 Found 280 hits with Last Name = 'minck' and Initial = 'ko'
Found 280 hits with Last Name = 'minck' and Initial = 'ko' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50010423
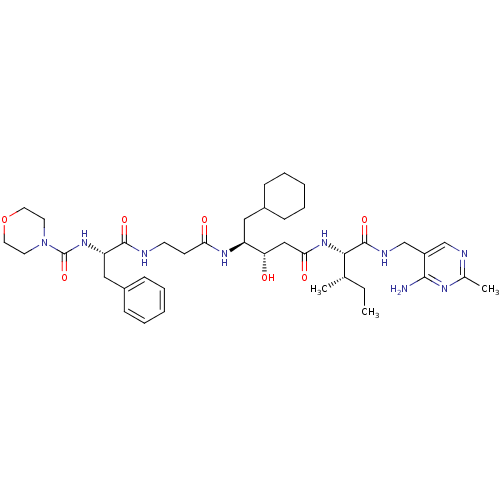
((Morpholinocarbonyl)-Phe-beta-Ala-ACHPA-Ile N-[(4-...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCNC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCOCC1)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C40H61N9O7/c1-4-26(2)36(39(54)44-25-30-24-43-27(3)45-37(30)41)48-35(52)23-33(50)31(21-28-11-7-5-8-12-28)46-34(51)15-16-42-38(53)32(22-29-13-9-6-10-14-29)47-40(55)49-17-19-56-20-18-49/h6,9-10,13-14,24,26,28,31-33,36,50H,4-5,7-8,11-12,15-23,25H2,1-3H3,(H,42,53)(H,44,54)(H,46,51)(H,47,55)(H,48,52)(H2,41,43,45)/t26-,31-,32-,33-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010416
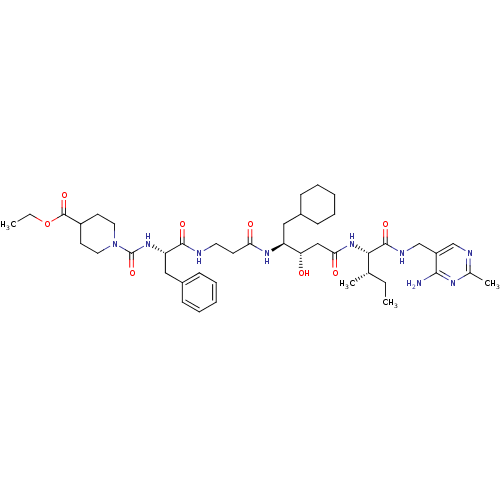
(CHEMBL324692 | [[4-(Ethoxycarbonyl)piperdino]carbo...)Show SMILES CCOC(=O)C1CCN(CC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCCC(=O)N[C@@H](CC1CCCCC1)[C@@H](O)CC(=O)N[C@@H]([C@@H](C)CC)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C44H67N9O8/c1-5-28(3)39(42(58)48-27-33-26-47-29(4)49-40(33)45)52-38(56)25-36(54)34(23-30-13-9-7-10-14-30)50-37(55)17-20-46-41(57)35(24-31-15-11-8-12-16-31)51-44(60)53-21-18-32(19-22-53)43(59)61-6-2/h8,11-12,15-16,26,28,30,32,34-36,39,54H,5-7,9-10,13-14,17-25,27H2,1-4H3,(H,46,57)(H,48,58)(H,50,55)(H,51,60)(H,52,56)(H2,45,47,49)/t28-,34-,35-,36-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010424

(BOC-Phe-Ala-ACHPA-Ile N-[(4-amino-2-methyl-5-pyrim...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C40H62N8O7/c1-8-24(2)34(38(53)43-23-29-22-42-26(4)45-35(29)41)48-33(50)21-32(49)30(19-27-15-11-9-12-16-27)46-36(51)25(3)44-37(52)31(20-28-17-13-10-14-18-28)47-39(54)55-40(5,6)7/h10,13-14,17-18,22,24-25,27,30-32,34,49H,8-9,11-12,15-16,19-21,23H2,1-7H3,(H,43,53)(H,44,52)(H,46,51)(H,47,54)(H,48,50)(H2,41,42,45)/t24-,25-,30-,31-,32-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010419
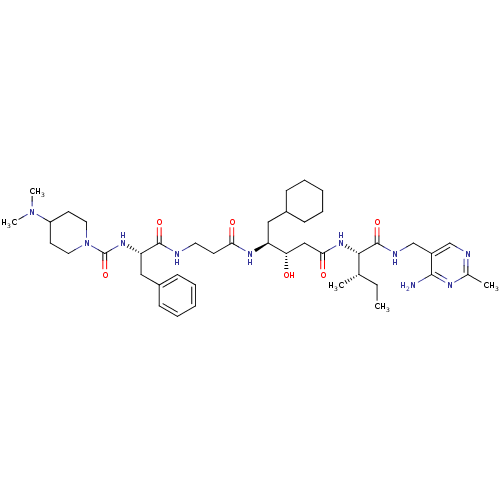
(4-Dimethylamino-piperidine-1-carboxylic acid {1-[2...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCNC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCC(CC1)N(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C43H68N10O6/c1-6-28(2)39(42(58)47-27-32-26-46-29(3)48-40(32)44)51-38(56)25-36(54)34(23-30-13-9-7-10-14-30)49-37(55)17-20-45-41(57)35(24-31-15-11-8-12-16-31)50-43(59)53-21-18-33(19-22-53)52(4)5/h8,11-12,15-16,26,28,30,33-36,39,54H,6-7,9-10,13-14,17-25,27H2,1-5H3,(H,45,57)(H,47,58)(H,49,55)(H,50,59)(H,51,56)(H2,44,46,48)/t28-,34-,35-,36-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010415
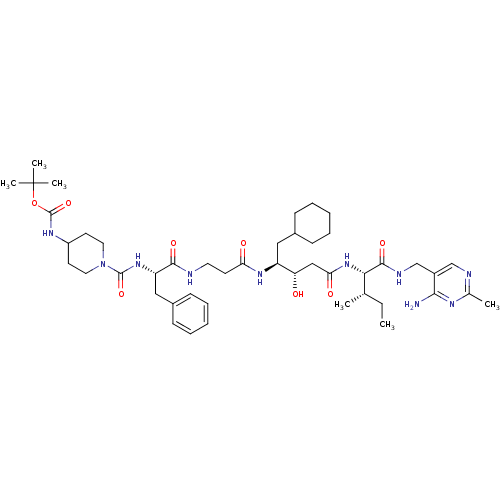
((1-{1-[2-(3-{1-[(4-Amino-2-methyl-pyrimidin-5-ylme...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCNC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCC(CC1)NC(=O)OC(C)(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C46H72N10O8/c1-7-29(2)40(43(61)50-28-33-27-49-30(3)51-41(33)47)55-39(59)26-37(57)35(24-31-14-10-8-11-15-31)53-38(58)18-21-48-42(60)36(25-32-16-12-9-13-17-32)54-44(62)56-22-19-34(20-23-56)52-45(63)64-46(4,5)6/h9,12-13,16-17,27,29,31,34-37,40,57H,7-8,10-11,14-15,18-26,28H2,1-6H3,(H,48,60)(H,50,61)(H,52,63)(H,53,58)(H,54,62)(H,55,59)(H2,47,49,51)/t29-,35-,36-,37-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285833
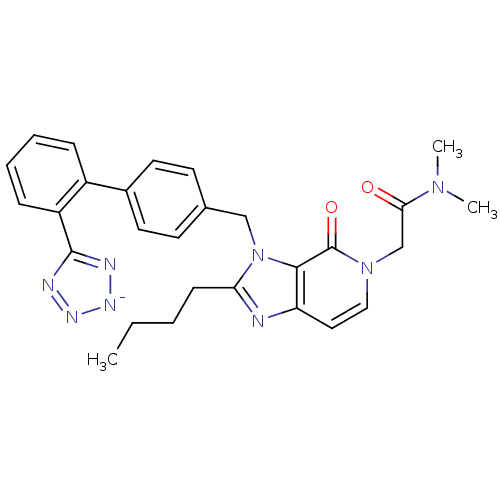
