

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50562760 (CHEMBL4750447) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50562761 (CHEMBL4782021) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50562759 (CHEMBL4783605) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50562758 (CHEMBL4755938) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50562757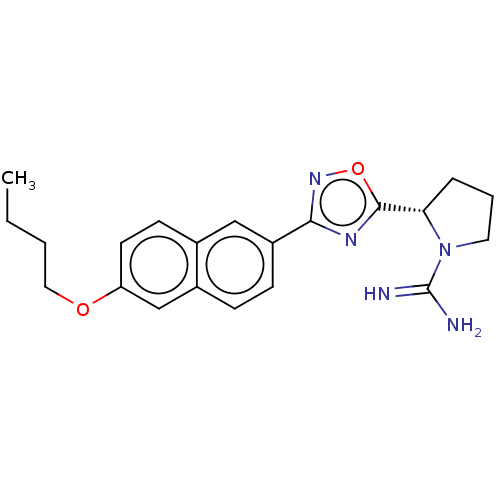 (CHEMBL3814580) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50177006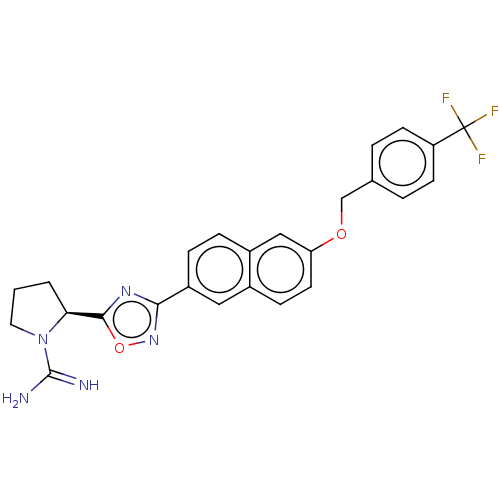 (CHEMBL3814849) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive-inhibition of recombinant human C-terminal His6-tagged Sphk1 expressed in baculovirus infected Sf21 insect cells using varying levels of ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50126591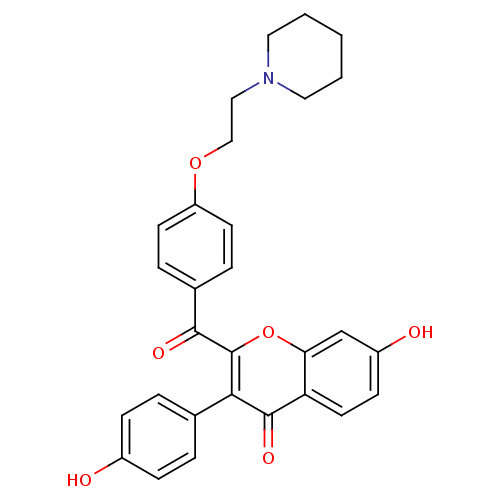 (7-Hydroxy-3-(4-hydroxy-phenyl)-2-[4-(2-piperidin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity for Estrogen receptor alpha | Bioorg Med Chem Lett 13: 1475-8 (2003) BindingDB Entry DOI: 10.7270/Q2KD1X9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50562757 (CHEMBL3814580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50562759 (CHEMBL4783605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50562760 (CHEMBL4750447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50562758 (CHEMBL4755938) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50285628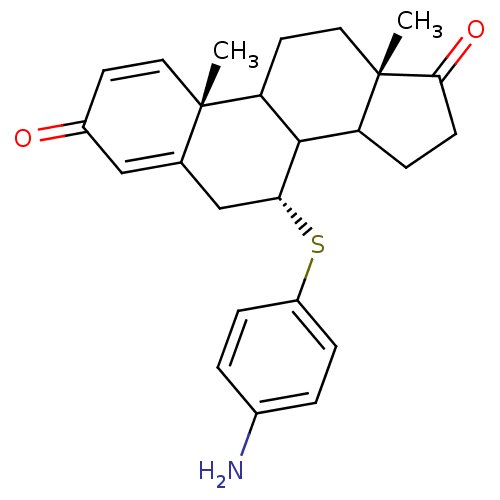 ((7R,10R,13S)-7-(4-Amino-phenylsulfanyl)-10,13-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of aromatase cytochrome P450 19A1 | Bioorg Med Chem Lett 5: 2513-2516 (1995) Article DOI: 10.1016/0960-894X(95)00440-5 BindingDB