

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499349 (CHEMBL4299370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499361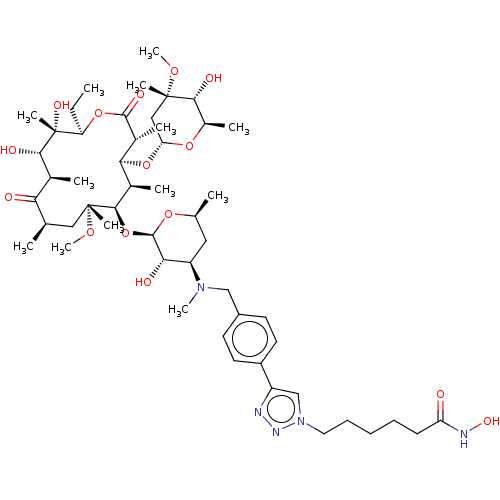 (CHEMBL4299435) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499346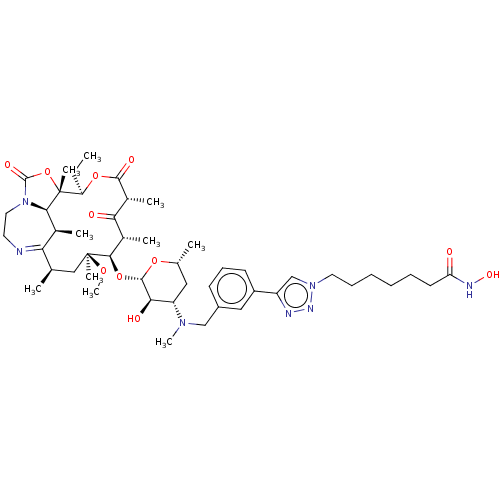 (CHEMBL3736314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499327 (CHEMBL4299447) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499350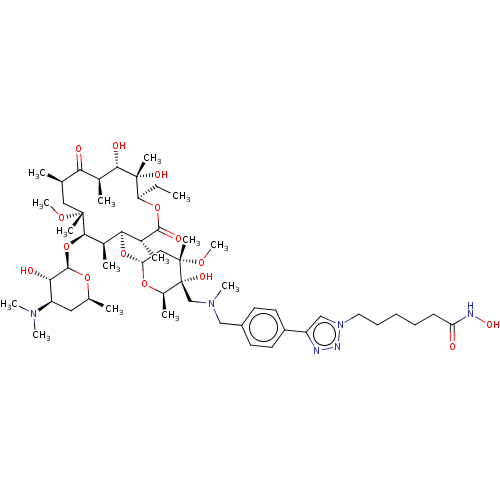 (CHEMBL4299426) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499330 (CHEMBL4299491) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499357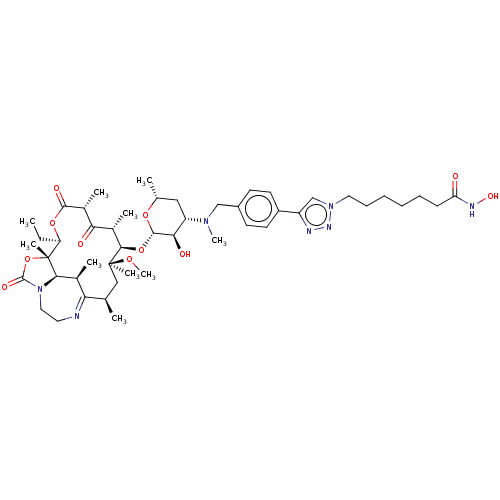 (CHEMBL3736342) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499331 (CHEMBL4299417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499320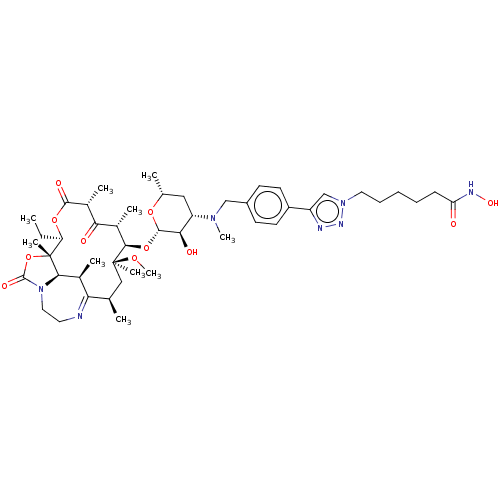 (CHEMBL3735736) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Rattus norvegicus) | BDBM50024270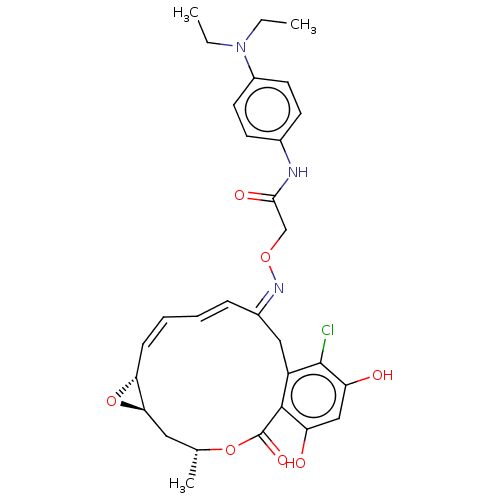 (CHEMBL85612) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure | J Med Chem 46: 2534-41 (2003) Article DOI: 10.1021/jm030110r