 Found 91 hits with Last Name = 'michelotti' and Initial = 'el'
Found 91 hits with Last Name = 'michelotti' and Initial = 'el' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482992

(CHEMBL1269927)Show SMILES CN(Cc1ccc2occc2c1)c1nc(O)nc(Nc2c(F)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C25H20FN5O2/c1-31(15-16-10-11-21-18(14-16)12-13-33-21)24-28-23(29-25(32)30-24)27-22-19(8-5-9-20(22)26)17-6-3-2-4-7-17/h2-14H,15H2,1H3,(H2,27,28,29,30,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50482992

(CHEMBL1269927)Show SMILES CN(Cc1ccc2occc2c1)c1nc(O)nc(Nc2c(F)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C25H20FN5O2/c1-31(15-16-10-11-21-18(14-16)12-13-33-21)24-28-23(29-25(32)30-24)27-22-19(8-5-9-20(22)26)17-6-3-2-4-7-17/h2-14H,15H2,1H3,(H2,27,28,29,30,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase L100I mutant |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482991
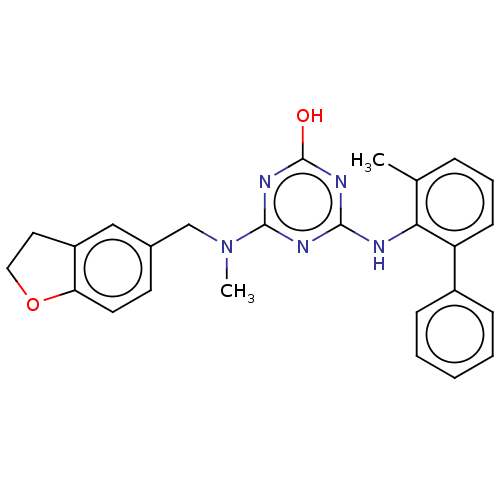
(CHEMBL1270036)Show SMILES CN(Cc1ccc2OCCc2c1)c1nc(O)nc(Nc2c(C)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C26H25N5O2/c1-17-7-6-10-21(19-8-4-3-5-9-19)23(17)27-24-28-25(30-26(32)29-24)31(2)16-18-11-12-22-20(15-18)13-14-33-22/h3-12,15H,13-14,16H2,1-2H3,(H2,27,28,29,30,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50482991

(CHEMBL1270036)Show SMILES CN(Cc1ccc2OCCc2c1)c1nc(O)nc(Nc2c(C)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C26H25N5O2/c1-17-7-6-10-21(19-8-4-3-5-9-19)23(17)27-24-28-25(30-26(32)29-24)31(2)16-18-11-12-22-20(15-18)13-14-33-22/h3-12,15H,13-14,16H2,1-2H3,(H2,27,28,29,30,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase L100I mutant |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482996
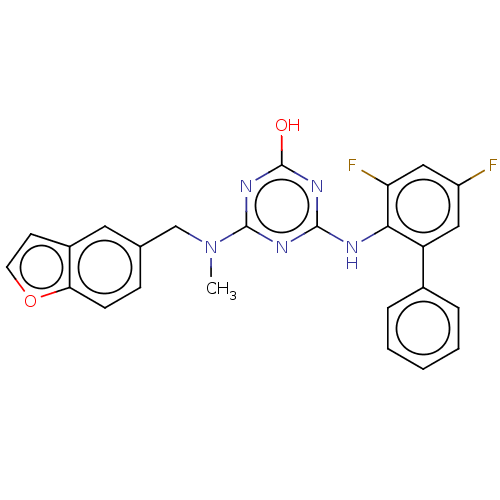
(CHEMBL1269928)Show SMILES CN(Cc1ccc2occc2c1)c1nc(O)nc(Nc2c(F)cc(F)cc2-c2ccccc2)n1 Show InChI InChI=1S/C25H19F2N5O2/c1-32(14-15-7-8-21-17(11-15)9-10-34-21)24-29-23(30-25(33)31-24)28-22-19(12-18(26)13-20(22)27)16-5-3-2-4-6-16/h2-13H,14H2,1H3,(H2,28,29,30,31,33) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483013
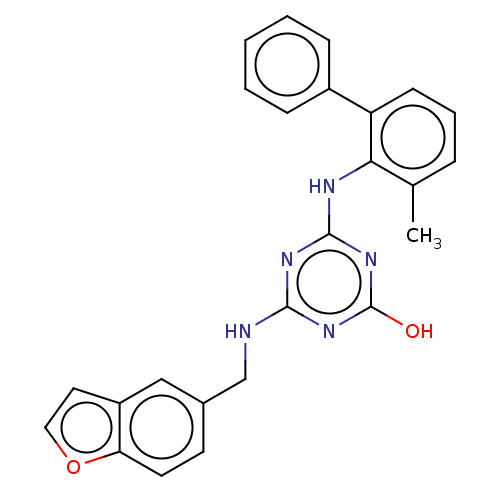
(CHEMBL1271250)Show SMILES Cc1cccc(-c2ccccc2)c1Nc1nc(O)nc(NCc2ccc3occc3c2)n1 Show InChI InChI=1S/C25H21N5O2/c1-16-6-5-9-20(18-7-3-2-4-8-18)22(16)27-24-28-23(29-25(31)30-24)26-15-17-10-11-21-19(14-17)12-13-32-21/h2-14H,15H2,1H3,(H3,26,27,28,29,30,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483007