Entry DOI: 10.7270/Q28915TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50562761 (CHEMBL4782021) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50032234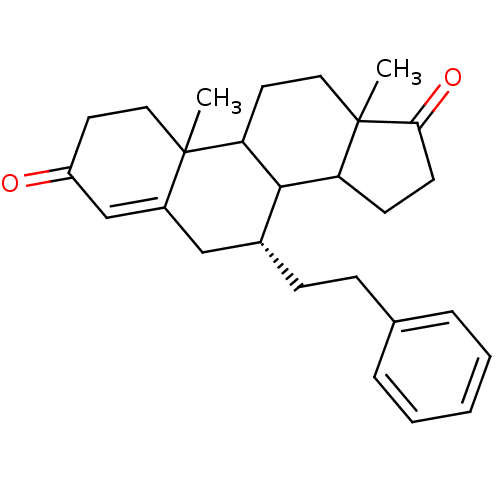 ((R)-10,13-Dimethyl-7-phenethyl-1,6,7,8,9,10,11,12,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Apparent binding affinity for human placental Cytochrome P450 19A1 | J Med Chem 38: 2842-50 (1995) BindingDB Entry DOI: 10.7270/Q29W0DH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50285627 ((7R,10R,13S)-7-Benzyl-10,13-dimethyl-1,6,7,8,9,10,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of aromatase cytochrome P450 19A1 | Bioorg Med Chem Lett 5: 2513-2516 (1995) Article DOI: 10.1016/0960-894X(95)00440-5 BindingDB Entry DOI: 10.7270/Q28915TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50285626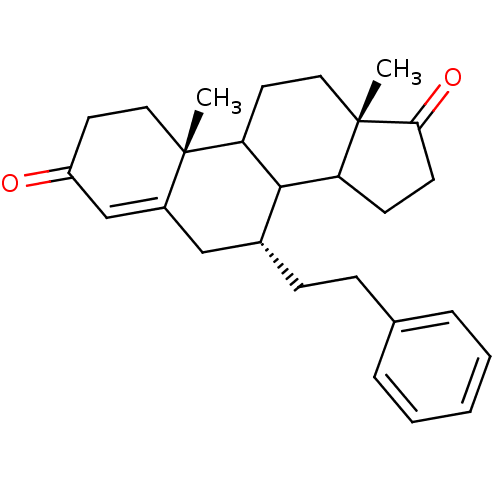 ((7R,10R,13S)-10,13-Dimethyl-7-phenethyl-1,6,7,8,9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of aromatase cytochrome P450 19A1 | Bioorg Med Chem Lett 5: 2513-2516 (1995) Article DOI: 10.1016/0960-894X(95)00440-5 BindingDB Entry DOI: 10.7270/Q28915TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50032231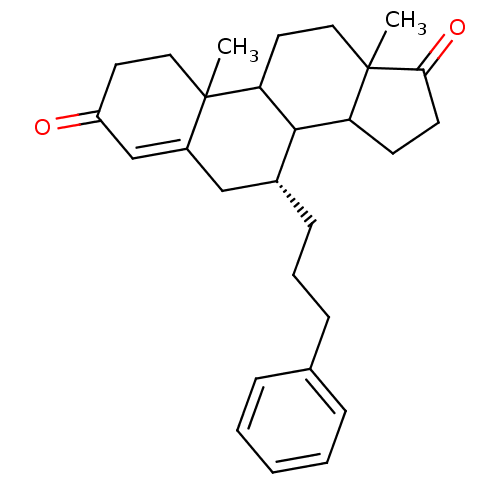 ((R)-10,13-Dimethyl-7-(3-phenyl-propyl)-1,6,7,8,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Apparent binding affinity for human placental Cytochrome P450 19A1 | J Med Chem 38: 2842-50 (1995) BindingDB Entry DOI: 10.7270/Q29W0DH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50285629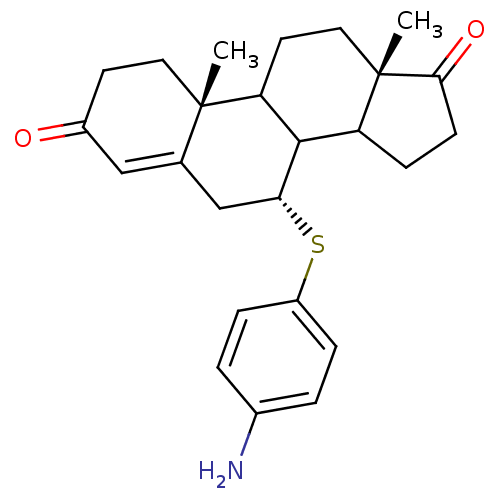 ((7R,10R,13S)-7-(4-Amino-phenylsulfanyl)-10,13-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of aromatase cytochrome