 Found 96 hits with Last Name = 'mellet' and Initial = 'm'
Found 96 hits with Last Name = 'mellet' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50421761
(CHEMBL2311113)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C42H63N5O10/c1-25(2)19-31(34(48)22-36(50)43-28(7)39(52)44-32(20-26(3)4)35(49)23-37(51)56-8)45-41(54)38(27(5)6)47-40(53)33(21-29-15-11-9-12-16-29)46-42(55)57-24-30-17-13-10-14-18-30/h9-18,25-28,31-35,38,48-49H,19-24H2,1-8H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53)/t28-,31-,32-,33-,34?,35?,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against monkey plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024163

(4-[2-(4-{2-[2-Benzyloxycarbonylamino-3-(1H-indol-3...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C44H64N6O10/c1-25(2)18-33(36(51)21-38(53)46-28(7)41(55)47-34(19-26(3)4)37(52)22-39(54)59-8)48-43(57)40(27(5)6)50-42(56)35(20-30-23-45-32-17-13-12-16-31(30)32)49-44(58)60-24-29-14-10-9-11-15-29/h9-17,23,25-28,33-37,40,45,51-52H,18-22,24H2,1-8H3,(H,46,53)(H,47,55)(H,48,57)(H,49,58)(H,50,56) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against man (human) plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024167
(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O9/c1-9-10-16-28(42-39(53)31(41-34(47)19-25(6)7)20-27-14-12-11-13-15-27)38(52)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(51)43-30(18-24(4)5)33(46)22-36(49)50/h11-15,23-26,28-33,45-46H,9-10,16-22H2,1-8H3,(H,40,48)(H,41,47)(H,42,53)(H,43,51)(H,44,52)(H,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024163

(4-[2-(4-{2-[2-Benzyloxycarbonylamino-3-(1H-indol-3...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C44H64N6O10/c1-25(2)18-33(36(51)21-38(53)46-28(7)41(55)47-34(19-26(3)4)37(52)22-39(54)59-8)48-43(57)40(27(5)6)50-42(56)35(20-30-23-45-32-17-13-12-16-31(30)32)49-44(58)60-24-29-14-10-9-11-15-29/h9-17,23,25-28,33-37,40,45,51-52H,18-22,24H2,1-8H3,(H,46,53)(H,47,55)(H,48,57)(H,49,58)(H,50,56) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of dog plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024163

(4-[2-(4-{2-[2-Benzyloxycarbonylamino-3-(1H-indol-3...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C44H64N6O10/c1-25(2)18-33(36(51)21-38(53)46-28(7)41(55)47-34(19-26(3)4)37(52)22-39(54)59-8)48-43(57)40(27(5)6)50-42(56)35(20-30-23-45-32-17-13-12-16-31(30)32)49-44(58)60-24-29-14-10-9-11-15-29/h9-17,23,25-28,33-37,40,45,51-52H,18-22,24H2,1-8H3,(H,46,53)(H,47,55)(H,48,57)(H,49,58)(H,50,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against man (human) plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025903
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C40H63N7O10/c1-23(2)15-28(32(48)19-34(50)43-25(5)36(52)44-29(16-24(3)4)33(49)20-35(51)56-9)45-38(54)31(18-27-21-41-22-42-27)46-37(53)30(17-26-13-11-10-12-14-26)47-39(55)57-40(6,7)8/h10-14,21-25,28-33,48-49H,15-20H2,1-9H3,(H,41,42)(H,43,50)(H,44,52)(H,45,54)(H,46,53)(H,47,55)/t25-,28-,29-,30-,31-,32?,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of man plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024167