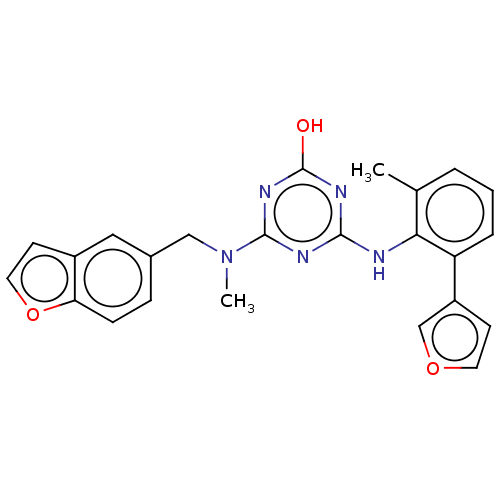
(CHEMBL1270035)Show SMILES CN(Cc1ccc2occc2c1)c1nc(O)nc(Nc2c(C)cccc2-c2ccoc2)n1 Show InChI InChI=1S/C24H21N5O3/c1-15-4-3-5-19(18-8-10-31-14-18)21(15)25-22-26-23(28-24(30)27-22)29(2)13-16-6-7-20-17(12-16)9-11-32-20/h3-12,14H,13H2,1-2H3,(H2,25,26,27,28,30) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483008
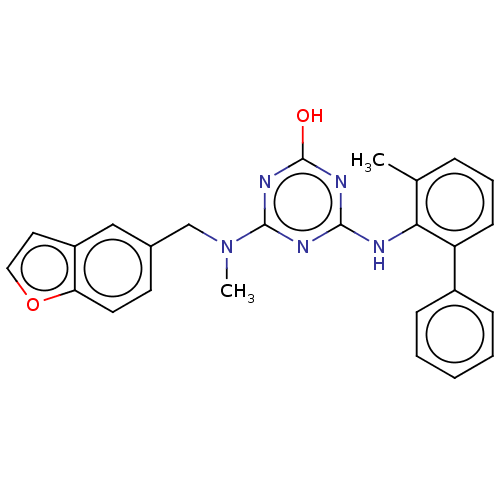
(CHEMBL1271460)Show SMILES CN(Cc1ccc2occc2c1)c1nc(O)nc(Nc2c(C)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C26H23N5O2/c1-17-7-6-10-21(19-8-4-3-5-9-19)23(17)27-24-28-25(30-26(32)29-24)31(2)16-18-11-12-22-20(15-18)13-14-33-22/h3-15H,16H2,1-2H3,(H2,27,28,29,30,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482995
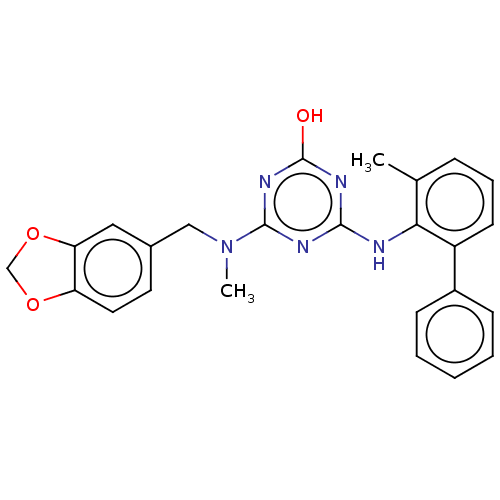
(CHEMBL1270141)Show SMILES CN(Cc1ccc2OCOc2c1)c1nc(O)nc(Nc2c(C)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C25H23N5O3/c1-16-7-6-10-19(18-8-4-3-5-9-18)22(16)26-23-27-24(29-25(31)28-23)30(2)14-17-11-12-20-21(13-17)33-15-32-20/h3-13H,14-15H2,1-2H3,(H2,26,27,28,29,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483009
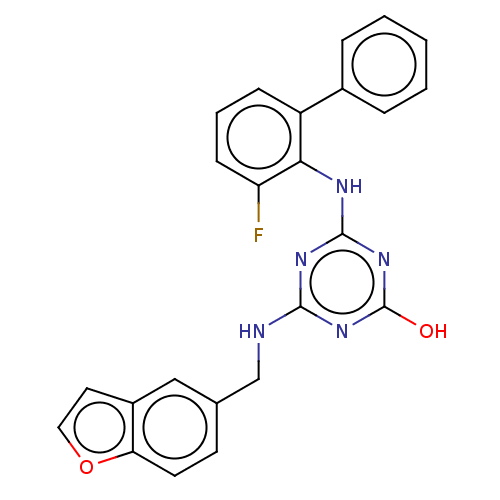
(CHEMBL1271251)Show SMILES Oc1nc(NCc2ccc3occc3c2)nc(Nc2c(F)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C24H18FN5O2/c25-19-8-4-7-18(16-5-2-1-3-6-16)21(19)27-23-28-22(29-24(31)30-23)26-14-15-9-10-20-17(13-15)11-12-32-20/h1-13H,14H2,(H3,26,27,28,29,30,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM13336
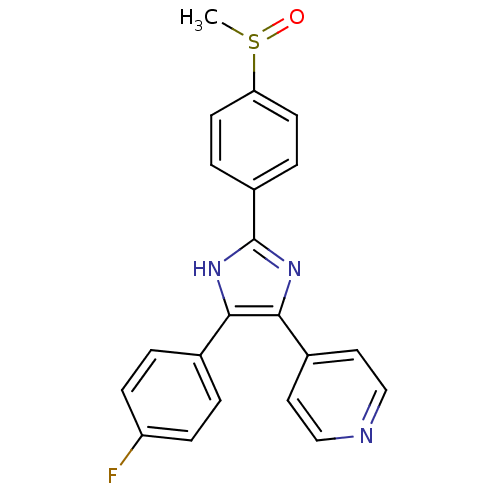
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174086

(CHEMBL198470 | N-(3-hydroxy-4-(1-hydroxy-2-methylp...)Show SMILES CC(C)(CO)c1ccc(CNC(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)cc1O Show InChI InChI=1S/C24H24ClNO4/c1-24(2,15-27)21-12-3-16(13-22(21)28)14-26-23(29)17-4-8-19(9-5-17)30-20-10-6-18(25)7-11-20/h3-13,27-28H,14-15H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174085
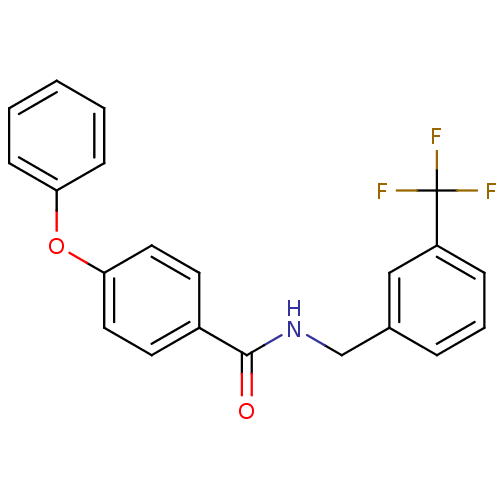
(CHEMBL198563 | N-(3-(trifluoromethyl)benzyl)-4-phe...)Show SMILES FC(F)(F)c1cccc(CNC(=O)c2ccc(Oc3ccccc3)cc2)c1 Show InChI InChI=1S/C21H16F3NO2/c22-21(23,24)17-6-4-5-15(13-17)14-25-20(26)16-9-11-19(12-10-16)27-18-7-2-1-3-8-18/h1-13H,14H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483001
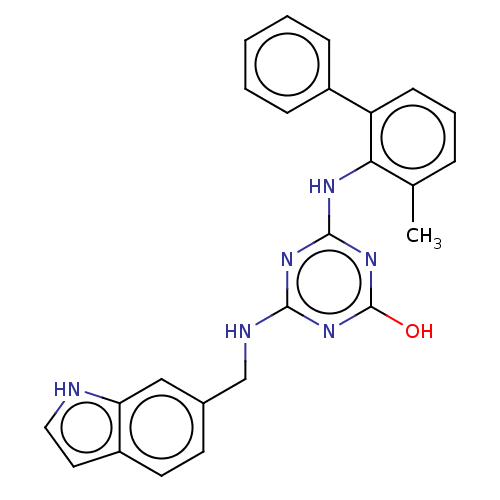