(1N,1N-dimethyl-2-(2-butyl-4-oxo-3-{4-[2-(1H-1,2,3,...)Show SMILES CCCCc1nc2ccn(CC(=O)N(C)C)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C28H29N8O2/c1-4-5-10-24-29-23-15-16-35(18-25(37)34(2)3)28(38)26(23)36(24)17-19-11-13-20(14-12-19)21-8-6-7-9-22(21)27-30-32-33-31-27/h6-9,11-16H,4-5,10,17-18H2,1-3H3/q-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010408
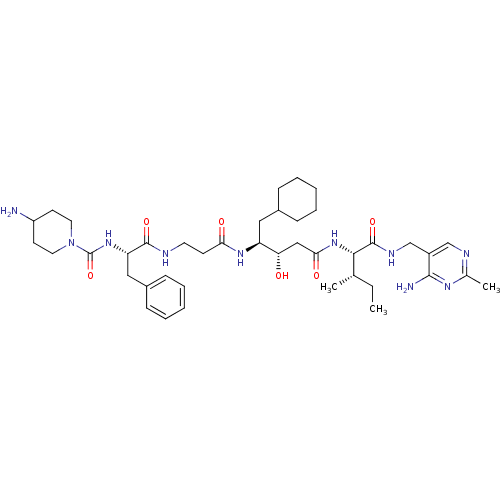
(4-Amino-piperidine-1-carboxylic acid {1-[2-(3-{1-[...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCNC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCC(N)CC1)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C41H64N10O6/c1-4-26(2)37(40(56)46-25-30-24-45-27(3)47-38(30)43)50-36(54)23-34(52)32(21-28-11-7-5-8-12-28)48-35(53)15-18-44-39(55)33(22-29-13-9-6-10-14-29)49-41(57)51-19-16-31(42)17-20-51/h6,9-10,13-14,24,26,28,31-34,37,52H,4-5,7-8,11-12,15-23,25,42H2,1-3H3,(H,44,55)(H,46,56)(H,48,53)(H,49,57)(H,50,54)(H2,43,45,47)/t26-,32-,33-,34-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010410
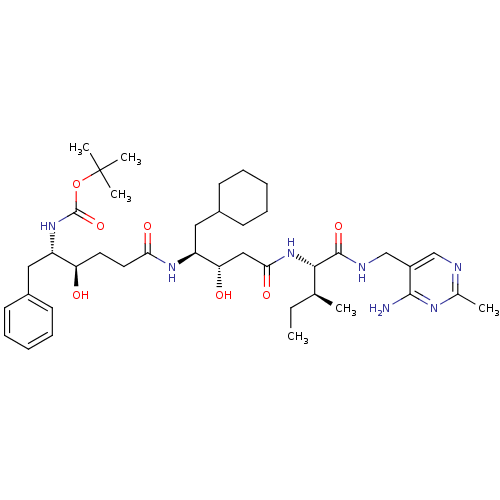
(CHEMBL323006 | [4-(3-{1-[(4-Amino-2-methyl-pyrimid...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C40H63N7O7/c1-7-25(2)36(38(52)43-24-29-23-42-26(3)44-37(29)41)47-35(51)22-33(49)31(21-28-16-12-9-13-17-28)45-34(50)19-18-32(48)30(20-27-14-10-8-11-15-27)46-39(53)54-40(4,5)6/h8,10-11,14-15,23,25,28,30-33,36,48-49H,7,9,12-13,16-22,24H2,1-6H3,(H,43,52)(H,45,50)(H,46,53)(H,47,51)(H2,41,42,44)/t25-,30-,31-,32+,33-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010431
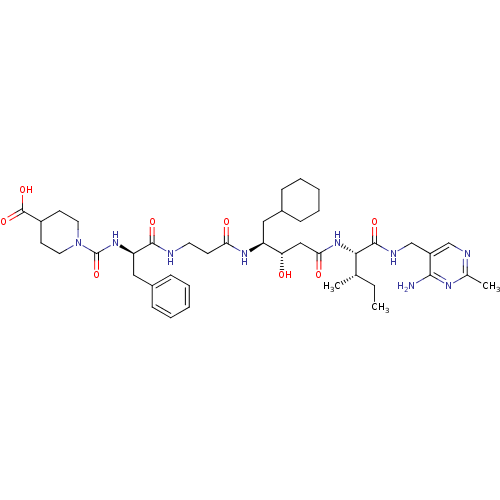
(CHEMBL320726 | [4-(Carboxypiperidino)carbonyl]-Phe...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCNC(=O)[C@@H](Cc1ccccc1)NC(=O)N1CCC(CC1)C(O)=O)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C42H63N9O8/c1-4-26(2)37(40(56)46-25-31-24-45-27(3)47-38(31)43)50-36(54)23-34(52)32(21-28-11-7-5-8-12-28)48-35(53)15-18-44-39(55)33(22-29-13-9-6-10-14-29)49-42(59)51-19-16-30(17-20-51)41(57)58/h6,9-10,13-14,24,26,28,30,32-34,37,52H,4-5,7-8,11-12,15-23,25H2,1-3H3,(H,44,55)(H,46,56)(H,48,53)(H,49,59)(H,50,54)(H,57,58)(H2,43,45,47)/t26-,32-,33+,34-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010421
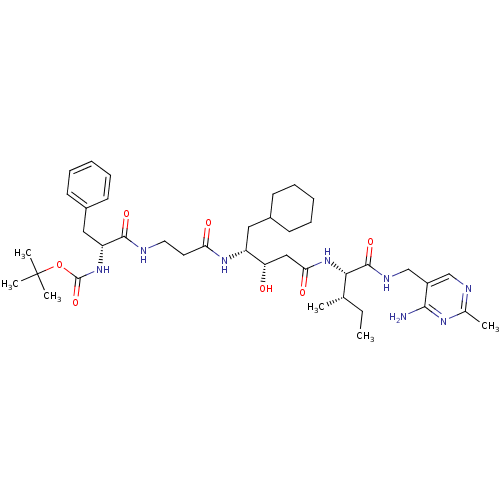
(BOC-Phe-beta-Ala-ACHPA-Ile N-[(4-amino-2-methyl-5-...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@@H](CC1CCCCC1)NC(=O)CCNC(=O)[C@@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C40H62N8O7/c1-7-25(2)35(38(53)44-24-29-23-43-26(3)45-36(29)41)48-34(51)22-32(49)30(20-27-14-10-8-11-15-27)46-33(50)18-19-42-37(52)31(21-28-16-12-9-13-17-28)47-39(54)55-40(4,5)6/h9,12-13,16-17,23,25,27,30-32,35,49H,7-8,10-11,14-15,18-22,24H2,1-6H3,(H,42,52)(H,44,53)(H,46,50)(H,47,54)(H,48,51)(H2,41,43,45)/t25-,30+,31+,32-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17941
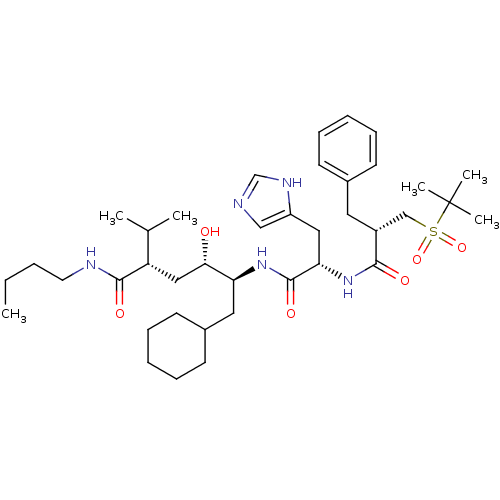
((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30-,32+,33+,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro inhibition of human renin. |
J Med Chem 37: 486-97 (1994)
BindingDB Entry DOI: 10.7270/Q2MW2HTW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50368156
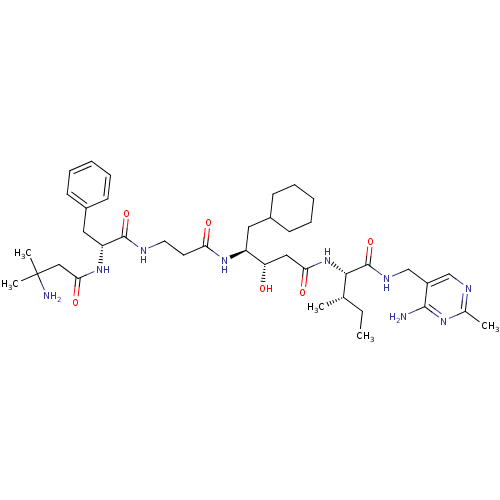
(CHEMBL1203096)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCNC(=O)[C@@H](Cc1ccccc1)NC(=O)CC(C)(C)N)C(=O)NCc1cnc(C)nc1N |r| Show InChI InChI=1S/C40H63N9O6/c1-6-25(2)36(39(55)45-24-29-23-44-26(3)46-37(29)41)49-34(52)21-32(50)30(19-27-13-9-7-10-14-27)47-33(51)17-18-43-38(54)31(20-28-15-11-8-12-16-28)48-35(53)22-40(4,5)42/h8,11-12,15-16,23,25,27,30-32,36,50H,6-7,9-10,13-14,17-22,24,42H2,1-5H3,(H,43,54)(H,45,55)(H,47,51)(H,48,53)(H,49,52)(H2,41,44,46)/t25-,30-,31+,32-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010426