(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O9/c1-9-10-16-28(42-39(53)31(41-34(47)19-25(6)7)20-27-14-12-11-13-15-27)38(52)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(51)43-30(18-24(4)5)33(46)22-36(49)50/h11-15,23-26,28-33,45-46H,9-10,16-22H2,1-8H3,(H,40,48)(H,41,47)(H,42,53)(H,43,51)(H,44,52)(H,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against man (human) plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024165
(4-[2-(4-{1-[1-Benzyl-2-(3-methyl-butyrylamino)-2-o...)Show SMILES CCC[C@H](N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC(=O)CC(C)C |r| Show InChI InChI=1S/C39H65N5O9/c1-10-14-28(38(51)43-31(20-27-15-12-11-13-16-27)39(52)44-34(47)19-25(6)7)41-29(17-23(2)3)32(45)21-35(48)40-26(8)37(50)42-30(18-24(4)5)33(46)22-36(49)53-9/h11-13,15-16,23-26,28-33,41,45-46H,10,14,17-22H2,1-9H3,(H,40,48)(H,42,50)(H,43,51)(H,44,47,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against monkey (Callithrix jacchus) plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50421761

(CHEMBL2311113)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C42H63N5O10/c1-25(2)19-31(34(48)22-36(50)43-28(7)39(52)44-32(20-26(3)4)35(49)23-37(51)56-8)45-41(54)38(27(5)6)47-40(53)33(21-29-15-11-9-12-16-29)46-42(55)57-24-30-17-13-10-14-18-30/h9-18,25-28,31-35,38,48-49H,19-24H2,1-8H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53)/t28-,31-,32-,33-,34?,35?,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against hog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024167

(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O9/c1-9-10-16-28(42-39(53)31(41-34(47)19-25(6)7)20-27-14-12-11-13-15-27)38(52)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(51)43-30(18-24(4)5)33(46)22-36(49)50/h11-15,23-26,28-33,45-46H,9-10,16-22H2,1-8H3,(H,40,48)(H,41,47)(H,42,53)(H,43,51)(H,44,52)(H,49,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against hog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50421761

(CHEMBL2311113)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C42H63N5O10/c1-25(2)19-31(34(48)22-36(50)43-28(7)39(52)44-32(20-26(3)4)35(49)23-37(51)56-8)45-41(54)38(27(5)6)47-40(53)33(21-29-15-11-9-12-16-29)46-42(55)57-24-30-17-13-10-14-18-30/h9-18,25-28,31-35,38,48-49H,19-24H2,1-8H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53)/t28-,31-,32-,33-,34?,35?,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024163

(4-[2-(4-{2-[2-Benzyloxycarbonylamino-3-(1H-indol-3...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C44H64N6O10/c1-25(2)18-33(36(51)21-38(53)46-28(7)41(55)47-34(19-26(3)4)37(52)22-39(54)59-8)48-43(57)40(27(5)6)50-42(56)35(20-30-23-45-32-17-13-12-16-31(30)32)49-44(58)60-24-29-14-10-9-11-15-29/h9-17,23,25-28,33-37,40,45,51-52H,18-22,24H2,1-8H3,(H,46,53)(H,47,55)(H,48,57)(H,49,58)(H,50,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against dog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024165

(4-[2-(4-{1-[1-Benzyl-2-(3-methyl-butyrylamino)-2-o...)Show SMILES CCC[C@H](N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC(=O)CC(C)C |r| Show InChI InChI=1S/C39H65N5O9/c1-10-14-28(38(51)43-31(20-27-15-12-11-13-16-27)39(52)44-34(47)19-25(6)7)41-29(17-23(2)3)32(45)21-35(48)40-26(8)37(50)42-30(18-24(4)5)33(46)22-36(49)53-9/h11-13,15-16,23-26,28-33,41,45-46H,10,14,17-22H2,1-9H3,(H,40,48)(H,42,50)(H,43,51)(H,44,47,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against man (human) plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024165

(4-[2-(4-{1-[1-Benzyl-2-(3-methyl-butyrylamino)-2-o...)Show SMILES CCC[C@H](N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC(=O)CC(C)C |r| Show InChI InChI=1S/C39H65N5O9/c1-10-14-28(38(51)43-31(20-27-15-12-11-13-16-27)39(52)44-34(47)19-25(6)7)41-29(17-23(2)3)32(45)21-35(48)40-26(8)37(50)42-30(18-24(4)5)33(46)22-36(49)53-9/h11-13,15-16,23-26,28-33,41,45-46H,10,14,17-22H2,1-9H3,(H,40,48)(H,42,50)(H,43,51)(H,44,47,52) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against hog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024167