(CHEMBL1271043)Show SMILES Cc1cccc(-c2ccccc2)c1Nc1nc(O)nc(NCc2ccc3cc[nH]c3c2)n1 Show InChI InChI=1S/C25H22N6O/c1-16-6-5-9-20(18-7-3-2-4-8-18)22(16)28-24-29-23(30-25(32)31-24)27-15-17-10-11-19-12-13-26-21(19)14-17/h2-14,26H,15H2,1H3,(H3,27,28,29,30,31,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174095

(CHEMBL198832 | N-(3-(trifluoromethoxy)benzyl)-4-ph...)Show SMILES FC(F)(F)Oc1cccc(CNC(=O)c2ccc(Oc3ccccc3)cc2)c1 Show InChI InChI=1S/C21H16F3NO3/c22-21(23,24)28-19-8-4-5-15(13-19)14-25-20(26)16-9-11-18(12-10-16)27-17-6-2-1-3-7-17/h1-13H,14H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174098

(CHEMBL197228 | N-((2,3-dihydro-1H-inden-5-yl)methy...)Show InChI InChI=1S/C23H21NO2/c25-23(24-16-17-9-10-18-5-4-6-20(18)15-17)19-11-13-22(14-12-19)26-21-7-2-1-3-8-21/h1-3,7-15H,4-6,16H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174090

(CHEMBL199074 | N-(4-(trifluoromethyl)benzyl)-4-phe...)Show SMILES FC(F)(F)c1ccc(CNC(=O)c2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C21H16F3NO2/c22-21(23,24)17-10-6-15(7-11-17)14-25-20(26)16-8-12-19(13-9-16)27-18-4-2-1-3-5-18/h1-13H,14H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174097
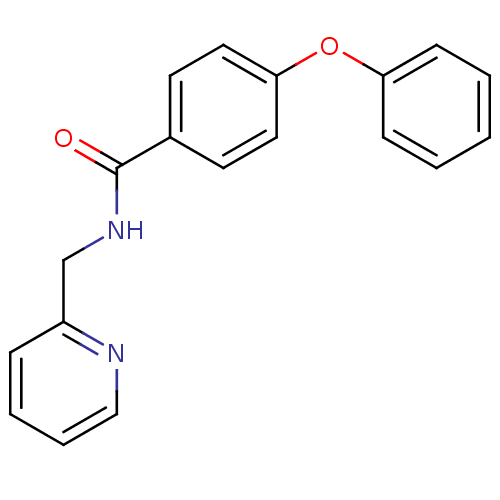
(4-phenoxy-N-(pyridin-2-ylmethyl)benzamide | CHEMBL...)Show InChI InChI=1S/C19H16N2O2/c22-19(21-14-16-6-4-5-13-20-16)15-9-11-18(12-10-15)23-17-7-2-1-3-8-17/h1-13H,14H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174096
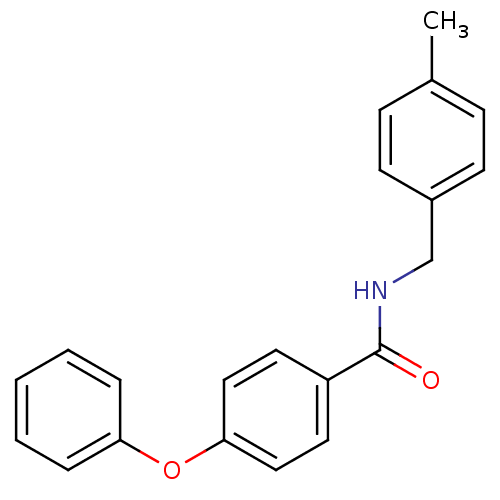
(CHEMBL199174 | N-(4-methyl-benzyl)-4-phenoxy-benza...)Show InChI InChI=1S/C21H19NO2/c1-16-7-9-17(10-8-16)15-22-21(23)18-11-13-20(14-12-18)24-19-5-3-2-4-6-19/h2-14H,15H2,1H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174092
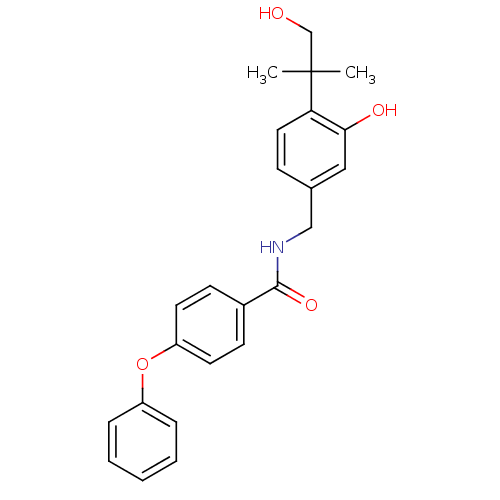
(CHEMBL199229 | N-(3-hydroxy-4-(1-hydroxy-2-methylp...)Show SMILES CC(C)(CO)c1ccc(CNC(=O)c2ccc(Oc3ccccc3)cc2)cc1O Show InChI InChI=1S/C24H25NO4/c1-24(2,16-26)21-13-8-17(14-22(21)27)15-25-23(28)18-9-11-20(12-10-18)29-19-6-4-3-5-7-19/h3-14,26-27H,15-16H2,1-2H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482993
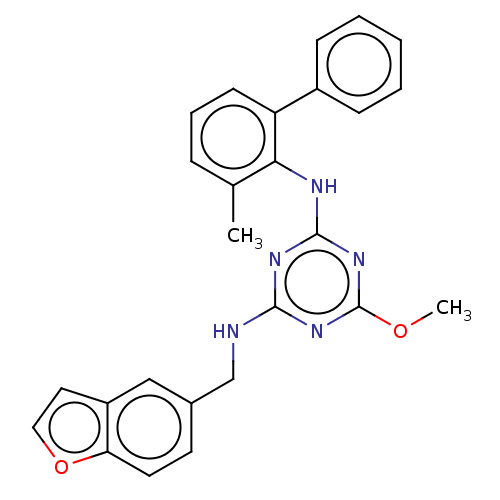
(CHEMBL1270346)Show SMILES COc1nc(NCc2ccc3occc3c2)nc(Nc2c(C)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C26H23N5O2/c1-17-7-6-10-21(19-8-4-3-5-9-19)23(17)28-25-29-24(30-26(31-25)32-2)27-16-18-11-12-22-20(15-18)13-14-33-22/h3-15H,16H2,1-2H3,(H2,27,28,29,30,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483003
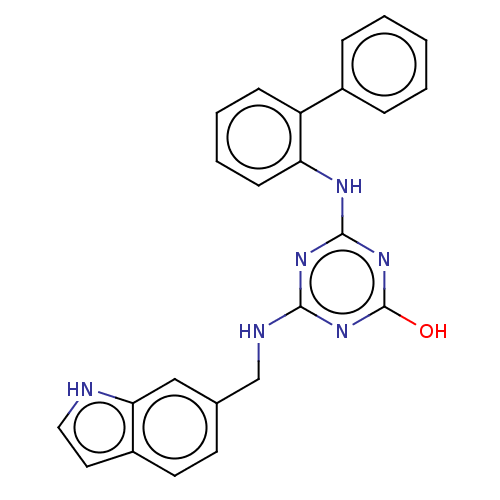