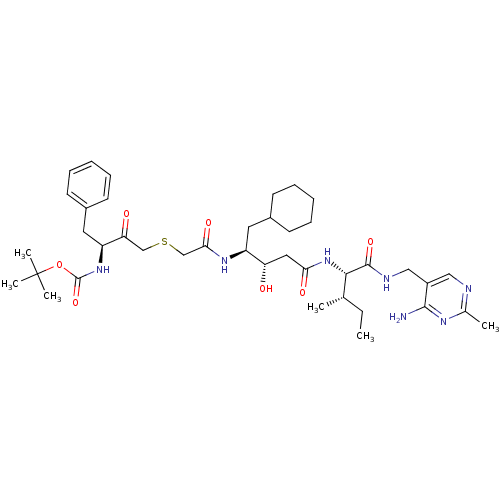
(CHEMBL324588 | {3-[(3-{1-[(4-Amino-2-methyl-pyrimi...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CSCC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C40H61N7O7S/c1-7-25(2)36(38(52)43-22-29-21-42-26(3)44-37(29)41)47-34(50)20-32(48)30(18-27-14-10-8-11-15-27)45-35(51)24-55-23-33(49)31(19-28-16-12-9-13-17-28)46-39(53)54-40(4,5)6/h9,12-13,16-17,21,25,27,30-32,36,48H,7-8,10-11,14-15,18-20,22-24H2,1-6H3,(H,43,52)(H,45,51)(H,46,53)(H,47,50)(H2,41,42,44)/t25-,30-,31-,32-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010433
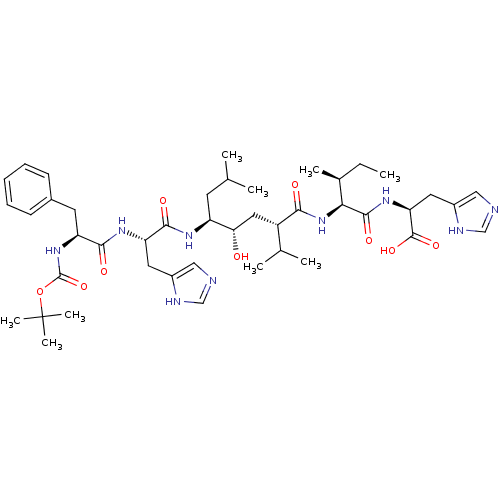
(BOC-Phe-His-Leu-psi-[CHOHCH2]Val-Ile-His-OH | CHEM...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O Show InChI InChI=1S/C44H67N9O9/c1-10-27(6)37(41(58)51-35(42(59)60)19-30-22-46-24-48-30)53-38(55)31(26(4)5)20-36(54)32(16-25(2)3)49-40(57)34(18-29-21-45-23-47-29)50-39(56)33(17-28-14-12-11-13-15-28)52-43(61)62-44(7,8)9/h11-15,21-27,31-37,54H,10,16-20H2,1-9H3,(H,45,47)(H,46,48)(H,49,57)(H,50,56)(H,51,58)(H,52,61)(H,53,55)(H,59,60)/t27-,31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50040384
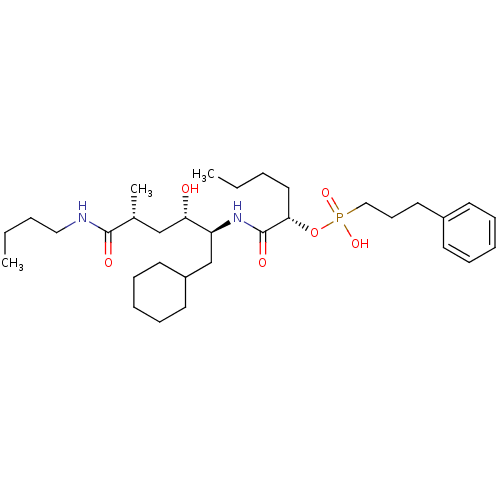
((3-Phenyl-propyl)-phosphonic acid mono-[(S)-1-((1S...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCC)OP(O)(=O)CCCc1ccccc1 Show InChI InChI=1S/C32H55N2O6P/c1-4-6-20-30(40-41(38,39)22-14-19-26-15-10-8-11-16-26)32(37)34-28(24-27-17-12-9-13-18-27)29(35)23-25(3)31(36)33-21-7-5-2/h8,10-11,15-16,25,27-30,35H,4-7,9,12-14,17-24H2,1-3H3,(H,33,36)(H,34,37)(H,38,39)/t25-,28+,29+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro inhibition of human renin. |
J Med Chem 37: 486-97 (1994)
BindingDB Entry DOI: 10.7270/Q2MW2HTW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010427
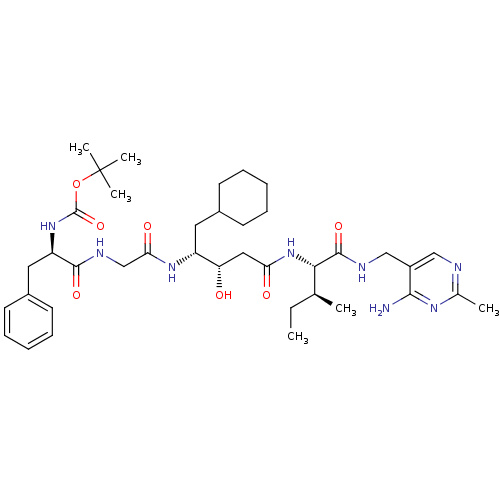
(BOC-Phe-Gly-ACHPA-Ile N-[(4-amino-2-methyl-5-pyrim...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@@H](CC1CCCCC1)NC(=O)CNC(=O)[C@@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C39H60N8O7/c1-7-24(2)34(37(52)42-22-28-21-41-25(3)44-35(28)40)47-32(49)20-31(48)29(18-26-14-10-8-11-15-26)45-33(50)23-43-36(51)30(19-27-16-12-9-13-17-27)46-38(53)54-39(4,5)6/h9,12-13,16-17,21,24,26,29-31,34,48H,7-8,10-11,14-15,18-20,22-23H2,1-6H3,(H,42,52)(H,43,51)(H,45,50)(H,46,53)(H,47,49)(H2,40,41,44)/t24-,29+,30+,31-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50002969
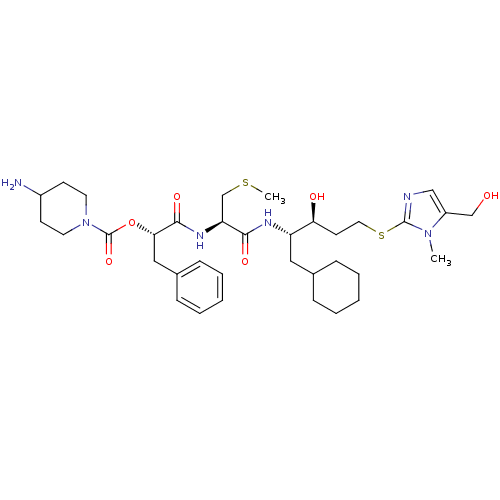
(4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...)Show SMILES CSC[C@H](NC(=O)[C@H](Cc1ccccc1)OC(=O)N1CCC(N)CC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)CCSc1ncc(CO)n1C Show InChI InChI=1S/C35H54N6O6S2/c1-40-27(22-42)21-37-34(40)49-18-15-30(43)28(19-24-9-5-3-6-10-24)38-32(44)29(23-48-2)39-33(45)31(20-25-11-7-4-8-12-25)47-35(46)41-16-13-26(36)14-17-41/h4,7-8,11-12,21,24,26,28-31,42-43H,3,5-6,9-10,13-20,22-23,36H2,1-2H3,(H,38,44)(H,39,45)/t28-,29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. |
J Med Chem 35: 3525-36 (1992)
BindingDB Entry DOI: 10.7270/Q2DB80S4 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50040390
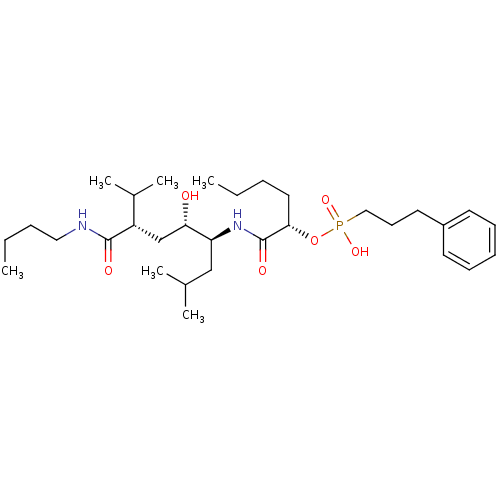