(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O9/c1-9-10-16-28(42-39(53)31(41-34(47)19-25(6)7)20-27-14-12-11-13-15-27)38(52)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(51)43-30(18-24(4)5)33(46)22-36(49)50/h11-15,23-26,28-33,45-46H,9-10,16-22H2,1-8H3,(H,40,48)(H,41,47)(H,42,53)(H,43,51)(H,44,52)(H,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against dog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024174
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C43H65N5O10/c1-26(2)20-31(35(49)24-37(51)44-28(5)39(53)45-32(21-27(3)4)36(50)25-38(52)57-9)46-40(54)33(22-29-16-12-10-13-17-29)47-41(55)34(23-30-18-14-11-15-19-30)48-42(56)58-43(6,7)8/h10-19,26-28,31-36,49-50H,20-25H2,1-9H3,(H,44,51)(H,45,53)(H,46,54)(H,47,55)(H,48,56) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against hog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024167

(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O9/c1-9-10-16-28(42-39(53)31(41-34(47)19-25(6)7)20-27-14-12-11-13-15-27)38(52)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(51)43-30(18-24(4)5)33(46)22-36(49)50/h11-15,23-26,28-33,45-46H,9-10,16-22H2,1-8H3,(H,40,48)(H,41,47)(H,42,53)(H,43,51)(H,44,52)(H,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against monkey plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025903

(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C40H63N7O10/c1-23(2)15-28(32(48)19-34(50)43-25(5)36(52)44-29(16-24(3)4)33(49)20-35(51)56-9)45-38(54)31(18-27-21-41-22-42-27)46-37(53)30(17-26-13-11-10-12-14-26)47-39(55)57-40(6,7)8/h10-14,21-25,28-33,48-49H,15-20H2,1-9H3,(H,41,42)(H,43,50)(H,44,52)(H,45,54)(H,46,53)(H,47,55)/t25-,28-,29-,30-,31-,32?,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of man plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024165

(4-[2-(4-{1-[1-Benzyl-2-(3-methyl-butyrylamino)-2-o...)Show SMILES CCC[C@H](N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC(=O)CC(C)C |r| Show InChI InChI=1S/C39H65N5O9/c1-10-14-28(38(51)43-31(20-27-15-12-11-13-16-27)39(52)44-34(47)19-25(6)7)41-29(17-23(2)3)32(45)21-35(48)40-26(8)37(50)42-30(18-24(4)5)33(46)22-36(49)53-9/h11-13,15-16,23-26,28-33,41,45-46H,10,14,17-22H2,1-9H3,(H,40,48)(H,42,50)(H,43,51)(H,44,47,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against monkey plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025903

(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C40H63N7O10/c1-23(2)15-28(32(48)19-34(50)43-25(5)36(52)44-29(16-24(3)4)33(49)20-35(51)56-9)45-38(54)31(18-27-21-41-22-42-27)46-37(53)30(17-26-13-11-10-12-14-26)47-39(55)57-40(6,7)8/h10-14,21-25,28-33,48-49H,15-20H2,1-9H3,(H,41,42)(H,43,50)(H,44,52)(H,45,54)(H,46,53)(H,47,55)/t25-,28-,29-,30-,31-,32?,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024174

(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C43H65N5O10/c1-26(2)20-31(35(49)24-37(51)44-28(5)39(53)45-32(21-27(3)4)36(50)25-38(52)57-9)46-40(54)33(22-29-16-12-10-13-17-29)47-41(55)34(23-30-18-14-11-15-19-30)48-42(56)58-43(6,7)8/h10-19,26-28,31-36,49-50H,20-25H2,1-9H3,(H,44,51)(H,45,53)(H,46,54)(H,47,55)(H,48,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against dog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421761