(CHEMBL1270839)Show SMILES Oc1nc(NCc2ccc3cc[nH]c3c2)nc(Nc2ccccc2-c2ccccc2)n1 Show InChI InChI=1S/C24H20N6O/c31-24-29-22(26-15-16-10-11-18-12-13-25-21(18)14-16)28-23(30-24)27-20-9-5-4-8-19(20)17-6-2-1-3-7-17/h1-14,25H,15H2,(H3,26,27,28,29,30,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482998
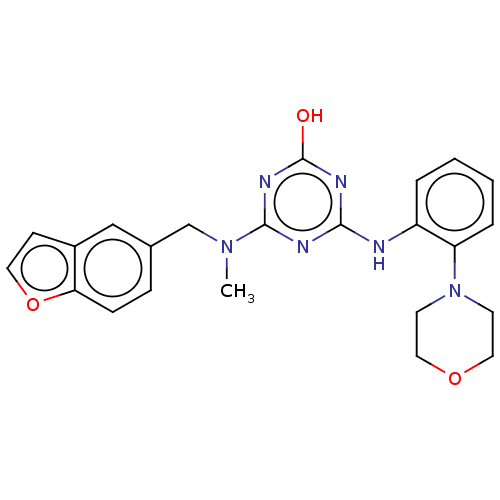
(CHEMBL1271352)Show SMILES CN(Cc1ccc2occc2c1)c1nc(O)nc(Nc2ccccc2N2CCOCC2)n1 Show InChI InChI=1S/C23H24N6O3/c1-28(15-16-6-7-20-17(14-16)8-11-32-20)22-25-21(26-23(30)27-22)24-18-4-2-3-5-19(18)29-9-12-31-13-10-29/h2-8,11,14H,9-10,12-13,15H2,1H3,(H2,24,25,26,27,30) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483006
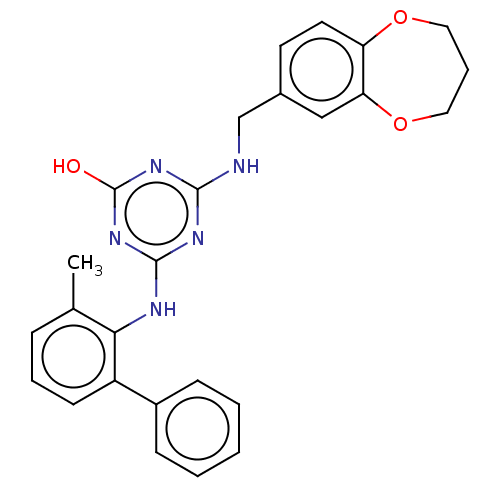
(CHEMBL1271148)Show SMILES Cc1cccc(-c2ccccc2)c1Nc1nc(O)nc(NCc2ccc3OCCCOc3c2)n1 Show InChI InChI=1S/C26H25N5O3/c1-17-7-5-10-20(19-8-3-2-4-9-19)23(17)28-25-29-24(30-26(32)31-25)27-16-18-11-12-21-22(15-18)34-14-6-13-33-21/h2-5,7-12,15H,6,13-14,16H2,1H3,(H3,27,28,29,30,31,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482999
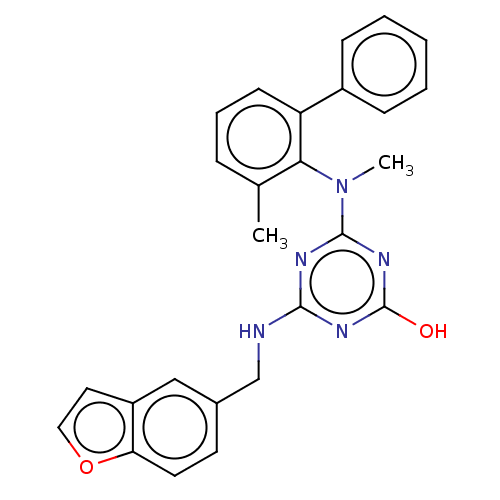
(CHEMBL1271351)Show SMILES CN(c1nc(O)nc(NCc2ccc3occc3c2)n1)c1c(C)cccc1-c1ccccc1 Show InChI InChI=1S/C26H23N5O2/c1-17-7-6-10-21(19-8-4-3-5-9-19)23(17)31(2)25-28-24(29-26(32)30-25)27-16-18-11-12-22-20(15-18)13-14-33-22/h3-15H,16H2,1-2H3,(H2,27,28,29,30,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174088
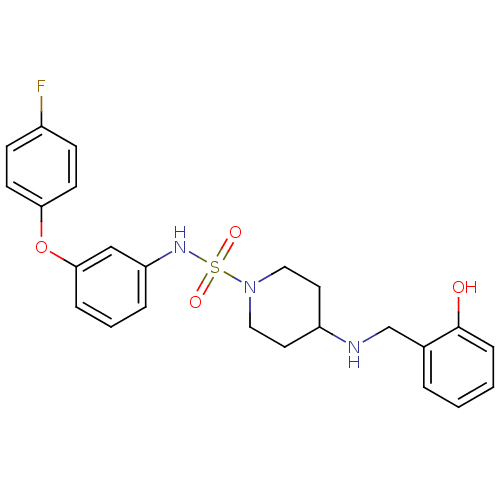
(4-(2-hydroxybenzylamino)-N-(3-(4-fluorophenoxy)phe...)Show SMILES Oc1ccccc1CNC1CCN(CC1)S(=O)(=O)Nc1cccc(Oc2ccc(F)cc2)c1 Show InChI InChI=1S/C24H26FN3O4S/c25-19-8-10-22(11-9-19)32-23-6-3-5-21(16-23)27-33(30,31)28-14-12-20(13-15-28)26-17-18-4-1-2-7-24(18)29/h1-11,16,20,26-27,29H,12-15,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482992

(CHEMBL1269927)Show SMILES CN(Cc1ccc2occc2c1)c1nc(O)nc(Nc2c(F)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C25H20FN5O2/c1-31(15-16-10-11-21-18(14-16)12-13-33-21)24-28-23(29-25(32)30-24)27-22-19(8-5-9-20(22)26)17-6-3-2-4-7-17/h2-14H,15H2,1H3,(H2,27,28,29,30,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y188L mutant |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482992

(CHEMBL1269927)Show SMILES CN(Cc1ccc2occc2c1)c1nc(O)nc(Nc2c(F)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C25H20FN5O2/c1-31(15-16-10-11-21-18(14-16)12-13-33-21)24-28-23(29-25(32)30-24)27-22-19(8-5-9-20(22)26)17-6-3-2-4-7-17/h2-14H,15H2,1H3,(H2,27,28,29,30,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V106A mutant |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482991