((3-Phenyl-propyl)-phosphonic acid mono-[(S)-1-((1S...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCCC)OP(O)(=O)CCCc1ccccc1)C(C)C Show InChI InChI=1S/C31H55N2O6P/c1-7-9-18-29(39-40(37,38)20-14-17-25-15-12-11-13-16-25)31(36)33-27(21-23(3)4)28(34)22-26(24(5)6)30(35)32-19-10-8-2/h11-13,15-16,23-24,26-29,34H,7-10,14,17-22H2,1-6H3,(H,32,35)(H,33,36)(H,37,38)/t26-,27-,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro inhibition of human renin. |
J Med Chem 37: 486-97 (1994)
BindingDB Entry DOI: 10.7270/Q2MW2HTW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010432
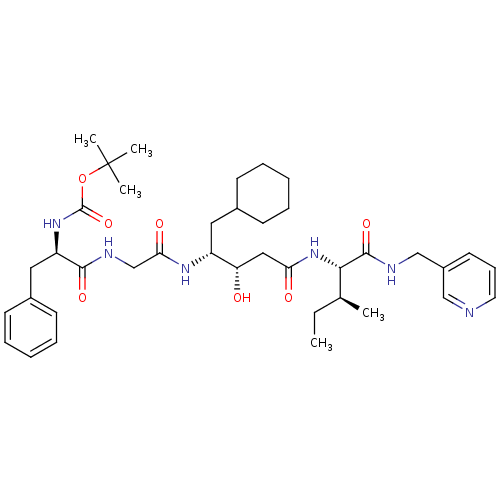
(BOC-Phe-Gly-ACHPA-Ile N-(3-Pyridylmethyl)amide | C...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@@H](CC1CCCCC1)NC(=O)CNC(=O)[C@@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1cccnc1 Show InChI InChI=1S/C39H58N6O7/c1-6-26(2)35(37(50)41-24-29-18-13-19-40-23-29)45-33(47)22-32(46)30(20-27-14-9-7-10-15-27)43-34(48)25-42-36(49)31(21-28-16-11-8-12-17-28)44-38(51)52-39(3,4)5/h8,11-13,16-19,23,26-27,30-32,35,46H,6-7,9-10,14-15,20-22,24-25H2,1-5H3,(H,41,50)(H,42,49)(H,43,48)(H,44,51)(H,45,47)/t26-,30+,31+,32-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285818
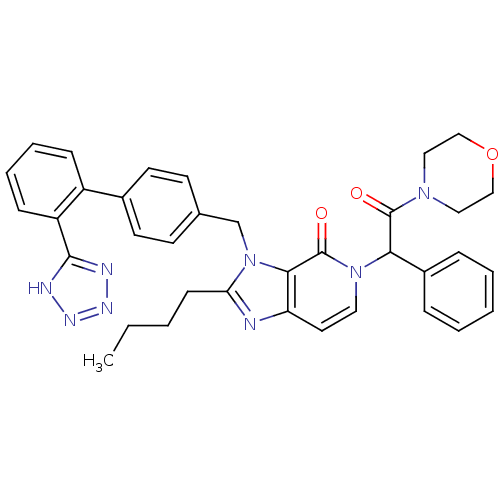
(2-Butyl-5-(2-morpholin-4-yl-2-oxo-1-phenyl-ethyl)-...)Show SMILES CCCCc1nc2ccn(C(C(=O)N3CCOCC3)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C36H36N8O3/c1-2-3-13-31-37-30-18-19-43(32(27-9-5-4-6-10-27)35(45)42-20-22-47-23-21-42)36(46)33(30)44(31)24-25-14-16-26(17-15-25)28-11-7-8-12-29(28)34-38-40-41-39-34/h4-12,14-19,32H,2-3,13,20-24H2,1H3,(H,38,39,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010429
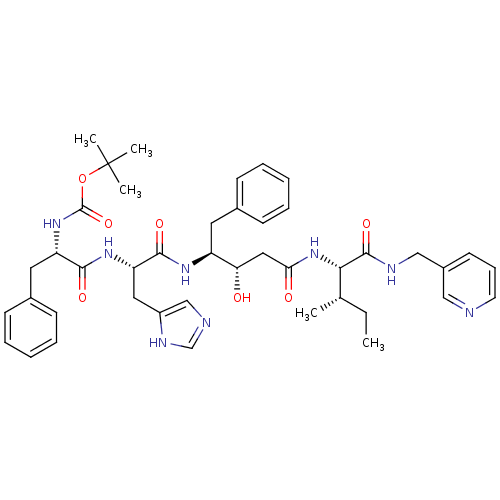
(CHEMBL323967 | {1-[1-(1-Cyclohexylmethyl-2-hydroxy...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1cccnc1 Show InChI InChI=1S/C43H56N8O7/c1-6-28(2)38(41(56)46-25-31-18-13-19-44-24-31)51-37(53)23-36(52)33(20-29-14-9-7-10-15-29)48-40(55)35(22-32-26-45-27-47-32)49-39(54)34(21-30-16-11-8-12-17-30)50-42(57)58-43(3,4)5/h7-19,24,26-28,33-36,38,52H,6,20-23,25H2,1-5H3,(H,45,47)(H,46,56)(H,48,55)(H,49,54)(H,50,57)(H,51,53)/t28-,33-,34-,35-,36-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285832
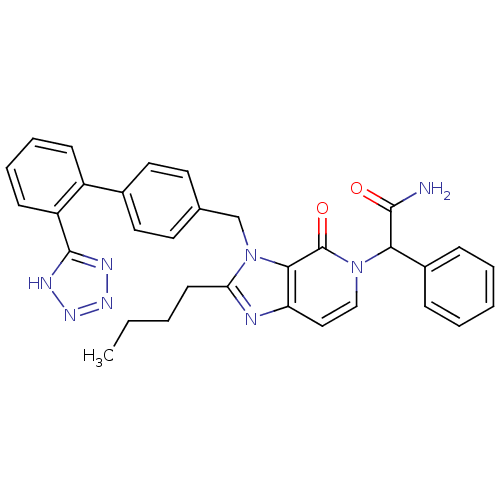
(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(C(C(N)=O)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C32H30N8O2/c1-2-3-13-27-34-26-18-19-39(28(30(33)41)23-9-5-4-6-10-23)32(42)29(26)40(27)20-21-14-16-22(17-15-21)24-11-7-8-12-25(24)31-35-37-38-36-31/h4-12,14-19,28H,2-3,13,20H2,1H3,(H2,33,41)(H,35,36,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285820
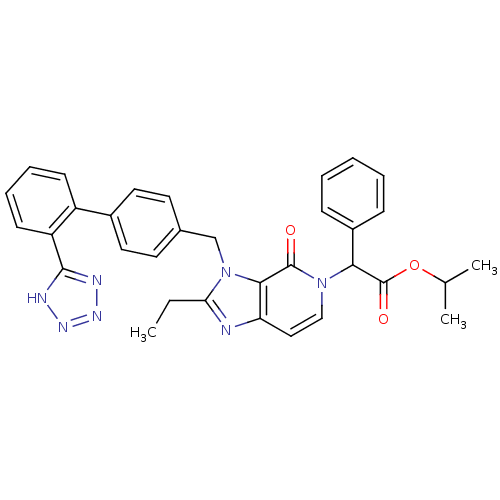
(CHEMBL88663 | {2-Ethyl-4-oxo-3-[2'-(1H-tetrazol-5-...)Show SMILES CCc1nc2ccn(C(C(=O)OC(C)C)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C33H31N7O3/c1-4-28-34-27-18-19-39(29(33(42)43-21(2)3)24-10-6-5-7-11-24)32(41)30(27)40(28)20-22-14-16-23(17-15-22)25-12-8-9-13-26(25)31-35-37-38-36-31/h5-19,21,29H,4,20H2,1-3H3,(H,35,36,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285829

(CHEMBL314603 | {4-Oxo-2-propyl-3-[2'-(1H-tetrazol-...)Show SMILES CCCc1nc2ccn(C(C(=O)OC(C)C)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C34H33N7O3/c1-4-10-29-35-28-19-20-40(30(34(43)44-22(2)3)25-11-6-5-7-12-25)33(42)31(28)41(29)21-23-15-17-24(18-16-23)26-13-8-9-14-27(26)32-36-38-39-37-32/h5-9,11-20,22,30H,4,10,21H2,1-3H3,(H,36,37,38,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285834
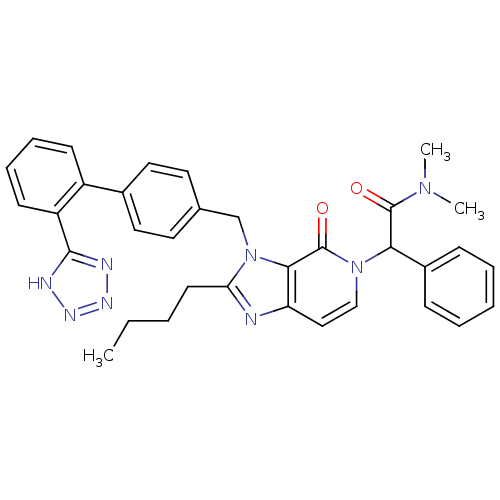