(CHEMBL2311113)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C42H63N5O10/c1-25(2)19-31(34(48)22-36(50)43-28(7)39(52)44-32(20-26(3)4)35(49)23-37(51)56-8)45-41(54)38(27(5)6)47-40(53)33(21-29-15-11-9-12-16-29)46-42(55)57-24-30-17-13-10-14-18-30/h9-18,25-28,31-35,38,48-49H,19-24H2,1-8H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53)/t28-,31-,32-,33-,34?,35?,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for human plasma renin. |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024190
(4-(2-{4-[2-[2-(2-Benzyloxycarbonylamino-ethanesulf...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)CCNC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C45H66N8O12S/c1-28(2)19-34(38(54)23-40(56)49-30(5)42(58)50-35(20-29(3)4)39(55)24-41(57)64-6)51-43(59)36(22-33-25-46-27-48-33)52-44(60)37(21-31-13-9-7-10-14-31)53-66(62,63)18-17-47-45(61)65-26-32-15-11-8-12-16-32/h7-16,25,27-30,34-39,53-55H,17-24,26H2,1-6H3,(H,46,48)(H,47,61)(H,49,56)(H,50,58)(H,51,59)(H,52,60) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024181
(4-(2-{4-[2-[2-tert-Butoxycarbonylamino-3-(1H-indol...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C42H64N8O10/c1-23(2)14-30(34(51)18-36(53)46-25(5)38(55)47-31(15-24(3)4)35(52)19-37(54)59-9)48-40(57)33(17-27-21-43-22-45-27)49-39(56)32(50-41(58)60-42(6,7)8)16-26-20-44-29-13-11-10-12-28(26)29/h10-13,20-25,30-35,44,51-52H,14-19H2,1-9H3,(H,43,45)(H,46,53)(H,47,55)(H,48,57)(H,49,56)(H,50,58) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024174

(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C43H65N5O10/c1-26(2)20-31(35(49)24-37(51)44-28(5)39(53)45-32(21-27(3)4)36(50)25-38(52)57-9)46-40(54)33(22-29-16-12-10-13-17-29)47-41(55)34(23-30-18-14-11-15-19-30)48-42(56)58-43(6,7)8/h10-19,26-28,31-36,49-50H,20-25H2,1-9H3,(H,44,51)(H,45,53)(H,46,54)(H,47,55)(H,48,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against monkey plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024181

(4-(2-{4-[2-[2-tert-Butoxycarbonylamino-3-(1H-indol...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C42H64N8O10/c1-23(2)14-30(34(51)18-36(53)46-25(5)38(55)47-31(15-24(3)4)35(52)19-37(54)59-9)48-40(57)33(17-27-21-43-22-45-27)49-39(56)32(50-41(58)60-42(6,7)8)16-26-20-44-29-13-11-10-12-28(26)29/h10-13,20-25,30-35,44,51-52H,14-19H2,1-9H3,(H,43,45)(H,46,53)(H,47,55)(H,48,57)(H,49,56)(H,50,58) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024192
(4-(2-{4-[2-(2-Acetylamino-3-phenyl-propionylamino)...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C40H59N5O9/c1-24(2)18-30(34(47)22-36(49)41-26(5)38(51)43-31(19-25(3)4)35(48)23-37(50)54-7)44-40(53)33(21-29-16-12-9-13-17-29)45-39(52)32(42-27(6)46)20-28-14-10-8-11-15-28/h8-17,24-26,30-35,47-48H,18-23H2,1-7H3,(H,41,49)(H,42,46)(H,43,51)(H,44,53)(H,45,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Cynomolgus monkey plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024181