(CHEMBL1270036)Show SMILES CN(Cc1ccc2OCCc2c1)c1nc(O)nc(Nc2c(C)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C26H25N5O2/c1-17-7-6-10-21(19-8-4-3-5-9-19)23(17)27-24-28-25(30-26(32)29-24)31(2)16-18-11-12-22-20(15-18)13-14-33-22/h3-12,15H,13-14,16H2,1-2H3,(H2,27,28,29,30,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y188L mutant |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482994
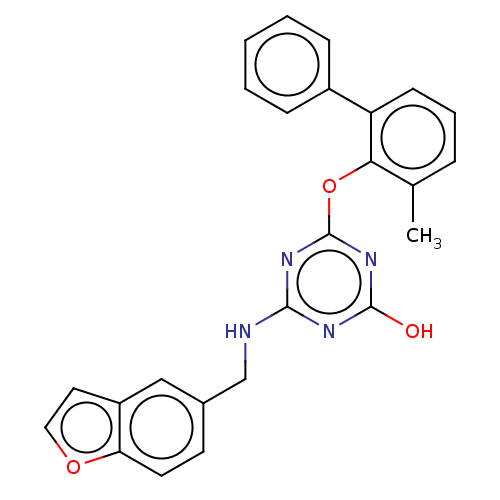
(CHEMBL1270142)Show SMILES Cc1cccc(-c2ccccc2)c1Oc1nc(O)nc(NCc2ccc3occc3c2)n1 Show InChI InChI=1S/C25H20N4O3/c1-16-6-5-9-20(18-7-3-2-4-8-18)22(16)32-25-28-23(27-24(30)29-25)26-15-17-10-11-21-19(14-17)12-13-31-21/h2-14H,15H2,1H3,(H2,26,27,28,29,30) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174100
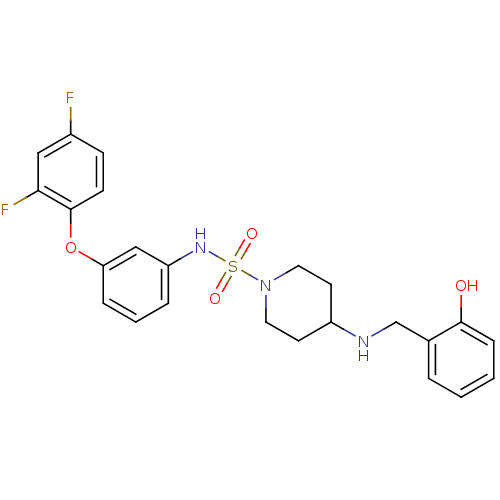
(4-(2-hydroxybenzylamino)-N-(3-(2,4-difluorophenoxy...)Show SMILES Oc1ccccc1CNC1CCN(CC1)S(=O)(=O)Nc1cccc(Oc2ccc(F)cc2F)c1 Show InChI InChI=1S/C24H25F2N3O4S/c25-18-8-9-24(22(26)14-18)33-21-6-3-5-20(15-21)28-34(31,32)29-12-10-19(11-13-29)27-16-17-4-1-2-7-23(17)30/h1-9,14-15,19,27-28,30H,10-13,16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174089
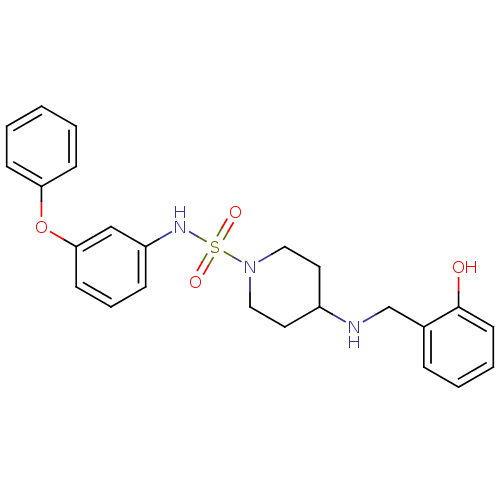
(4-(2-hydroxybenzylamino)-N-(3-phenoxyphenyl)piperi...)Show SMILES Oc1ccccc1CNC1CCN(CC1)S(=O)(=O)Nc1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C24H27N3O4S/c28-24-12-5-4-7-19(24)18-25-20-13-15-27(16-14-20)32(29,30)26-21-8-6-11-23(17-21)31-22-9-2-1-3-10-22/h1-12,17,20,25-26,28H,13-16,18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174094
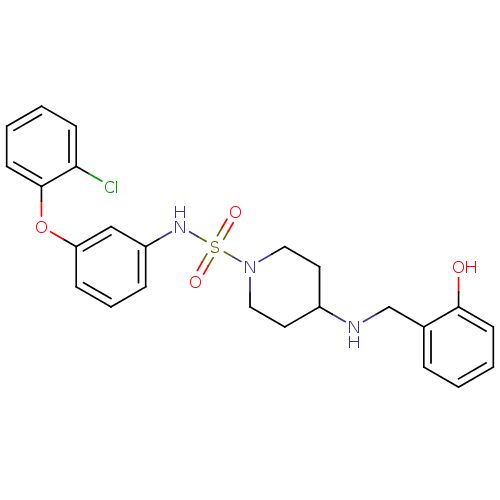
(4-(2-hydroxybenzylamino)-N-(3-(2-chlorophenoxy)phe...)Show SMILES Oc1ccccc1CNC1CCN(CC1)S(=O)(=O)Nc1cccc(Oc2ccccc2Cl)c1 Show InChI InChI=1S/C24H26ClN3O4S/c25-22-9-2-4-11-24(22)32-21-8-5-7-20(16-21)27-33(30,31)28-14-12-19(13-15-28)26-17-18-6-1-3-10-23(18)29/h1-11,16,19,26-27,29H,12-15,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174093
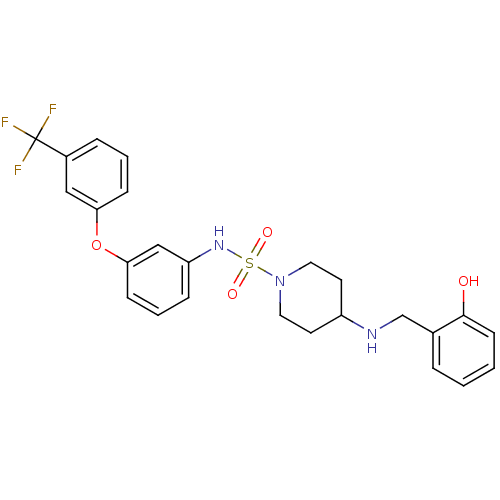
(4-(2-hydroxybenzylamino)-N-(3-(3-(trifluoromethyl)...)Show SMILES Oc1ccccc1CNC1CCN(CC1)S(=O)(=O)Nc1cccc(Oc2cccc(c2)C(F)(F)F)c1 Show InChI InChI=1S/C25H26F3N3O4S/c26-25(27,28)19-6-3-8-22(15-19)35-23-9-4-7-21(16-23)30-36(33,34)31-13-11-20(12-14-31)29-17-18-5-1-2-10-24(18)32/h1-10,15-16,20,29-30,32H,11-14,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174099
