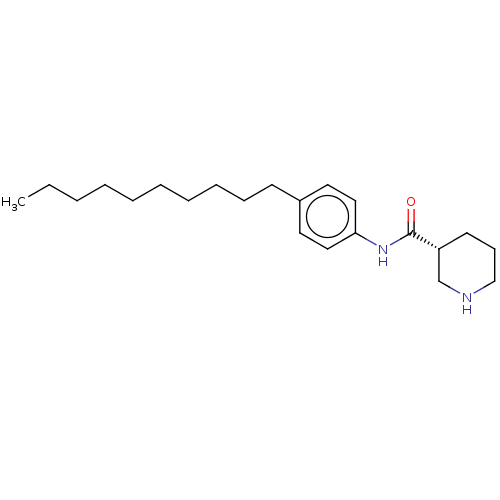 (US20230331683, Compound 2a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein spinster homolog 1 (Human) | BDBM627429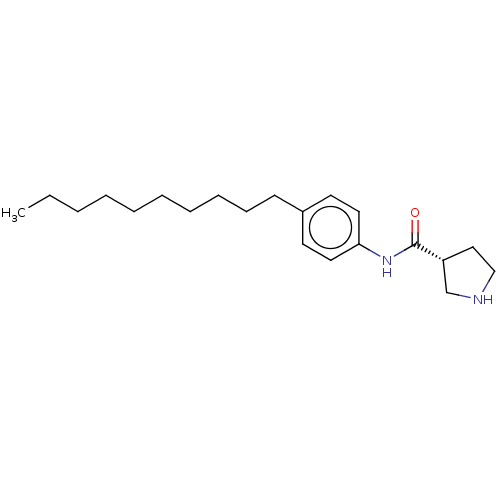 (US20230331683, Compound 2b) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein spinster homolog 1 (Human) | BDBM627430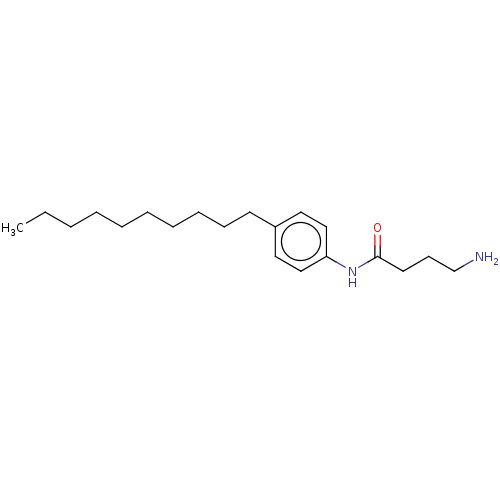 (US20230331683, Compound 2c) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein spinster homolog 1 (Human) | BDBM627431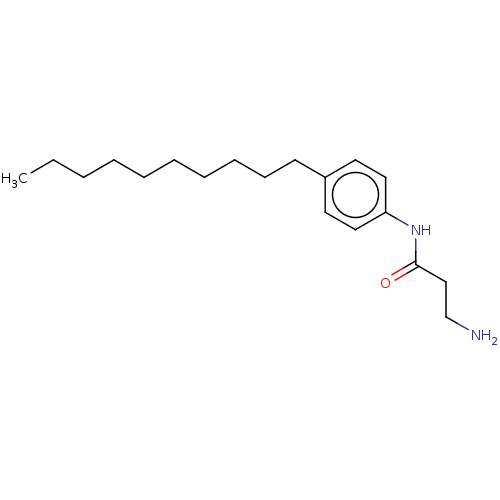 (US20230331683, Compound 2d) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein spinster homolog 1 (Human) | BDBM627432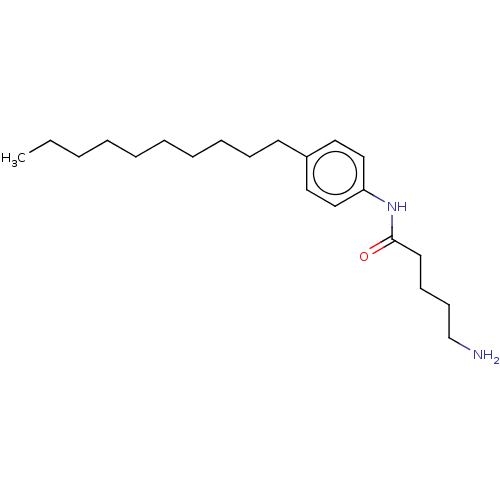 (US20230331683, Compound 2e) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein spinster homolog 1 (Human) | BDBM627433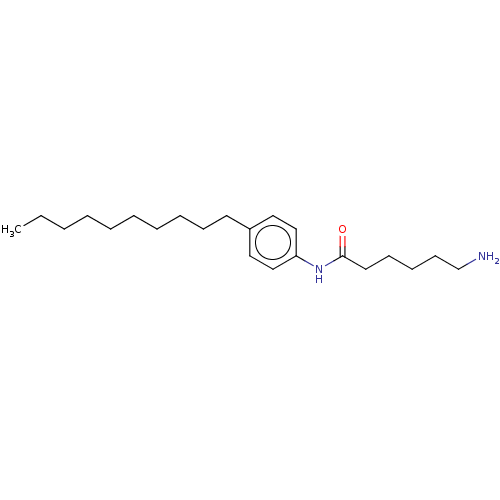 (US20230331683, Compound 2f) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein spinster homolog 1 (Human) | BDBM627434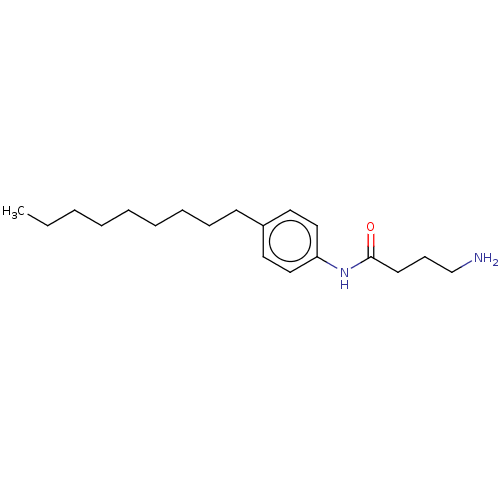 (US20230331683, Compound 2g) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein spinster homolog 1 (Human) | BDBM627435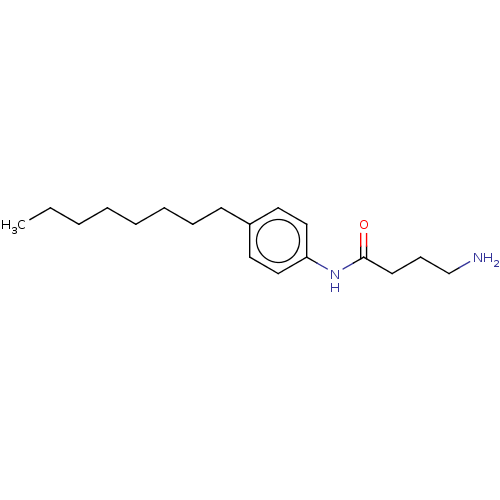 (US20230331683, Compound 2h) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 142 total ) | Next | Last >> |