(4-(2-{4-[2-[2-tert-Butoxycarbonylamino-3-(1H-indol...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C42H64N8O10/c1-23(2)14-30(34(51)18-36(53)46-25(5)38(55)47-31(15-24(3)4)35(52)19-37(54)59-9)48-40(57)33(17-27-21-43-22-45-27)49-39(56)32(50-41(58)60-42(6,7)8)16-26-20-44-29-13-11-10-12-28(26)29/h10-13,20-25,30-35,44,51-52H,14-19H2,1-9H3,(H,43,45)(H,46,53)(H,47,55)(H,48,57)(H,49,56)(H,50,58) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024169
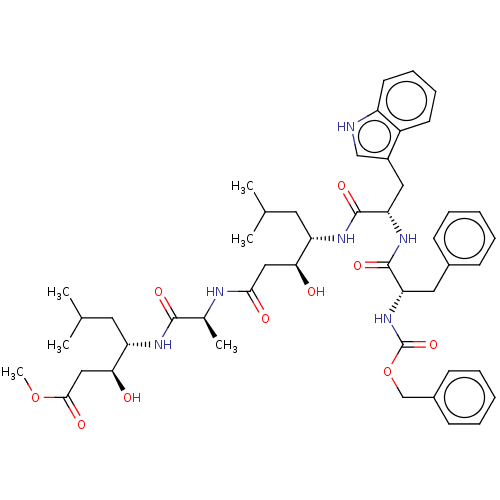
(4-(2-{4-[2-(2-Benzyloxycarbonylamino-3-phenyl-prop...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C48H64N6O10/c1-29(2)21-37(41(55)25-43(57)50-31(5)45(59)51-38(22-30(3)4)42(56)26-44(58)63-6)52-47(61)40(24-34-27-49-36-20-14-13-19-35(34)36)53-46(60)39(23-32-15-9-7-10-16-32)54-48(62)64-28-33-17-11-8-12-18-33/h7-20,27,29-31,37-42,49,55-56H,21-26,28H2,1-6H3,(H,50,57)(H,51,59)(H,52,61)(H,53,60)(H,54,62) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50025903

(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C40H63N7O10/c1-23(2)15-28(32(48)19-34(50)43-25(5)36(52)44-29(16-24(3)4)33(49)20-35(51)56-9)45-38(54)31(18-27-21-41-22-42-27)46-37(53)30(17-26-13-11-10-12-14-26)47-39(55)57-40(6,7)8/h10-14,21-25,28-33,48-49H,15-20H2,1-9H3,(H,41,42)(H,43,50)(H,44,52)(H,45,54)(H,46,53)(H,47,55)/t25-,28-,29-,30-,31-,32?,33?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of hog man plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024163

(4-[2-(4-{2-[2-Benzyloxycarbonylamino-3-(1H-indol-3...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C44H64N6O10/c1-25(2)18-33(36(51)21-38(53)46-28(7)41(55)47-34(19-26(3)4)37(52)22-39(54)59-8)48-43(57)40(27(5)6)50-42(56)35(20-30-23-45-32-17-13-12-16-31(30)32)49-44(58)60-24-29-14-10-9-11-15-29/h9-17,23,25-28,33-37,40,45,51-52H,18-22,24H2,1-8H3,(H,46,53)(H,47,55)(H,48,57)(H,49,58)(H,50,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024163

(4-[2-(4-{2-[2-Benzyloxycarbonylamino-3-(1H-indol-3...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C44H64N6O10/c1-25(2)18-33(36(51)21-38(53)46-28(7)41(55)47-34(19-26(3)4)37(52)22-39(54)59-8)48-43(57)40(27(5)6)50-42(56)35(20-30-23-45-32-17-13-12-16-31(30)32)49-44(58)60-24-29-14-10-9-11-15-29/h9-17,23,25-28,33-37,40,45,51-52H,18-22,24H2,1-8H3,(H,46,53)(H,47,55)(H,48,57)(H,49,58)(H,50,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022516

(Boc-Trp-Trp-Sta-Ala-Sta-OMe | CHEMBL3142766)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C Show InChI InChI=1S/C47H67N7O10/c1-26(2)18-35(39(55)22-41(57)50-28(5)43(59)51-36(19-27(3)4)40(56)23-42(58)63-9)52-44(60)37(20-29-24-48-33-16-12-10-14-31(29)33)53-45(61)38(54-46(62)64-47(6,7)8)21-30-25-49-34-17-13-11-15-32(30)34/h10-17,24-28,35-40,48-49,55-56H,18-23H2,1-9H3,(H,50,57)(H,51,59)(H,52,60)(H,53,61)(H,54,62)/t28-,35?,36?,37-,38-,39?,40?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022516