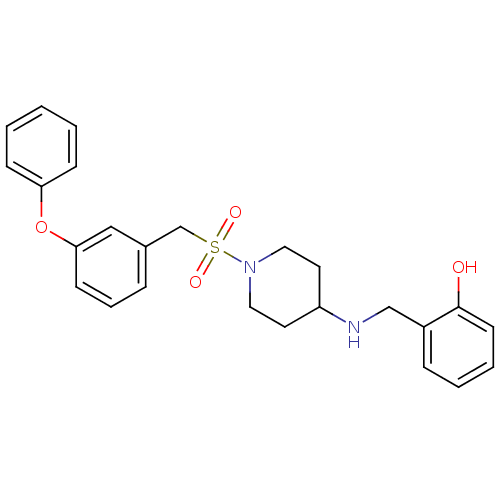
(2-((1-(3-phenoxybenzylsulfonyl)piperidin-4-ylamino...)Show SMILES Oc1ccccc1CNC1CCN(CC1)S(=O)(=O)Cc1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C25H28N2O4S/c28-25-12-5-4-8-21(25)18-26-22-13-15-27(16-14-22)32(29,30)19-20-7-6-11-24(17-20)31-23-9-2-1-3-10-23/h1-12,17,22,26,28H,13-16,18-19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174087
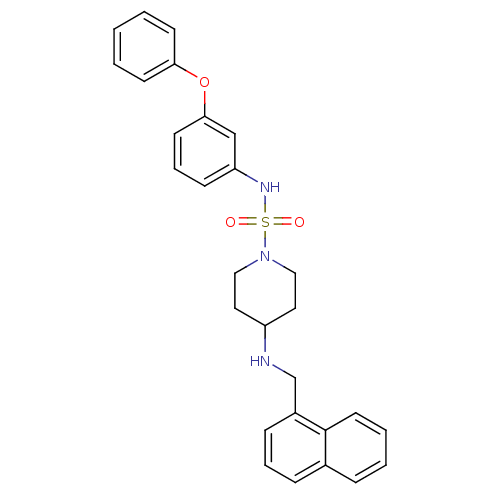
(4-(naphthalen-1-ylmethylamino)-N-(3-phenoxyphenyl)...)Show SMILES O=S(=O)(Nc1cccc(Oc2ccccc2)c1)N1CCC(CC1)NCc1cccc2ccccc12 Show InChI InChI=1S/C28H29N3O3S/c32-35(33,30-25-11-7-14-27(20-25)34-26-12-2-1-3-13-26)31-18-16-24(17-19-31)29-21-23-10-6-9-22-8-4-5-15-28(22)23/h1-15,20,24,29-30H,16-19,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50174091
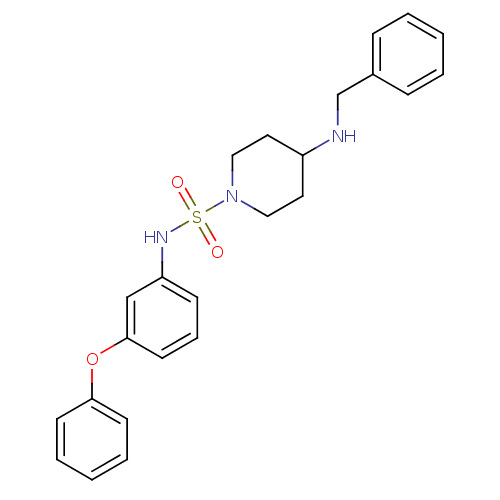
(4-(benzylamino)-N-(3-phenoxyphenyl)piperidine-1-su...)Show SMILES O=S(=O)(Nc1cccc(Oc2ccccc2)c1)N1CCC(CC1)NCc1ccccc1 Show InChI InChI=1S/C24H27N3O3S/c28-31(29,26-22-10-7-13-24(18-22)30-23-11-5-2-6-12-23)27-16-14-21(15-17-27)25-19-20-8-3-1-4-9-20/h1-13,18,21,25-26H,14-17,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Locus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38alpha MAPK |
Bioorg Med Chem Lett 15: 5274-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.038
BindingDB Entry DOI: 10.7270/Q24J0DP9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483005
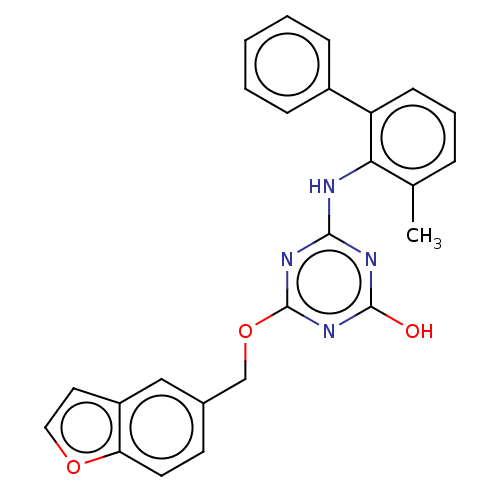
(CHEMBL1270245)Show SMILES Cc1cccc(-c2ccccc2)c1Nc1nc(O)nc(OCc2ccc3occc3c2)n1 Show InChI InChI=1S/C25H20N4O3/c1-16-6-5-9-20(18-7-3-2-4-8-18)22(16)26-23-27-24(30)29-25(28-23)32-15-17-10-11-21-19(14-17)12-13-31-21/h2-14H,15H2,1H3,(H2,26,27,28,29,30) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482991

(CHEMBL1270036)Show SMILES CN(Cc1ccc2OCCc2c1)c1nc(O)nc(Nc2c(C)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C26H25N5O2/c1-17-7-6-10-21(19-8-4-3-5-9-19)23(17)27-24-28-25(30-26(32)29-24)31(2)16-18-11-12-22-20(15-18)13-14-33-22/h3-12,15H,13-14,16H2,1-2H3,(H2,27,28,29,30,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V106A mutant |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483011
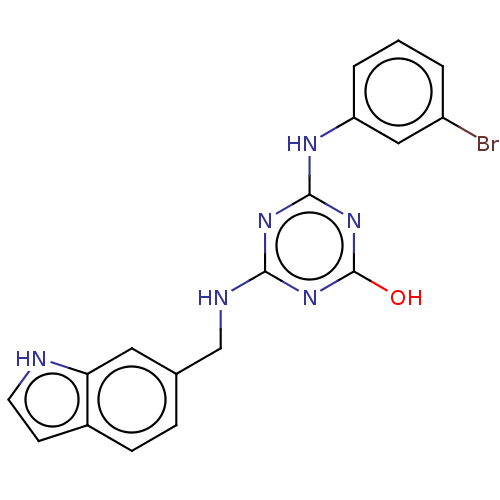