(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(C(C(=O)N(C)C)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C34H34N8O2/c1-4-5-15-29-35-28-20-21-41(30(33(43)40(2)3)25-11-7-6-8-12-25)34(44)31(28)42(29)22-23-16-18-24(19-17-23)26-13-9-10-14-27(26)32-36-38-39-37-32/h6-14,16-21,30H,4-5,15,22H2,1-3H3,(H,36,37,38,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50450370
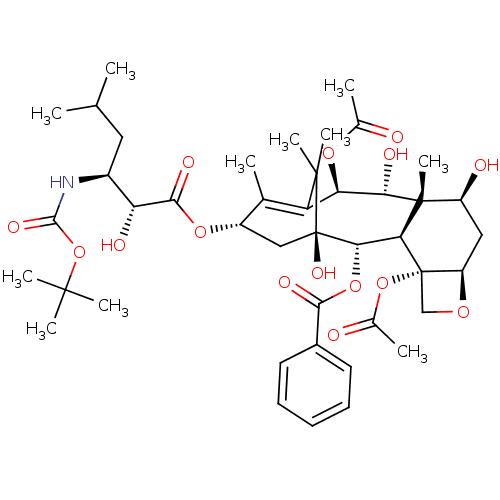
(CHEMBL313691)Show SMILES [H][C@@]12C[C@H](O)[C@@]3(C)[C@@H](O)[C@H](OC(C)=O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](O)[C@H](CC(C)C)NC(=O)OC(C)(C)C |c:14| Show InChI InChI=1S/C43H61NO15/c1-21(2)17-26(44-38(52)59-39(6,7)8)31(48)37(51)56-27-19-43(53)35(57-36(50)25-15-13-12-14-16-25)33-41(11,28(47)18-29-42(33,20-54-29)58-24(5)46)34(49)32(55-23(4)45)30(22(27)3)40(43,9)10/h12-16,21,26-29,31-35,47-49,53H,17-20H2,1-11H3,(H,44,52)/t26-,27-,28-,29+,31+,32+,33-,34-,35-,41+,42-,43+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50040388
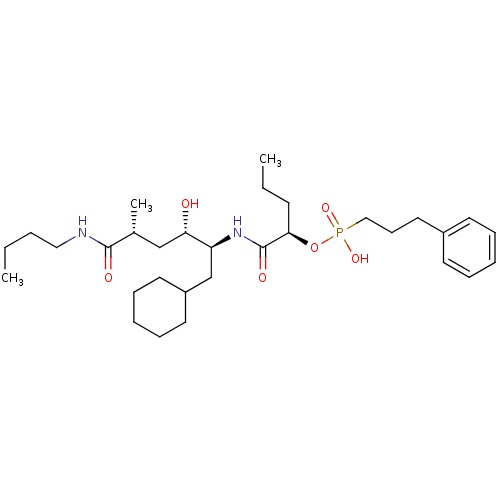
((3-Phenyl-propyl)-phosphonic acid mono-[(R)-1-((1S...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@@H](CCC)OP(O)(=O)CCCc1ccccc1 Show InChI InChI=1S/C31H53N2O6P/c1-4-6-20-32-30(35)24(3)22-28(34)27(23-26-17-11-8-12-18-26)33-31(36)29(14-5-2)39-40(37,38)21-13-19-25-15-9-7-10-16-25/h7,9-10,15-16,24,26-29,34H,4-6,8,11-14,17-23H2,1-3H3,(H,32,35)(H,33,36)(H,37,38)/t24-,27+,28+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro inhibition of human renin. |
J Med Chem 37: 486-97 (1994)
BindingDB Entry DOI: 10.7270/Q2MW2HTW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285813
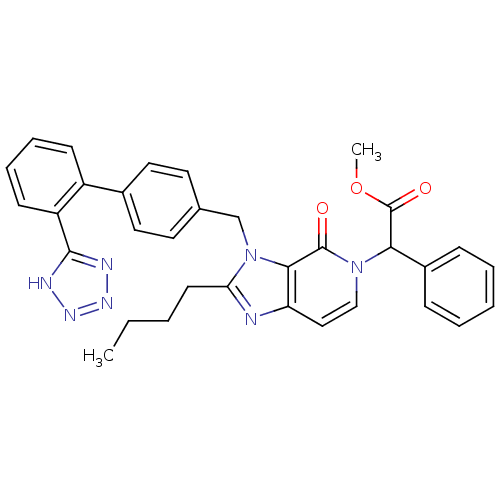
(CHEMBL316063 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5...)Show SMILES CCCCc1nc2ccn(C(C(=O)OC)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C33H31N7O3/c1-3-4-14-28-34-27-19-20-39(29(33(42)43-2)24-10-6-5-7-11-24)32(41)30(27)40(28)21-22-15-17-23(18-16-22)25-12-8-9-13-26(25)31-35-37-38-36-31/h5-13,15-20,29H,3-4,14,21H2,1-2H3,(H,35,36,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285821
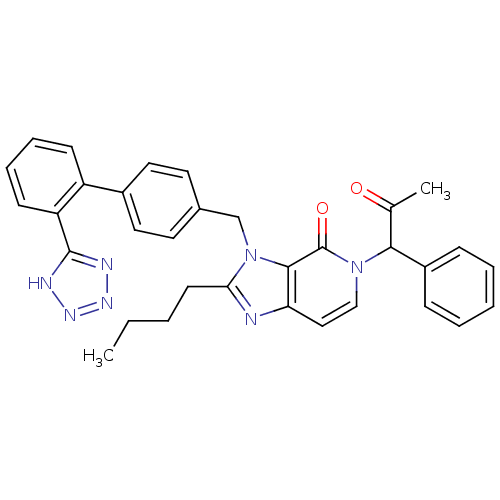
(2-Butyl-5-(2-oxo-1-phenyl-propyl)-3-[2'-(1H-tetraz...)Show SMILES CCCCc1nc2ccn(C(C(C)=O)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C33H31N7O2/c1-3-4-14-29-34-28-19-20-39(30(22(2)41)25-10-6-5-7-11-25)33(42)31(28)40(29)21-23-15-17-24(18-16-23)26-12-8-9-13-27(26)32-35-37-38-36-32/h5-13,15-20,30H,3-4,14,21H2,1-2H3,(H,35,36,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010417
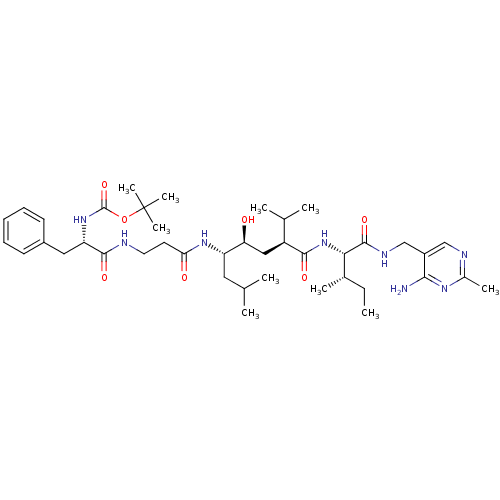
(BOC-Phe-beta-Ala-Leu-psi[CHOHCH2]Val-Ile N-[(4-ami...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)CCNC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C41H66N8O7/c1-11-26(6)35(39(54)45-23-29-22-44-27(7)46-36(29)42)49-37(52)30(25(4)5)21-33(50)31(19-24(2)3)47-34(51)17-18-43-38(53)32(20-28-15-13-12-14-16-28)48-40(55)56-41(8,9)10/h12-16,22,24-26,30-33,35,50H,11,17-21,23H2,1-10H3,(H,43,53)(H,45,54)(H,47,51)(H,48,55)(H,49,52)(H2,42,44,46)/t26-,30-,31-,32-,33-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010413
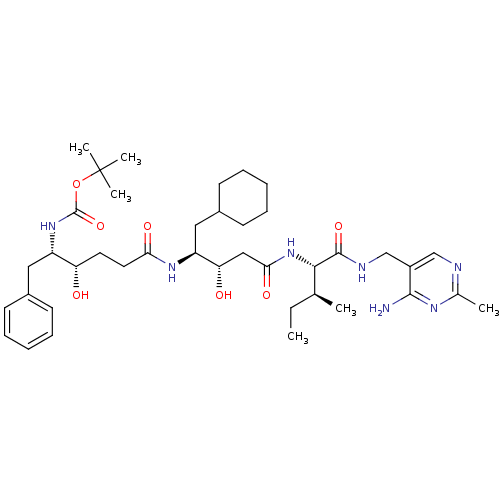
(CHEMBL324624 | [4-(3-{1-[(4-Amino-2-methyl-pyrimid...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CC[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C40H63N7O7/c1-7-25(2)36(38(52)43-24-29-23-42-26(3)44-37(29)41)47-35(51)22-33(49)31(21-28-16-12-9-13-17-28)45-34(50)19-18-32(48)30(20-27-14-10-8-11-15-27)46-39(53)54-40(4,5)6/h8,10-11,14-15,23,25,28,30-33,36,48-49H,7,9,12-13,16-22,24H2,1-6H3,(H,43,52)(H,45,50)(H,46,53)(H,47,51)(H2,41,42,44)/t25-,30-,31-,32-,33-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285816