(Boc-Trp-Trp-Sta-Ala-Sta-OMe | CHEMBL3142766)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C Show InChI InChI=1S/C47H67N7O10/c1-26(2)18-35(39(55)22-41(57)50-28(5)43(59)51-36(19-27(3)4)40(56)23-42(58)63-9)52-44(60)37(20-29-24-48-33-16-12-10-14-31(29)33)53-45(61)38(54-46(62)64-47(6,7)8)21-30-25-49-34-17-13-11-15-32(30)34/h10-17,24-28,35-40,48-49,55-56H,18-23H2,1-9H3,(H,50,57)(H,51,59)(H,52,60)(H,53,61)(H,54,62)/t28-,35?,36?,37-,38-,39?,40?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against man plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024182
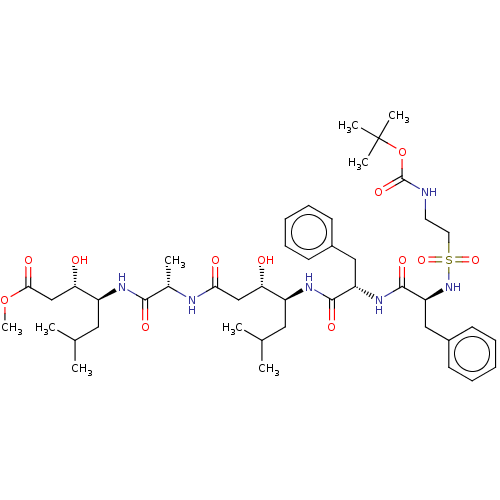
(4-[2-(4-{2-[2-(2-tert-Butoxycarbonylamino-ethanesu...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)CCNC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C45H70N6O12S/c1-28(2)22-33(37(52)26-39(54)47-30(5)41(56)48-34(23-29(3)4)38(53)27-40(55)62-9)49-42(57)35(24-31-16-12-10-13-17-31)50-43(58)36(25-32-18-14-11-15-19-32)51-64(60,61)21-20-46-44(59)63-45(6,7)8/h10-19,28-30,33-38,51-53H,20-27H2,1-9H3,(H,46,59)(H,47,54)(H,48,56)(H,49,57)(H,50,58) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421761

(CHEMBL2311113)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C42H63N5O10/c1-25(2)19-31(34(48)22-36(50)43-28(7)39(52)44-32(20-26(3)4)35(49)23-37(51)56-8)45-41(54)38(27(5)6)47-40(53)33(21-29-15-11-9-12-16-29)46-42(55)57-24-30-17-13-10-14-18-30/h9-18,25-28,31-35,38,48-49H,19-24H2,1-8H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53)/t28-,31-,32-,33-,34?,35?,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421761

(CHEMBL2311113)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C42H63N5O10/c1-25(2)19-31(34(48)22-36(50)43-28(7)39(52)44-32(20-26(3)4)35(49)23-37(51)56-8)45-41(54)38(27(5)6)47-40(53)33(21-29-15-11-9-12-16-29)46-42(55)57-24-30-17-13-10-14-18-30/h9-18,25-28,31-35,38,48-49H,19-24H2,1-8H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53)/t28-,31-,32-,33-,34?,35?,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024174

(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C43H65N5O10/c1-26(2)20-31(35(49)24-37(51)44-28(5)39(53)45-32(21-27(3)4)36(50)25-38(52)57-9)46-40(54)33(22-29-16-12-10-13-17-29)47-41(55)34(23-30-18-14-11-15-19-30)48-42(56)58-43(6,7)8/h10-19,26-28,31-36,49-50H,20-25H2,1-9H3,(H,44,51)(H,45,53)(H,46,54)(H,47,55)(H,48,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50279761
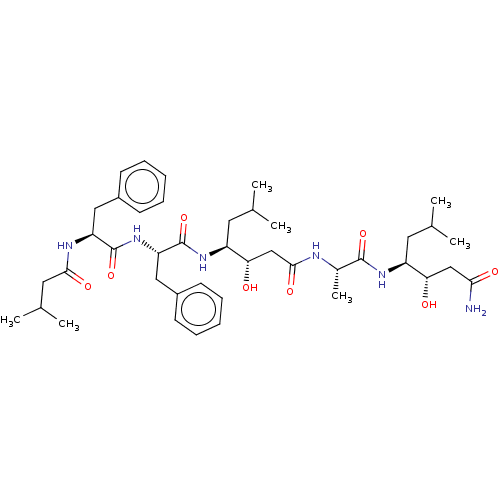
(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)[C@@H](O)CC(N)=O |r| Show InChI InChI=1S/C42H64N6O8/c1-25(2)18-31(35(49)23-37(43)51)46-40(54)28(7)44-39(53)24-36(50)32(19-26(3)4)47-42(56)34(22-30-16-12-9-13-17-30)48-41(55)33(45-38(52)20-27(5)6)21-29-14-10-8-11-15-29/h8-17,25-28,31-36,49-50H,18-24H2,1-7H3,(H2,43,51)(H,44,53)(H,45,52)(H,46,54)(H,47,56)(H,48,55) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024174