(CHEMBL1270940)Show SMILES Oc1nc(NCc2ccc3cc[nH]c3c2)nc(Nc2cccc(Br)c2)n1 Show InChI InChI=1S/C18H15BrN6O/c19-13-2-1-3-14(9-13)22-17-23-16(24-18(26)25-17)21-10-11-4-5-12-6-7-20-15(12)8-11/h1-9,20H,10H2,(H3,21,22,23,24,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483002
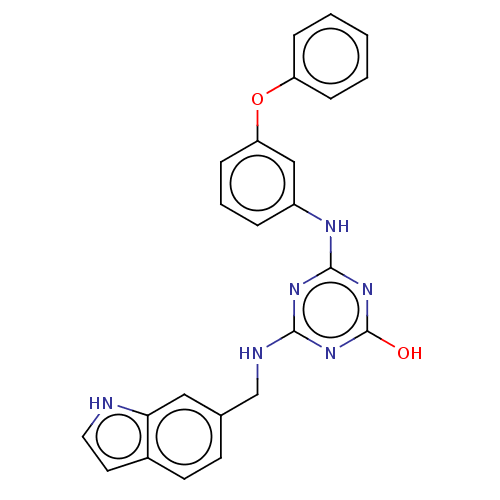
(CHEMBL1270941)Show SMILES Oc1nc(NCc2ccc3cc[nH]c3c2)nc(Nc2cccc(Oc3ccccc3)c2)n1 Show InChI InChI=1S/C24H20N6O2/c31-24-29-22(26-15-16-9-10-17-11-12-25-21(17)13-16)28-23(30-24)27-18-5-4-8-20(14-18)32-19-6-2-1-3-7-19/h1-14,25H,15H2,(H3,26,27,28,29,30,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483000
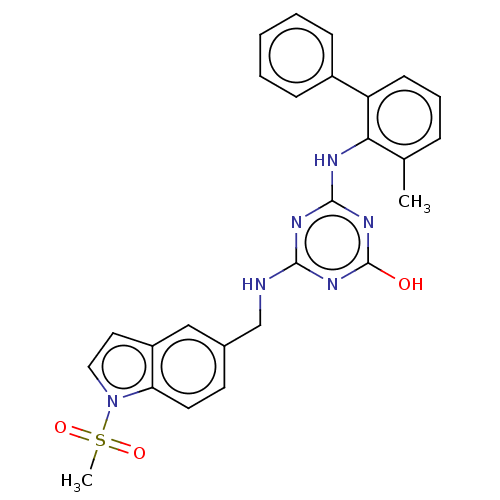
(CHEMBL1271147)Show SMILES Cc1cccc(-c2ccccc2)c1Nc1nc(O)nc(NCc2ccc3n(ccc3c2)S(C)(=O)=O)n1 Show InChI InChI=1S/C26H24N6O3S/c1-17-7-6-10-21(19-8-4-3-5-9-19)23(17)28-25-29-24(30-26(33)31-25)27-16-18-11-12-22-20(15-18)13-14-32(22)36(2,34)35/h3-15H,16H2,1-2H3,(H3,27,28,29,30,31,33) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482997
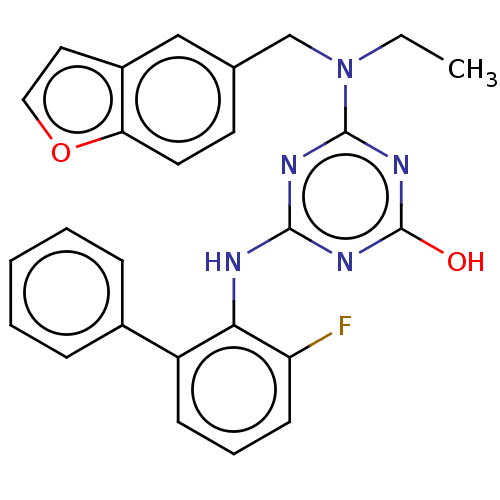
(CHEMBL1271459)Show SMILES CCN(Cc1ccc2occc2c1)c1nc(O)nc(Nc2c(F)cccc2-c2ccccc2)n1 Show InChI InChI=1S/C26H22FN5O2/c1-2-32(16-17-11-12-22-19(15-17)13-14-34-22)25-29-24(30-26(33)31-25)28-23-20(9-6-10-21(23)27)18-7-4-3-5-8-18/h3-15H,2,16H2,1H3,(H2,28,29,30,31,33) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483004
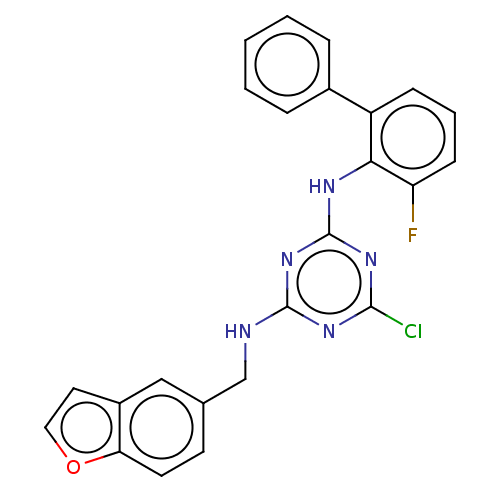
(CHEMBL1270345)Show SMILES Fc1cccc(-c2ccccc2)c1Nc1nc(Cl)nc(NCc2ccc3occc3c2)n1 Show InChI InChI=1S/C24H17ClFN5O/c25-22-29-23(27-14-15-9-10-20-17(13-15)11-12-32-20)31-24(30-22)28-21-18(7-4-8-19(21)26)16-5-2-1-3-6-16/h1-13H,14H2,(H2,27,28,29,30,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483012
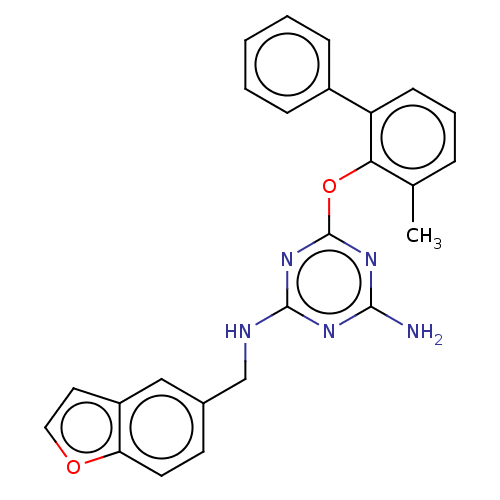
(CHEMBL1270246)Show SMILES Cc1cccc(-c2ccccc2)c1Oc1nc(N)nc(NCc2ccc3occc3c2)n1 Show InChI InChI=1S/C25H21N5O2/c1-16-6-5-9-20(18-7-3-2-4-8-18)22(16)32-25-29-23(26)28-24(30-25)27-15-17-10-11-21-19(14-17)12-13-31-21/h2-14H,15H2,1H3,(H3,26,27,28,29,30) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483010
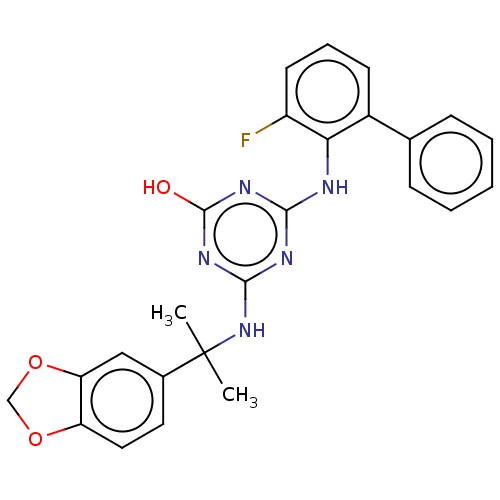
(CHEMBL1271044)Show SMILES CC(C)(Nc1nc(O)nc(Nc2c(F)cccc2-c2ccccc2)n1)c1ccc2OCOc2c1 Show InChI InChI=1S/C25H22FN5O3/c1-25(2,16-11-12-19-20(13-16)34-14-33-19)31-23-28-22(29-24(32)30-23)27-21-17(9-6-10-18(21)26)15-7-4-3-5-8-15/h3-13H,14H2,1-2H3,(H3,27,28,29,30,31,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase |
Bioorg Med Chem Lett 20: 6592-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.034
BindingDB Entry DOI: 10.7270/Q2NG4TG2 |