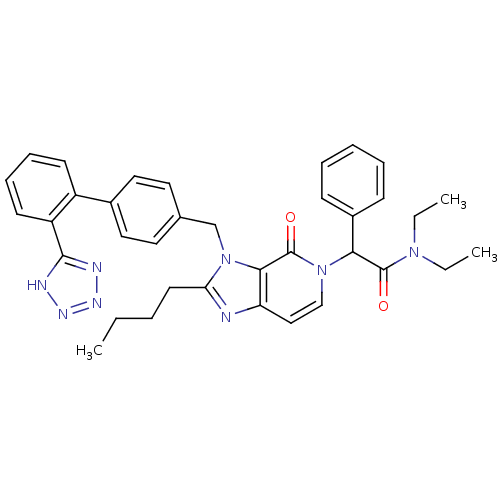
(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(C(C(=O)N(CC)CC)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C36H38N8O2/c1-4-7-17-31-37-30-22-23-43(32(27-13-9-8-10-14-27)35(45)42(5-2)6-3)36(46)33(30)44(31)24-25-18-20-26(21-19-25)28-15-11-12-16-29(28)34-38-40-41-39-34/h8-16,18-23,32H,4-7,17,24H2,1-3H3,(H,38,39,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50040383
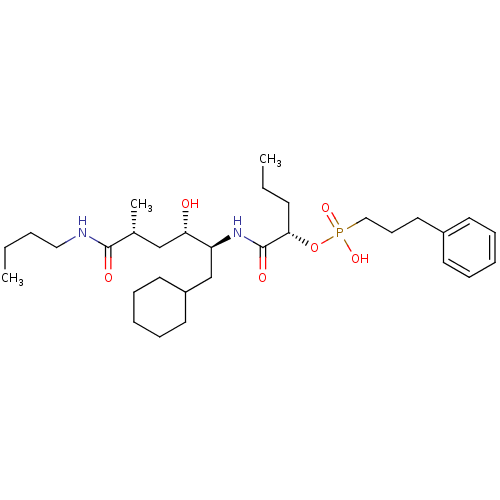
((3-Phenyl-propyl)-phosphonic acid mono-[(S)-1-((1S...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCC)OP(O)(=O)CCCc1ccccc1 Show InChI InChI=1S/C31H53N2O6P/c1-4-6-20-32-30(35)24(3)22-28(34)27(23-26-17-11-8-12-18-26)33-31(36)29(14-5-2)39-40(37,38)21-13-19-25-15-9-7-10-16-25/h7,9-10,15-16,24,26-29,34H,4-6,8,11-14,17-23H2,1-3H3,(H,32,35)(H,33,36)(H,37,38)/t24-,27+,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro inhibition of human renin. |
J Med Chem 37: 486-97 (1994)
BindingDB Entry DOI: 10.7270/Q2MW2HTW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285835
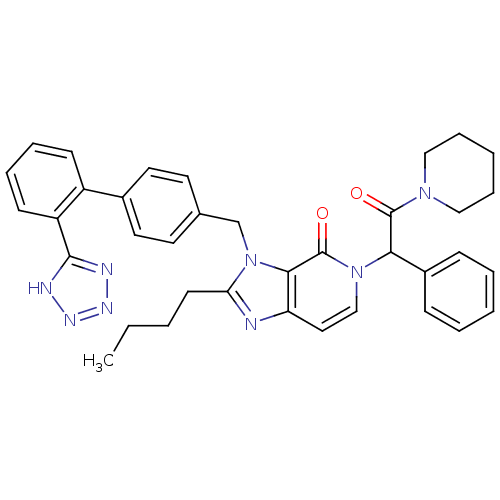
(2-Butyl-5-(2-oxo-1-phenyl-2-piperidin-1-yl-ethyl)-...)Show SMILES CCCCc1nc2ccn(C(C(=O)N3CCCCC3)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C37H38N8O2/c1-2-3-16-32-38-31-21-24-44(33(28-12-6-4-7-13-28)36(46)43-22-10-5-11-23-43)37(47)34(31)45(32)25-26-17-19-27(20-18-26)29-14-8-9-15-30(29)35-39-41-42-40-35/h4,6-9,12-15,17-21,24,33H,2-3,5,10-11,16,22-23,25H2,1H3,(H,39,40,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50368157
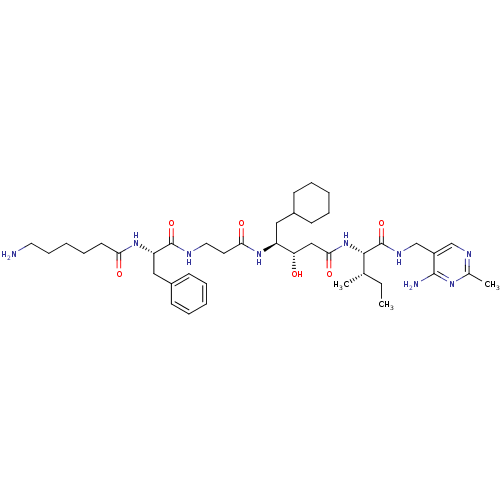
(CHEMBL1203095)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCNC(=O)[C@H](Cc1ccccc1)NC(=O)CCCCCN)C(=O)NCc1cnc(C)nc1N |r| Show InChI InChI=1S/C41H65N9O6/c1-4-27(2)38(41(56)46-26-31-25-45-28(3)47-39(31)43)50-37(54)24-34(51)32(22-29-14-8-5-9-15-29)48-36(53)19-21-44-40(55)33(23-30-16-10-6-11-17-30)49-35(52)18-12-7-13-20-42/h6,10-11,16-17,25,27,29,32-34,38,51H,4-5,7-9,12-15,18-24,26,42H2,1-3H3,(H,44,55)(H,46,56)(H,48,53)(H,49,52)(H,50,54)(H2,43,45,47)/t27-,32-,33-,34-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50368155
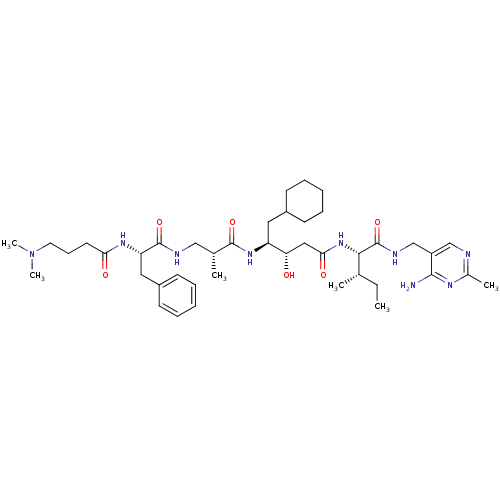
(CHEMBL1203094)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](C)CNC(=O)[C@H](Cc1ccccc1)NC(=O)CCCN(C)C)C(=O)NCc1cnc(C)nc1N |r| Show InChI InChI=1S/C42H67N9O6/c1-7-27(2)38(42(57)46-26-32-25-44-29(4)47-39(32)43)50-37(54)23-35(52)33(21-30-15-10-8-11-16-30)49-40(55)28(3)24-45-41(56)34(22-31-17-12-9-13-18-31)48-36(53)19-14-20-51(5)6/h9,12-13,17-18,25,27-28,30,33-35,38,52H,7-8,10-11,14-16,19-24,26H2,1-6H3,(H,45,56)(H,46,57)(H,48,53)(H,49,55)(H,50,54)(H2,43,44,47)/t27-,28+,33-,34-,35-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
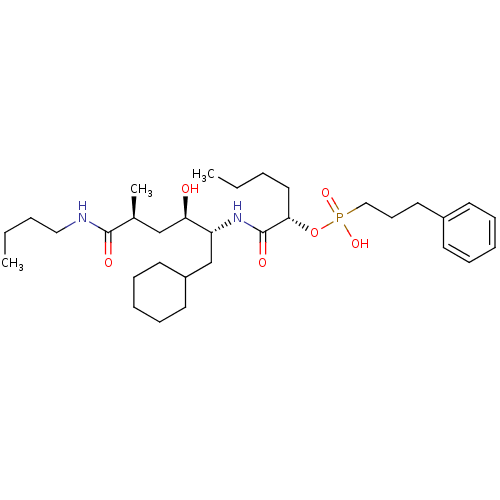
Renin
(Homo sapiens (Human)) | BDBM50040382
((3-Phenyl-propyl)-phosphonic acid mono-[(S)-1-((1R...)Show SMILES CCCCNC(=O)[C@@H](C)C[C@@H](O)[C@@H](CC1CCCCC1)NC(=O)[C@H](CCCC)OP(O)(=O)CCCc1ccccc1 Show InChI InChI=1S/C32H55N2O6P/c1-4-6-20-30(40-41(38,39)22-14-19-26-15-10-8-11-16-26)32(37)34-28(24-27-17-12-9-13-18-27)29(35)23-25(3)31(36)33-21-7-5-2/h8,10-11,15-16,25,27-30,35H,4-7,9,12-14,17-24H2,1-3H3,(H,33,36)(H,34,37)(H,38,39)/t25-,28+,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro inhibition of human renin. |
J Med Chem 37: 486-97 (1994)
BindingDB Entry DOI: 10.7270/Q2MW2HTW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50002985