(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C43H65N5O10/c1-26(2)20-31(35(49)24-37(51)44-28(5)39(53)45-32(21-27(3)4)36(50)25-38(52)57-9)46-40(54)33(22-29-16-12-10-13-17-29)47-41(55)34(23-30-18-14-11-15-19-30)48-42(56)58-43(6,7)8/h10-19,26-28,31-36,49-50H,20-25H2,1-9H3,(H,44,51)(H,45,53)(H,46,54)(H,47,55)(H,48,56) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024193
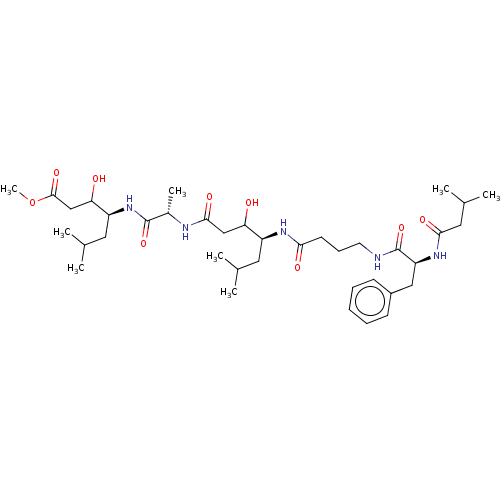
(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{4-[2-(3-meth...)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)CCCNC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C |r| Show InChI InChI=1S/C38H63N5O9/c1-23(2)17-28(31(44)21-35(48)40-26(7)37(50)43-29(18-24(3)4)32(45)22-36(49)52-8)41-33(46)15-12-16-39-38(51)30(42-34(47)19-25(5)6)20-27-13-10-9-11-14-27/h9-11,13-14,23-26,28-32,44-45H,12,15-22H2,1-8H3,(H,39,51)(H,40,48)(H,41,46)(H,42,47)(H,43,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024178

(4-(2-{4-[2-(3-Adamantan-1-yl-2-tert-butoxycarbonyl...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CC12CC3CC(CC(C3)C1)C2)NC(=O)OC(C)(C)C |r,TLB:46:47:51:45.44.50,THB:46:45:51:47.52.48,48:47:44:49.51.50,48:49:44:47.52.46| Show InChI InChI=1S/C44H73N7O10/c1-24(2)10-31(35(52)16-37(54)47-26(5)39(56)48-32(11-25(3)4)36(53)17-38(55)60-9)49-40(57)33(15-30-22-45-23-46-30)50-41(58)34(51-42(59)61-43(6,7)8)21-44-18-27-12-28(19-44)14-29(13-27)20-44/h22-29,31-36,52-53H,10-21H2,1-9H3,(H,45,46)(H,47,54)(H,48,56)(H,49,57)(H,50,58)(H,51,59) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024178

(4-(2-{4-[2-(3-Adamantan-1-yl-2-tert-butoxycarbonyl...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CC12CC3CC(CC(C3)C1)C2)NC(=O)OC(C)(C)C |r,TLB:46:47:51:45.44.50,THB:46:45:51:47.52.48,48:47:44:49.51.50,48:49:44:47.52.46| Show InChI InChI=1S/C44H73N7O10/c1-24(2)10-31(35(52)16-37(54)47-26(5)39(56)48-32(11-25(3)4)36(53)17-38(55)60-9)49-40(57)33(15-30-22-45-23-46-30)50-41(58)34(51-42(59)61-43(6,7)8)21-44-18-27-12-28(19-44)14-29(13-27)20-44/h22-29,31-36,52-53H,10-21H2,1-9H3,(H,45,46)(H,47,54)(H,48,56)(H,49,57)(H,50,58)(H,51,59) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against dog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024184