P450 19A1 | Bioorg Med Chem Lett 5: 2513-2516 (1995) Article DOI: 10.1016/0960-894X(95)00440-5 BindingDB Entry DOI: 10.7270/Q28915TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50032228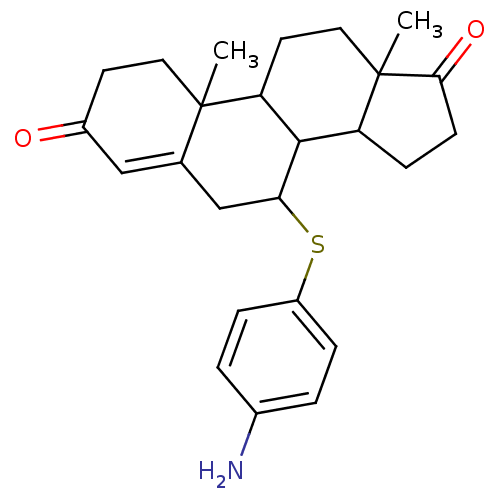 (7-(4-Amino-phenylsulfanyl)-10,13-dimethyl-1,6,7,8,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Apparent binding affinity for human placental Cytochrome P450 19A1 | J Med Chem 38: 2842-50 (1995) BindingDB Entry DOI: 10.7270/Q29W0DH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50010070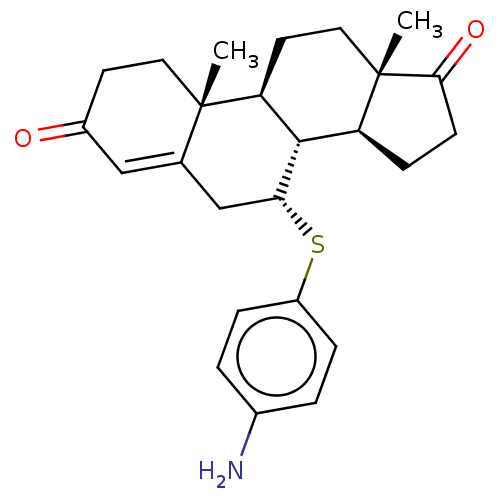 (CHEMBL3245357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene... | J Med Chem 21: 1007-11 (1979) BindingDB Entry DOI: 10.7270/Q2ZK5J6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50032232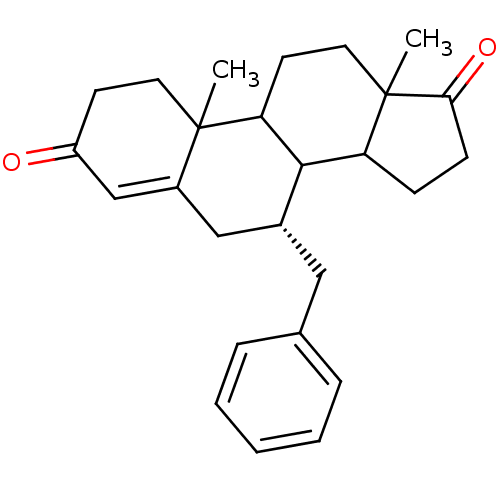 ((R)-7-Benzyl-10,13-dimethyl-1,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Apparent binding affinity for human placental Cytochrome P450 19A1 | J Med Chem 38: 2842-50 (1995) BindingDB Entry DOI: 10.7270/Q29W0DH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50177006 (CHEMBL3814849) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113121 BindingDB Entry DOI: 10.7270/Q2F193FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50010069 (CHEMBL3245356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene... | J Med Chem 21: 1007-11 (1979) BindingDB Entry DOI: 10.7270/Q2ZK5J6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50032229 ((7R,17S)-17-Hydroxy-10,13-dimethyl-7-phenethyl-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Apparent binding affinity for human placental Cytochrome P450 19A1 | J Med Chem 38: 2842-50 (1995) BindingDB Entry