(4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...)Show SMILES CSC[C@H](NC(=O)[C@H](Cc1ccccc1)OC(=O)N1CCC(N)CC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)CCSc1nccs1 Show InChI InChI=1S/C33H49N5O5S3/c1-44-22-27(30(40)36-26(20-23-8-4-2-5-9-23)28(39)14-18-45-32-35-15-19-46-32)37-31(41)29(21-24-10-6-3-7-11-24)43-33(42)38-16-12-25(34)13-17-38/h3,6-7,10-11,15,19,23,25-29,39H,2,4-5,8-9,12-14,16-18,20-22,34H2,1H3,(H,36,40)(H,37,41)/t26-,27-,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. |
J Med Chem 35: 3525-36 (1992)
BindingDB Entry DOI: 10.7270/Q2DB80S4 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010428
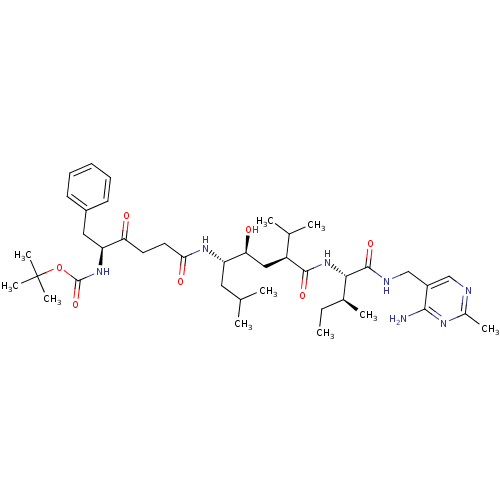
(BOC-Phe-psi[COCH2]Gly-Leu-psi-[CHOHCH2]Val-Ile N-[...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C41H65N7O7/c1-11-26(6)36(39(53)44-23-29-22-43-27(7)45-37(29)42)48-38(52)30(25(4)5)21-34(50)31(19-24(2)3)46-35(51)18-17-33(49)32(20-28-15-13-12-14-16-28)47-40(54)55-41(8,9)10/h12-16,22,24-26,30-32,34,36,50H,11,17-21,23H2,1-10H3,(H,44,53)(H,46,51)(H,47,54)(H,48,52)(H2,42,43,45)/t26-,30-,31-,32-,34-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010412
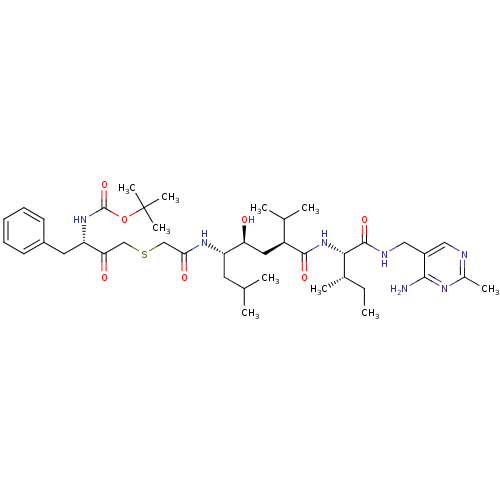
(BOC-Phe-psi[COCH2S]Gly-Leu-psi-[CHOHCH2]Val-Ile N-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)CSCC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C41H65N7O7S/c1-11-26(6)36(39(53)44-21-29-20-43-27(7)45-37(29)42)48-38(52)30(25(4)5)19-33(49)31(17-24(2)3)46-35(51)23-56-22-34(50)32(18-28-15-13-12-14-16-28)47-40(54)55-41(8,9)10/h12-16,20,24-26,30-33,36,49H,11,17-19,21-23H2,1-10H3,(H,44,53)(H,46,51)(H,47,54)(H,48,52)(H2,42,43,45)/t26-,30-,31-,32-,33-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010425
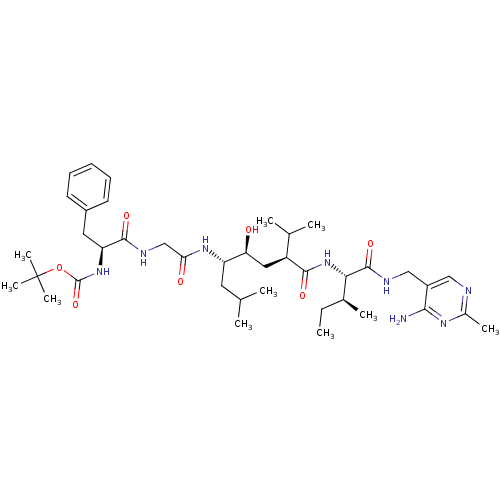
(BOC-Phe-Gly-Leu-psi[CHOHCH2]Gly-Ile N-[(4-amino-2-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C40H64N8O7/c1-11-25(6)34(38(53)43-21-28-20-42-26(7)45-35(28)41)48-36(51)29(24(4)5)19-32(49)30(17-23(2)3)46-33(50)22-44-37(52)31(18-27-15-13-12-14-16-27)47-39(54)55-40(8,9)10/h12-16,20,23-25,29-32,34,49H,11,17-19,21-22H2,1-10H3,(H,43,53)(H,44,52)(H,46,50)(H,47,54)(H,48,51)(H2,41,42,45)/t25-,29-,30-,31-,32-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010409
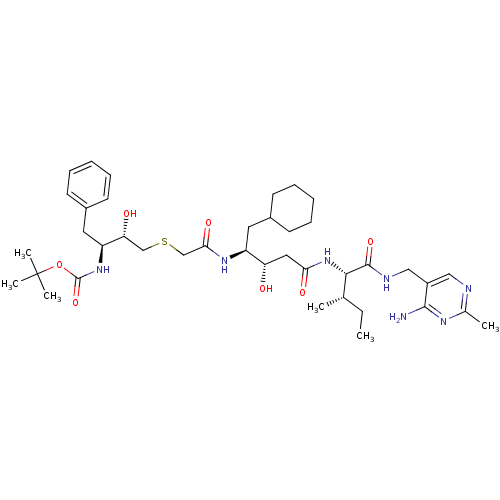
(CHEMBL112091 | {3-[(3-{1-[(4-Amino-2-methyl-pyrimi...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CSC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C40H63N7O7S/c1-7-25(2)36(38(52)43-22-29-21-42-26(3)44-37(29)41)47-34(50)20-32(48)30(18-27-14-10-8-11-15-27)45-35(51)24-55-23-33(49)31(19-28-16-12-9-13-17-28)46-39(53)54-40(4,5)6/h9,12-13,16-17,21,25,27,30-33,36,48-49H,7-8,10-11,14-15,18-20,22-24H2,1-6H3,(H,43,52)(H,45,51)(H,46,53)(H,47,50)(H2,41,42,44)/t25-,30-,31-,32-,33+,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285826
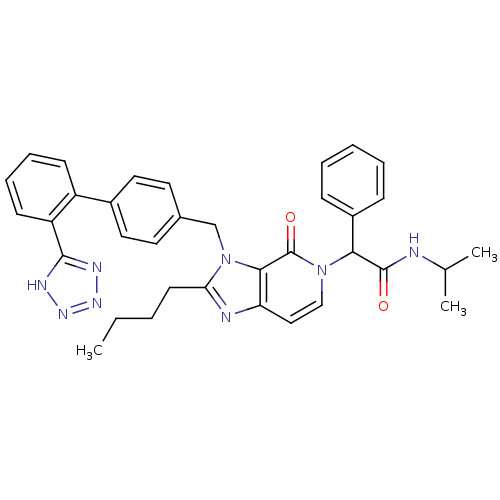
(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(C(C(=O)NC(C)C)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C35H36N8O2/c1-4-5-15-30-37-29-20-21-42(31(34(44)36-23(2)3)26-11-7-6-8-12-26)35(45)32(29)43(30)22-24-16-18-25(19-17-24)27-13-9-10-14-28(27)33-38-40-41-39-33/h6-14,16-21,23,31H,4-5,15,22H2,1-3H3,(H,36,44)(H,38,39,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010405
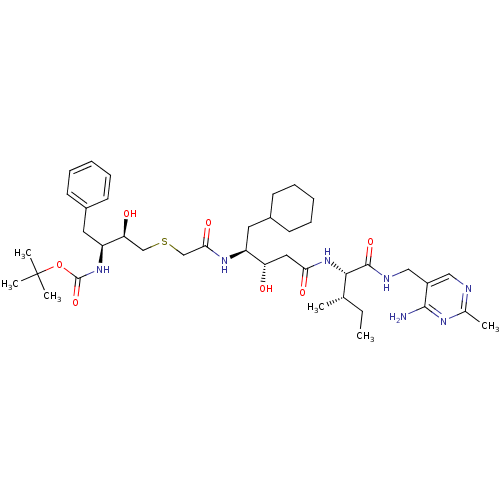