(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES CCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C36H59N5O10/c1-9-13-25(38-35(48)28(41-36(49)51-8)18-24-14-11-10-12-15-24)34(47)40-26(16-21(2)3)29(42)19-31(44)37-23(6)33(46)39-27(17-22(4)5)30(43)20-32(45)50-7/h10-12,14-15,21-23,25-30,42-43H,9,13,16-20H2,1-8H3,(H,37,44)(H,38,48)(H,39,46)(H,40,47)(H,41,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024167

(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O9/c1-9-10-16-28(42-39(53)31(41-34(47)19-25(6)7)20-27-14-12-11-13-15-27)38(52)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(51)43-30(18-24(4)5)33(46)22-36(49)50/h11-15,23-26,28-33,45-46H,9-10,16-22H2,1-8H3,(H,40,48)(H,41,47)(H,42,53)(H,43,51)(H,44,52)(H,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024187
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O10/c1-10-11-17-27(41-37(52)30(20-26-15-13-12-14-16-26)44-38(53)54-39(7,8)9)36(51)43-28(18-23(2)3)31(45)21-33(47)40-25(6)35(50)42-29(19-24(4)5)32(46)22-34(48)49/h12-16,23-25,27-32,45-46H,10-11,17-22H2,1-9H3,(H,40,47)(H,41,52)(H,42,50)(H,43,51)(H,44,53)(H,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024189
(4-[2-(4-{2-[2-(Adamantan-1-yloxycarbonylamino)-3-p...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC12CC3CC(CC(C3)C1)C2 |r,TLB:57:58:62:56.55.61,61:60:63:56.55.57,61:56:63:60.62.59,THB:57:56:62:58.63.59| Show InChI InChI=1S/C49H71N5O10/c1-29(2)17-37(41(55)24-43(57)50-31(5)45(59)51-38(18-30(3)4)42(56)25-44(58)63-6)52-46(60)39(22-32-13-9-7-10-14-32)53-47(61)40(23-33-15-11-8-12-16-33)54-48(62)64-49-26-34-19-35(27-49)21-36(20-34)28-49/h7-16,29-31,34-42,55-56H,17-28H2,1-6H3,(H,50,57)(H,51,59)(H,52,60)(H,53,61)(H,54,62) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024195
(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C |r| Show InChI InChI=1S/C43H65N5O9/c1-26(2)19-32(36(49)24-39(52)44-29(7)41(54)46-33(20-27(3)4)37(50)25-40(53)57-8)47-43(56)35(23-31-17-13-10-14-18-31)48-42(55)34(45-38(51)21-28(5)6)22-30-15-11-9-12-16-30/h9-18,26-29,32-37,49-50H,19-25H2,1-8H3,(H,44,52)(H,45,51)(H,46,54)(H,47,56)(H,48,55) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024177
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-methyl-pe...)Show SMILES CC[C@H](C)[C@H](NC(=O)OC(C)(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C37H65N7O10/c1-12-22(6)32(44-36(52)54-37(8,9)10)35(51)43-27(15-24-18-38-19-39-24)34(50)42-25(13-20(2)3)28(45)16-30(47)40-23(7)33(49)41-26(14-21(4)5)29(46)17-31(48)53-11/h18-23,25-29,32,45-46H,12-17H2,1-11H3,(H,38,39)(H,40,47)(H,41,49)(H,42,50)(H,43,51)(H,44,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against dog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024177

(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-methyl-pe...)Show SMILES CC[C@H](C)[C@H](NC(=O)OC(C)(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C37H65N7O10/c1-12-22(6)32(44-36(52)54-37(8,9)10)35(51)43-27(15-24-18-38-19-39-24)34(50)42-25(13-20(2)3)28(45)16-30(47)40-23(7)33(49)41-26(14-21(4)5)29(46)17-31(48)53-11/h18-23,25-29,32,45-46H,12-17H2,1-11H3,(H,38,39)(H,40,47)(H,41,49)(H,42,50)(H,43,51)(H,44,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against man (human) plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data