DOI: 10.7270/Q29W0DH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9911 (3-Phenyl-7-(4-biphenylmethoxy)-2-[(4-pyridylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | -44.0 | 79 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | Bioorg Med Chem 13: 6571-7 (2005) Article DOI: 10.1016/j.bmc.2005.07.038 BindingDB Entry DOI: 10.7270/Q2PK0DCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50032233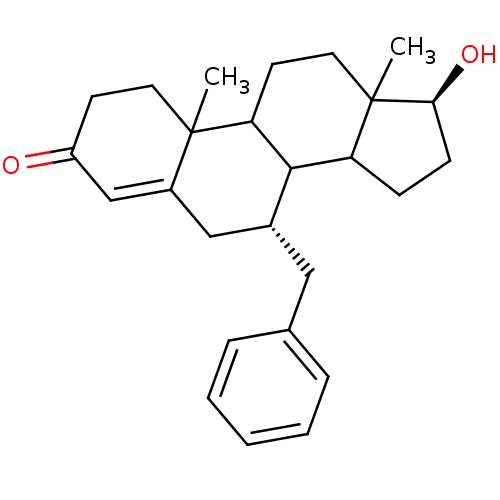 ((7R,17S)-7-Benzyl-17-hydroxy-10,13-dimethyl-1,2,6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Apparent binding affinity for human placental Cytochrome P450 19A1 | J Med Chem 38: 2842-50 (1995) BindingDB Entry DOI: 10.7270/Q29W0DH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50032227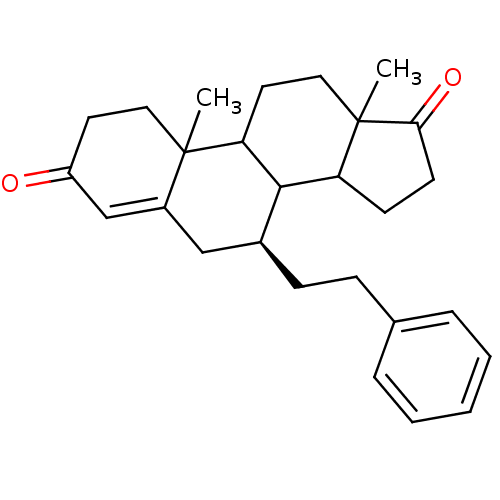 ((S)-10,13-Dimethyl-7-phenethyl-1,6,7,8,9,10,11,12,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Apparent binding affinity for human placental Cytochrome P450 19A1 | J Med Chem 38: 2842-50 (1995) BindingDB Entry DOI: 10.7270/Q29W0DH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50032230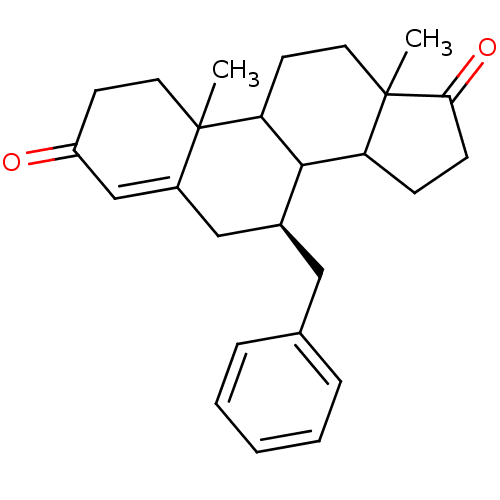 ((S)-7-Benzyl-10,13-dimethyl-1,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Apparent binding affinity for human placental Cytochrome P450 19A1 | J Med Chem 38: 2842-50 (1995) BindingDB Entry DOI: 10.7270/Q29W0DH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50010067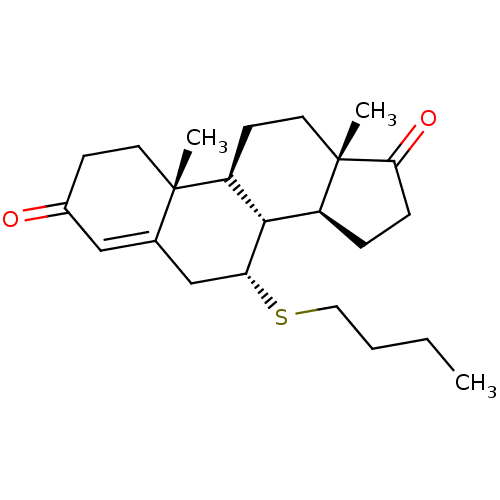 (CHEMBL3245348) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene... | J Med Chem 21: 1007-11 (1979) BindingDB Entry DOI: 10.7270/Q2ZK5J6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014562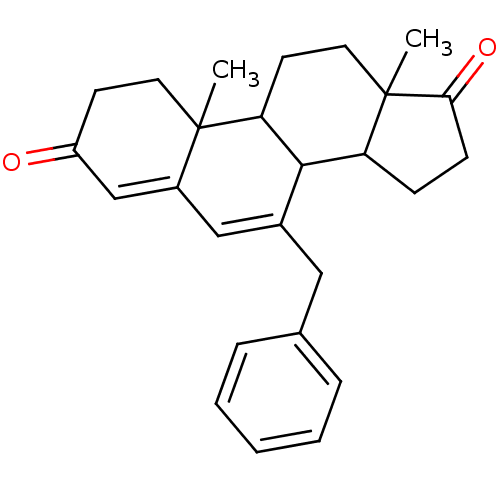 (7-Benzyl-10,13-dimethyl-1,8,9,10,11,12,13,14,15,16...