More data for this
Ligand-Target Pair | |
Tubulin polymerization-promoting protein
(Bos taurus) | BDBM50014846
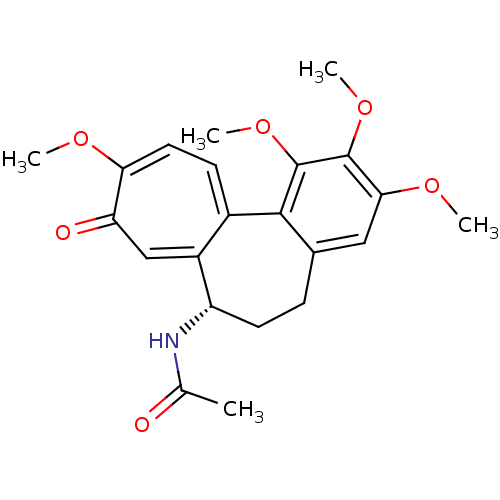
((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...)Show SMILES COc1cc2CC[C@H](NC(C)=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
Rohm and Haas Company
Curated by ChEMBL
| Assay Description
Inhibition of Bos taurus (bovine) brain tubulin polymerization |
Bioorg Med Chem Lett 11: 1393-6 (2001)
BindingDB Entry DOI: 10.7270/Q20V8C18 |
More data for this
Ligand-Target Pair | |
Tubulin polymerization-promoting protein
(Bos taurus) | BDBM50099938
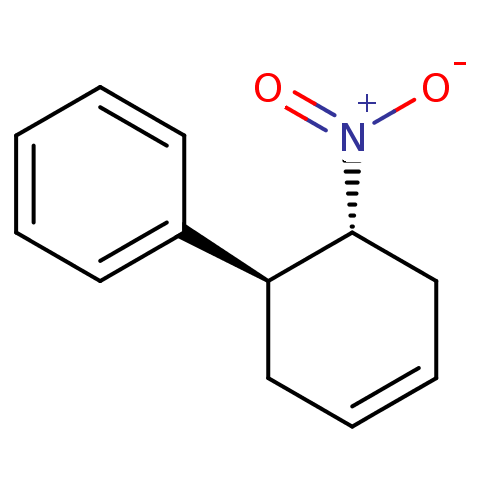
(((1S,6R)-6-Nitro-cyclohex-3-enyl)-benzene | CHEMBL...)Show SMILES [O-][N+](=O)[C@@H]1CC=CC[C@H]1c1ccccc1 |c:5| Show InChI InChI=1S/C12H13NO2/c14-13(15)12-9-5-4-8-11(12)10-6-2-1-3-7-10/h1-7,11-12H,8-9H2/t11-,12+/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Rohm and Haas Company
Curated by ChEMBL
| Assay Description
Inhibition of Bos taurus (bovine) brain tubulin polymerization |
Bioorg Med Chem Lett 11: 1393-6 (2001)
BindingDB Entry DOI: 10.7270/Q20V8C18 |
More data for this
Ligand-Target Pair | |
Tubulin polymerization-promoting protein
(Bos taurus) | BDBM50099944

(1-Methoxy-3-((1S,6R)-6-nitro-cyclohex-3-enyl)-benz...)Show SMILES COc1cccc(c1)[C@@H]1CC=CC[C@H]1[N+]([O-])=O |c:11| Show InChI InChI=1S/C13H15NO3/c1-17-11-6-4-5-10(9-11)12-7-2-3-8-13(12)14(15)16/h2-6,9,12-13H,7-8H2,1H3/t12-,13+/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Rohm and Haas Company
Curated by ChEMBL
| Assay Description
Inhibition of Bos taurus (bovine) brain tubulin polymerization |
Bioorg Med Chem Lett 11: 1393-6 (2001)
BindingDB Entry DOI: 10.7270/Q20V8C18 |
More data for this
Ligand-Target Pair | |
Tubulin polymerization-promoting protein
(Bos taurus) | BDBM50099946

(1,2,3-Trimethoxy-4-(6-nitro-cyclohex-3-enyl)-benze...)Show SMILES COc1ccc(C2CC=CC[C@H]2[N+]([O-])=O)c(OC)c1OC |c:8| Show InChI InChI=1S/C15H19NO5/c1-19-13-9-8-11(14(20-2)15(13)21-3)10-6-4-5-7-12(10)16(17)18/h4-5,8-10,12H,6-7H2,1-3H3/t10?,12-/m1/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Rohm and Haas Company
Curated by ChEMBL
| Assay Description
Inhibition of Bos taurus (bovine) brain tubulin polymerization |
Bioorg Med Chem Lett 11: 1393-6 (2001)
BindingDB Entry DOI: 10.7270/Q20V8C18 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data