(CHEMBL113593 | {3-[(3-{1-[(4-Amino-2-methyl-pyrimi...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CSC[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C40H63N7O7S/c1-7-25(2)36(38(52)43-22-29-21-42-26(3)44-37(29)41)47-34(50)20-32(48)30(18-27-14-10-8-11-15-27)45-35(51)24-55-23-33(49)31(19-28-16-12-9-13-17-28)46-39(53)54-40(4,5)6/h9,12-13,16-17,21,25,27,30-33,36,48-49H,7-8,10-11,14-15,18-20,22-24H2,1-6H3,(H,43,52)(H,45,51)(H,46,53)(H,47,50)(H2,41,42,44)/t25-,30-,31-,32-,33-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285814
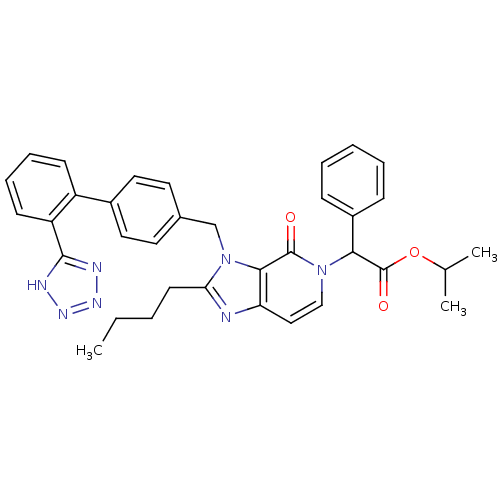
(CHEMBL88170 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-...)Show SMILES CCCCc1nc2ccn(C(C(=O)OC(C)C)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C35H35N7O3/c1-4-5-15-30-36-29-20-21-41(31(35(44)45-23(2)3)26-11-7-6-8-12-26)34(43)32(29)42(30)22-24-16-18-25(19-17-24)27-13-9-10-14-28(27)33-37-39-40-38-33/h6-14,16-21,23,31H,4-5,15,22H2,1-3H3,(H,37,38,39,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285822
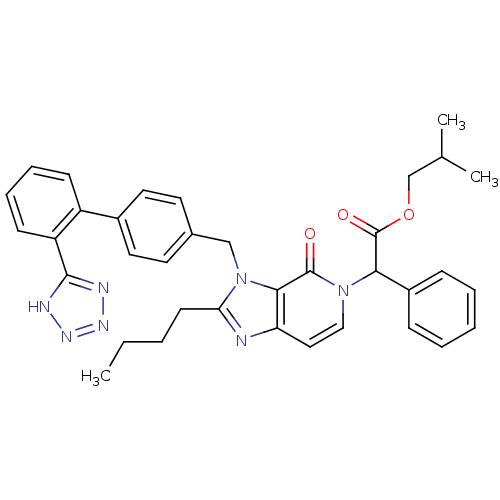
(CHEMBL420607 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5...)Show SMILES CCCCc1nc2ccn(C(C(=O)OCC(C)C)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C36H37N7O3/c1-4-5-15-31-37-30-20-21-42(32(27-11-7-6-8-12-27)36(45)46-23-24(2)3)35(44)33(30)43(31)22-25-16-18-26(19-17-25)28-13-9-10-14-29(28)34-38-40-41-39-34/h6-14,16-21,24,32H,4-5,15,22-23H2,1-3H3,(H,38,39,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285812
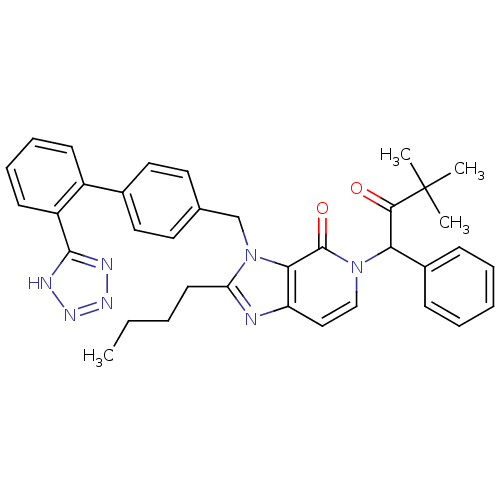
(2-Butyl-5-(3,3-dimethyl-2-oxo-1-phenyl-butyl)-3-[2...)Show SMILES CCCCc1nc2ccn(C(C(=O)C(C)(C)C)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C36H37N7O2/c1-5-6-16-30-37-29-21-22-42(31(33(44)36(2,3)4)26-12-8-7-9-13-26)35(45)32(29)43(30)23-24-17-19-25(20-18-24)27-14-10-11-15-28(27)34-38-40-41-39-34/h7-15,17-22,31H,5-6,16,23H2,1-4H3,(H,38,39,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285815
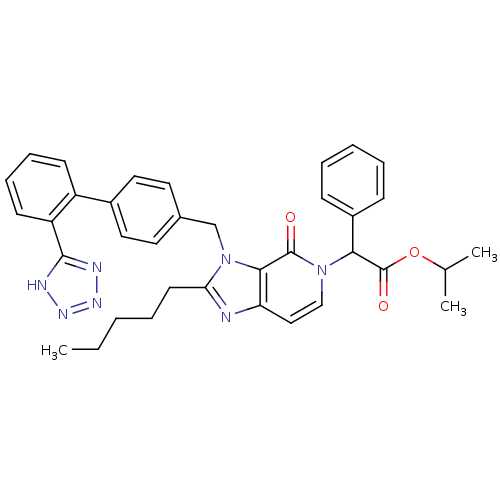
(CHEMBL88600 | {4-Oxo-2-pentyl-3-[2'-(1H-tetrazol-5...)Show SMILES CCCCCc1nc2ccn(C(C(=O)OC(C)C)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C36H37N7O3/c1-4-5-7-16-31-37-30-21-22-42(32(36(45)46-24(2)3)27-12-8-6-9-13-27)35(44)33(30)43(31)23-25-17-19-26(20-18-25)28-14-10-11-15-29(28)34-38-40-41-39-34/h6,8-15,17-22,24,32H,4-5,7,16,23H2,1-3H3,(H,38,39,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50010406
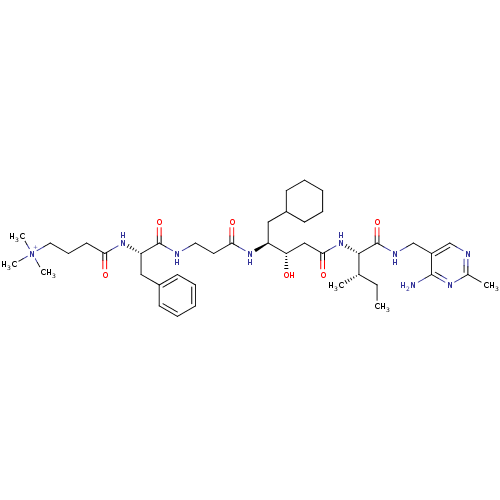
(CHEMBL325329 | [4-(trimethylammonio)butyryl]-Phe-b...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCNC(=O)[C@H](Cc1ccccc1)NC(=O)CCC[N+](C)(C)C)C(=O)NCc1cnc(C)nc1N Show InChI InChI=1S/C42H67N9O6/c1-7-28(2)39(42(57)46-27-32-26-45-29(3)47-40(32)43)50-38(55)25-35(52)33(23-30-15-10-8-11-16-30)48-37(54)20-21-44-41(56)34(24-31-17-12-9-13-18-31)49-36(53)19-14-22-51(4,5)6/h9,12-13,17-18,26,28,30,33-35,39,52H,7-8,10-11,14-16,19-25,27H2,1-6H3,(H6-,43,44,45,46,47,48,49,50,53,54,55,56,57)/p+1/t28-,33-,34-,35-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt
Curated by ChEMBL
| Assay Description
In vitro for inhibitory activity against human renin |
J Med Chem 34: 3267-80 (1991)
BindingDB Entry DOI: 10.7270/Q2G161FT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285825
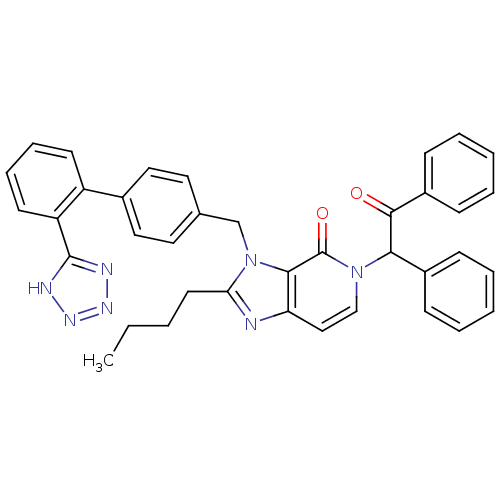
(2-Butyl-5-(2-oxo-1,2-diphenyl-ethyl)-3-[2'-(1H-tet...)Show SMILES CCCCc1nc2ccn(C(C(=O)c3ccccc3)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C38H33N7O2/c1-2-3-18-33-39-32-23-24-44(34(28-12-6-4-7-13-28)36(46)29-14-8-5-9-15-29)38(47)35(32)45(33)25-26-19-21-27(22-20-26)30-16-10-11-17-31(30)37-40-42-43-41-37/h4-17,19-24,34H,2-3,18,25H2,1H3,(H,40,41,42,43) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data