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description In vitro inhibition of human placental cytochrome P450 19A1 | J Med Chem 33: 101-5 (1990) BindingDB Entry DOI: 10.7270/Q2KP8141 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50010068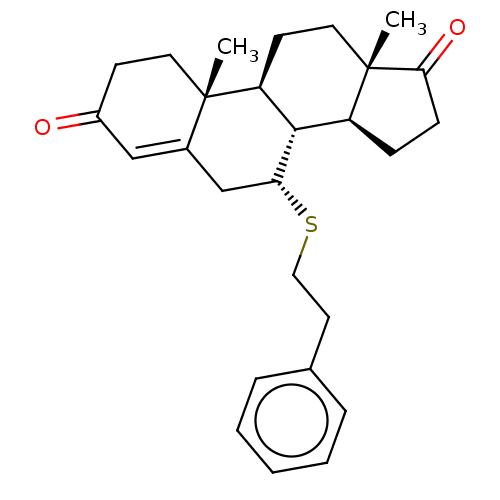 (CHEMBL3245353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene... | J Med Chem 21: 1007-11 (1979) BindingDB Entry DOI: 10.7270/Q2ZK5J6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50010071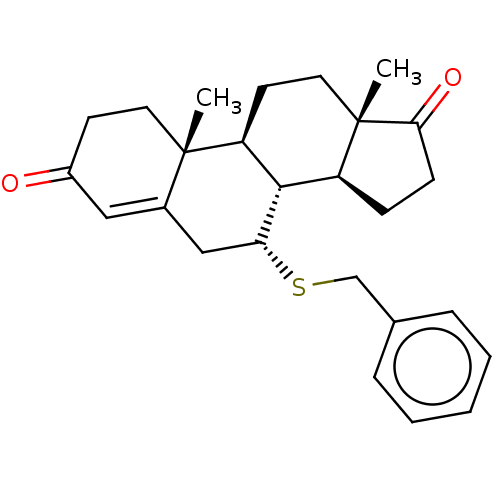 (CHEMBL3245350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition of human placental microsomal aromatase assessed as [C14]estrone/[C14]estradiol formation using 0.075 to 0.515 uM [C14]androstene... | J Med Chem 21: 1007-11 (1979) BindingDB Entry DOI: 10.7270/Q2ZK5J6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9903 (3-Phenyl-7-(beta-naphthylmethoxy)-2-[(4-pyridylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 74 | -42.3 | 90 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | Bioorg Med Chem 13: 6571-7 (2005) Article DOI: 10.1016/j.bmc.2005.07.038 BindingDB Entry DOI: 10.7270/Q2PK0DCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014565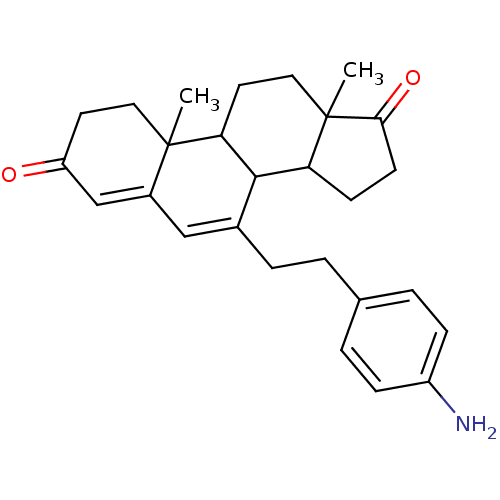 (7-[2-(4-Amino-phenyl)-ethyl]-10,13-dimethyl-1,8,9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description In vitro inhibition of human placental cytochrome P450 19A1 | J Med Chem 33: 101-5 (1990) BindingDB Entry DOI: 10.7270/Q2KP8141 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014559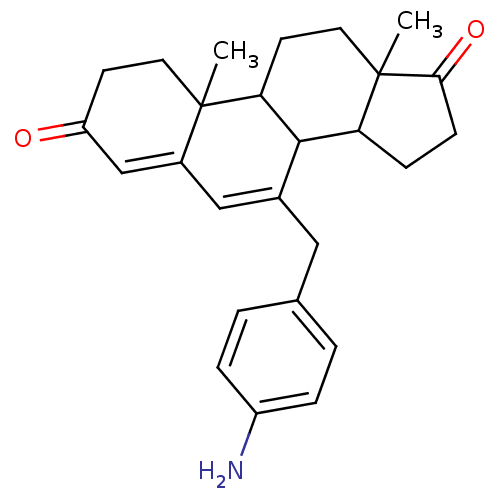 (7-(4-Amino-benzyl)-10,13-dimethyl-1,8,9,10,11,12,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description In vitro inhibition of human placental cytochrome P450 19A1 | J Med Chem 33: 101-5 (1990) BindingDB Entry DOI: 10.7270/Q2KP8141 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014560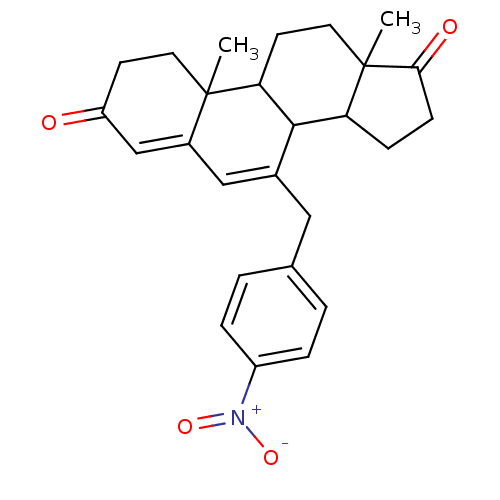 (10,13-Dimethyl-7-(4-nitro-benzyl)-1,8,9,10,11,12,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description In vitro inhibition of human placental cytochrome P450 19A1 | J Med Chem 33: 101-5 (1990) BindingDB Entry DOI: 10.7270/Q2KP8141 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014563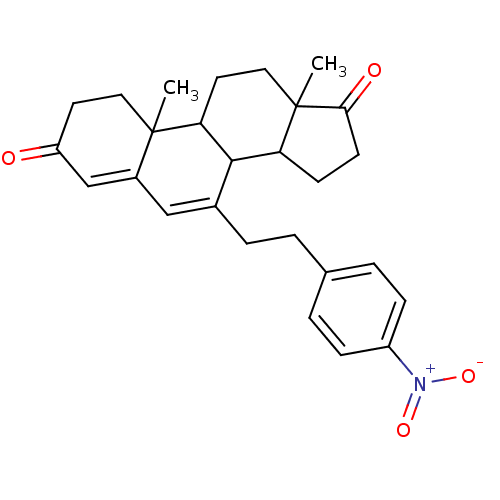 (10,13-Dimethyl-7-[2-(4-nitro-phenyl)-ethyl]-1,8,9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description In vitro inhibition of human placental cytochrome P450 19A1 | J Med Chem 33: 101-5 (1990) BindingDB Entry DOI: 10.7270/Q2KP8141 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9902 (3-Phenyl-7-(alpha-naphthylmethoxy)-2-[(4-pyridylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 114 | -41.2 | 112 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | Bioorg Med Chem 13: 6571-7 (2005) Article DOI: 10.1016/j.bmc.2005.07.038 BindingDB Entry DOI: 10.7270/Q2PK0DCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014561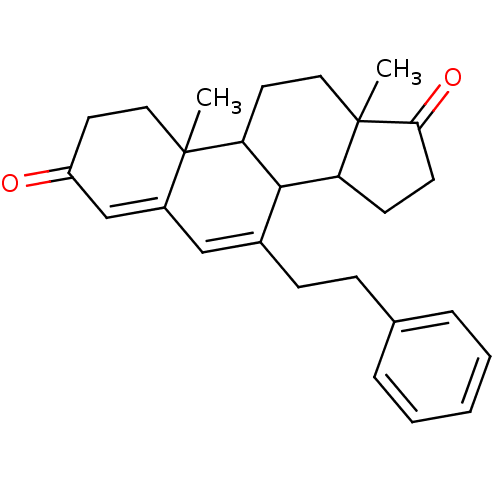 (10,13-Dimethyl-7-phenethyl-1,8,9,10,11,12,13,14,15...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description In vitro inhibition of human placental cytochrome P450 19A1 | J Med Chem 33: 101-5 (1990) BindingDB Entry DOI: 10.7270/Q2KP8141 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9454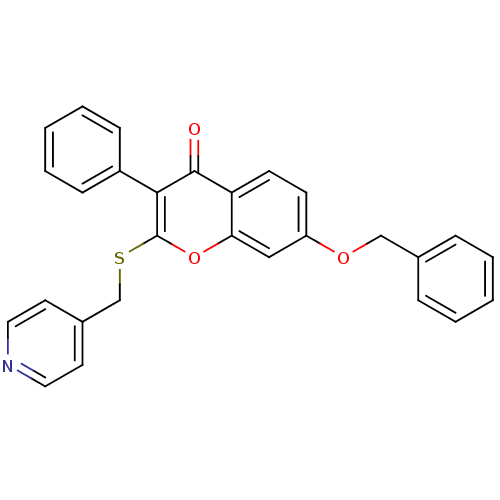 (7-(benzyloxy)-3-phenyl-2-[(pyridin-4-ylmethyl)sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | -39.5 | 210 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | J Med Chem 47: 4032-40 (2004) Article DOI: 10.1021/jm0306024 BindingDB Entry DOI: 10.7270/Q2RJ4GP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9893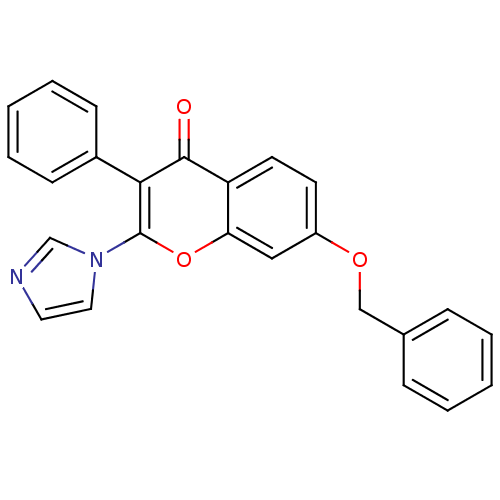 (2-(1H-Imidazol-1-yl)-3-phenyl-7-(benzyloxy)-4H-1-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | -39.2 | 520 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | Bioorg Med Chem 13: 4063-70 (2005) Article DOI: 10.1016/j.bmc.2005.03.050 BindingDB Entry DOI: 10.7270/Q2TB154R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9459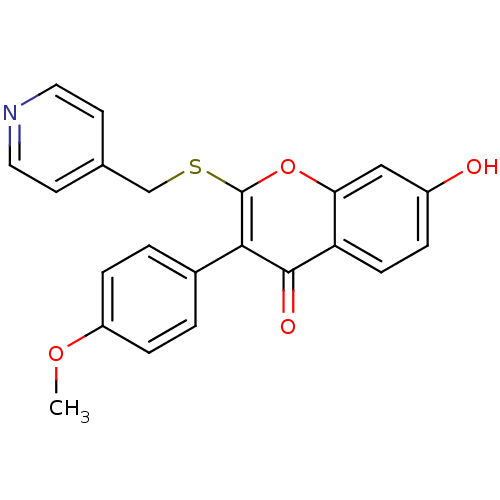 (7-Hydroxy-3-(4-methoxyphenyl)-2-[(4-pyridylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 260 | -39.1 | 220 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | J Med Chem 47: 4032-40 (2004) Article DOI: 10.1021/jm0306024 BindingDB Entry DOI: 10.7270/Q2RJ4GP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9458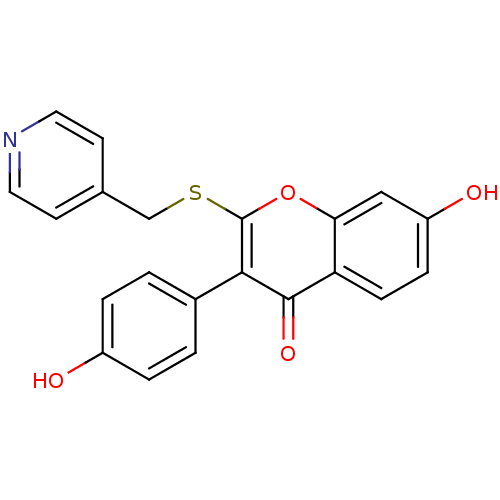 (7-Hydroxy-3-(4-hydroxyphenyl)-2-[(4-pyridylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | -38.6 | 280 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | J Med Chem 47: 4032-40 (2004) Article DOI: 10.1021/jm0306024 BindingDB Entry DOI: 10.7270/Q2RJ4GP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9891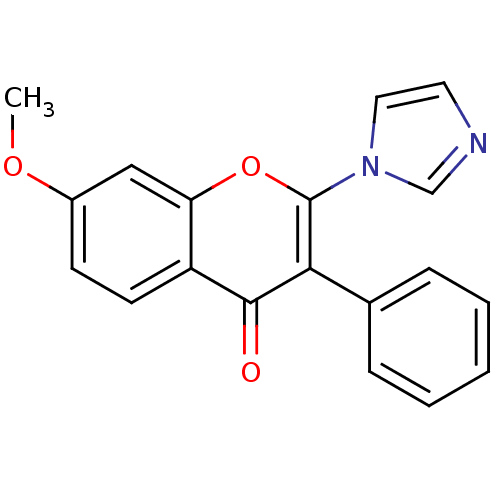 (2-(1H-Imidazol-1-yl)-7-methoxy-3-phenyl-4H-1-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 680 | -36.6 | 770 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | Bioorg Med Chem 13: 4063-70 (2005) Article DOI: 10.1016/j.bmc.2005.03.050 BindingDB Entry DOI: 10.7270/Q2TB154R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9449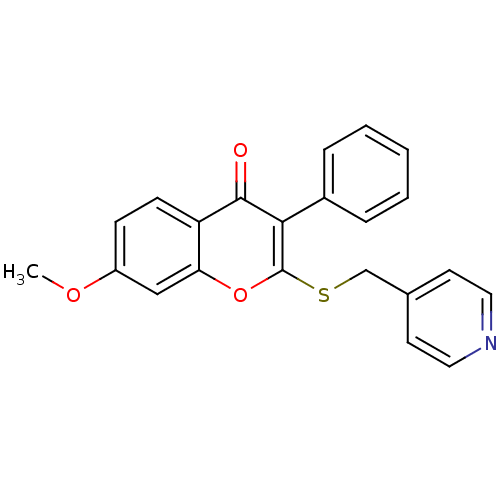 (7-Methoxy-3-phenyl-2-[(4-pyridylmethyl)thio]-4H-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | -35.9 | 1.60E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | J Med Chem 47: 4032-40 (2004) Article DOI: 10.1021/jm0306024 BindingDB Entry DOI: 10.7270/Q2RJ4GP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9452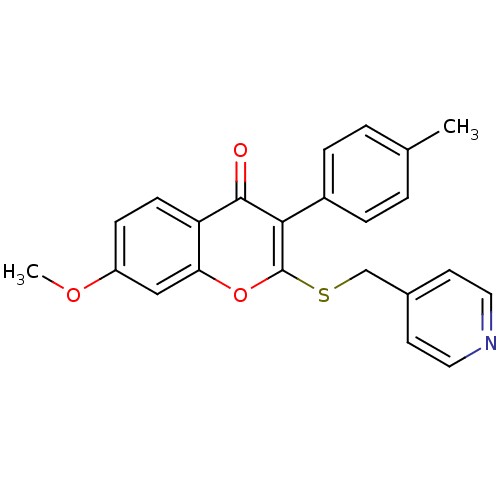 (7-Methoxy-3-(4-methylphenyl)-2-[(4-pyridylmethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | -35.2 | 3.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | J Med Chem 47: 4032-40 (2004) Article DOI: 10.1021/jm0306024 BindingDB Entry DOI: 10.7270/Q2RJ4GP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.41E+3 | -34.7 | 2.80E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | Bioorg Med Chem 13: 4063-70 (2005) Article DOI: 10.1016/j.bmc.2005.03.050 BindingDB Entry DOI: 10.7270/Q2TB154R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.41E+3 | -34.7 | 2.80E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | Bioorg Med Chem 13: 6571-7 (2005) Article DOI: 10.1016/j.bmc.2005.07.038 BindingDB Entry DOI: 10.7270/Q2PK0DCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.41E+3 | -34.7 | 2.80E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Ohio State University | Assay Description Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-... | J Med Chem 47: 4032-40 (2004) Article DOI: 10.1021/jm0306024 BindingDB Entry DOI: 10.7270/Q2RJ4GP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 836 total ) | Next | Last >> |