BindingDB Entry DOI: 10.7270/Q2H41QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499345 (CHEMBL4299449) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Rattus norvegicus) | BDBM50128843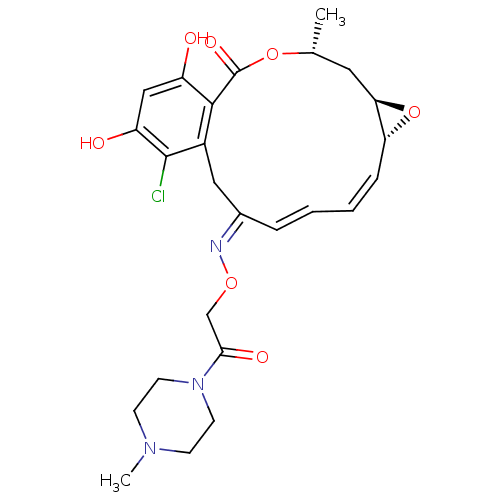 (16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxa-tricy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr | J Med Chem 46: 2534-41 (2003) Article DOI: 10.1021/jm030110r BindingDB Entry DOI: 10.7270/Q2H41QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499362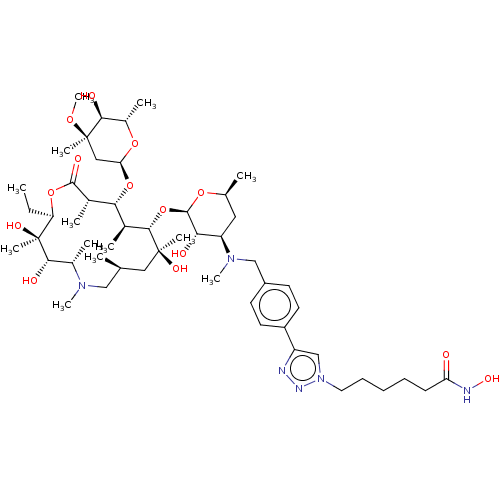 (CHEMBL4299470) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Rattus norvegicus) | BDBM50128852 (2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure | J Med Chem 46: 2534-41 (2003) Article DOI: 10.1021/jm030110r BindingDB Entry DOI: 10.7270/Q2H41QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499343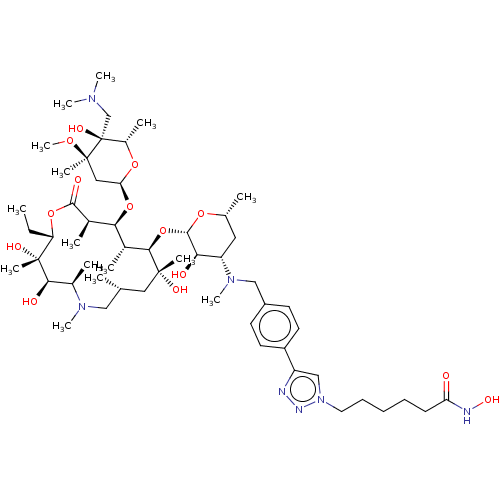 (CHEMBL4299468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499327 (CHEMBL4299447) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Rattus norvegicus) | BDBM50128851 (2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure | J Med Chem 46: 2534-41 (2003) Article DOI: 10.1021/jm030110r BindingDB Entry DOI: 10.7270/Q2H41QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499319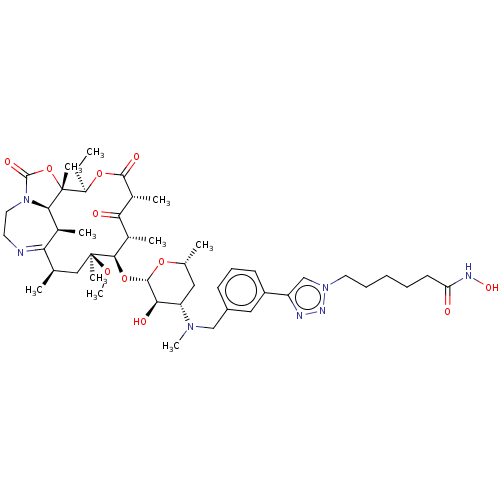 (CHEMBL3735212) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499358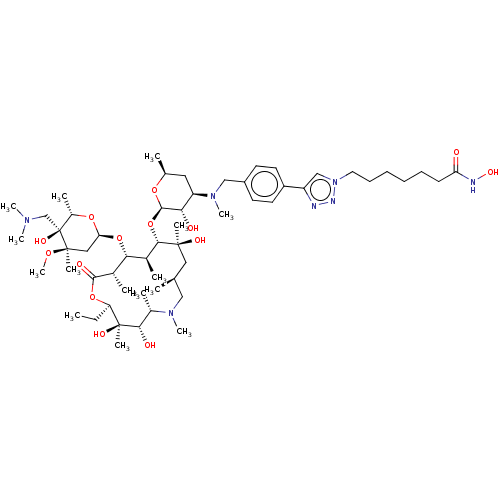 (CHEMBL4299467) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499357 (CHEMBL3736342) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499349 (CHEMBL4299370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Rattus norvegicus) | BDBM50128859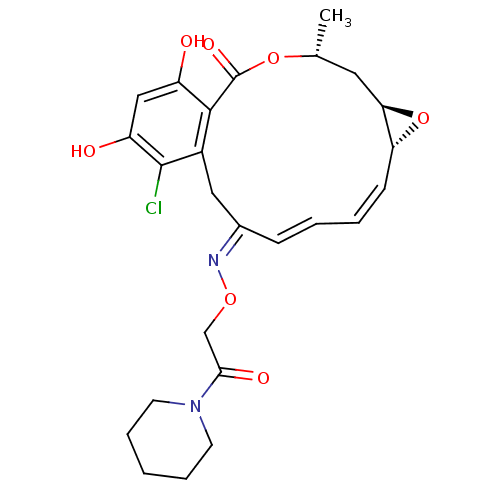 (16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxa-tricy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure | J Med Chem 46: 2534-41 (2003) Article DOI: 10.1021/jm030110r BindingDB Entry DOI: 10.7270/Q2H41QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499355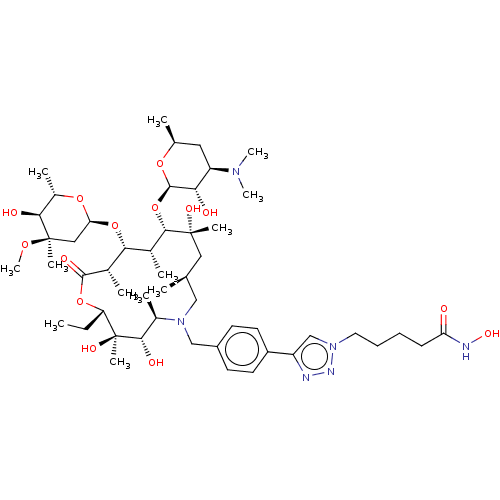 (CHEMBL4299469) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499365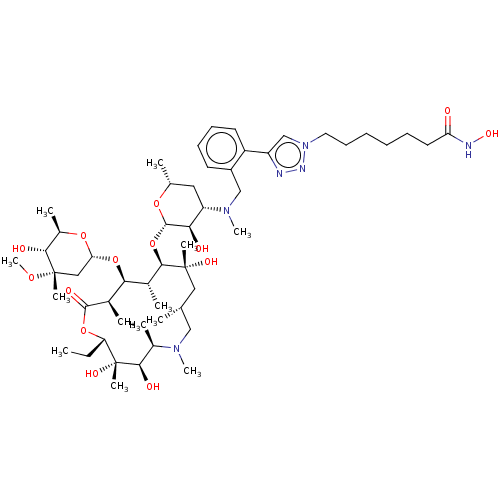 (CHEMBL4299472) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499325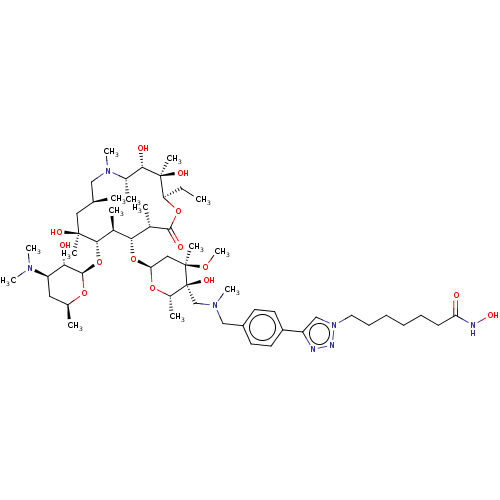 (CHEMBL4299488) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499348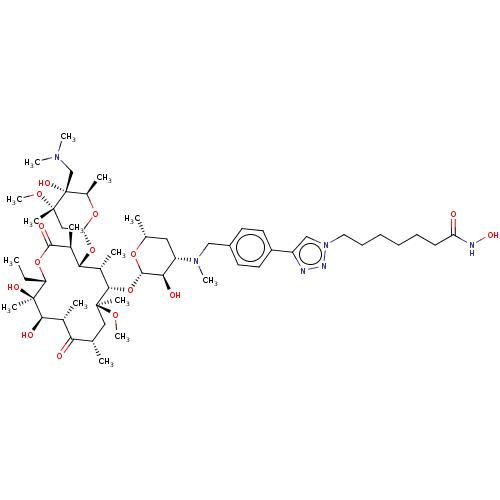 (CHEMBL4299407) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499323 (CHEMBL4299428) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499336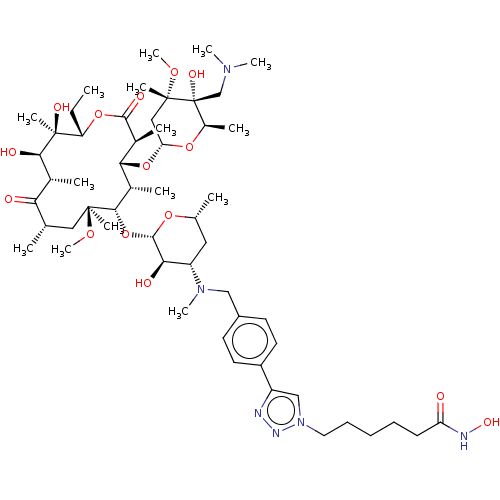 (CHEMBL4299384) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499348 (CHEMBL4299407) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499321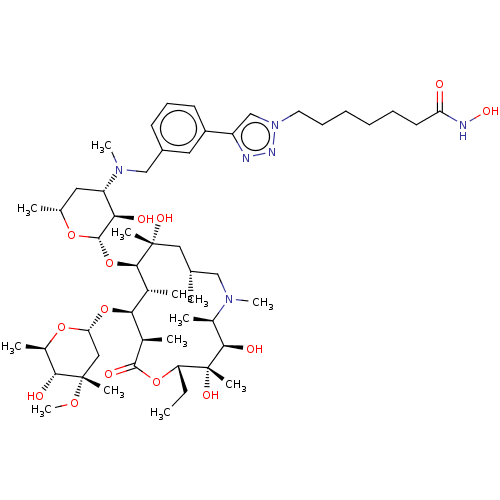 (CHEMBL4299419) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Rattus norvegicus) | BDBM50128846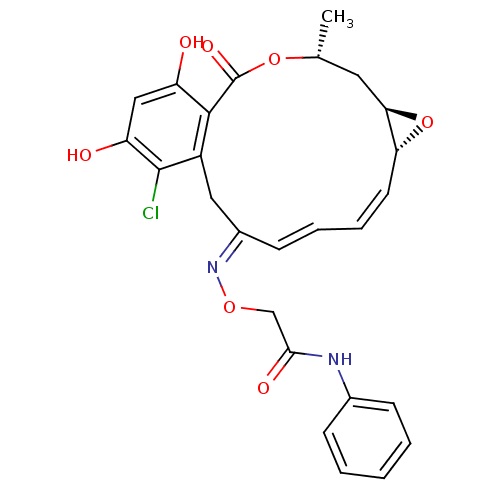 (2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure | J Med Chem 46: 2534-41 (2003) Article DOI: 10.1021/jm030110r BindingDB Entry DOI: 10.7270/Q2H41QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499338 (CHEMBL4299411) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499325 (CHEMBL4299488) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Rattus norvegicus) | BDBM50128850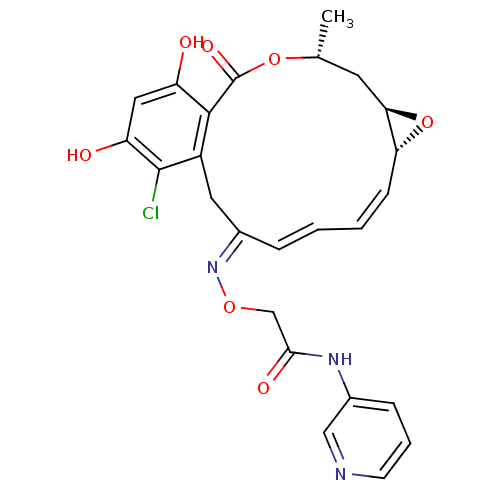 (2-(16-Chloro-17,19-dihydroxy-4-methyl-2-oxo-3,7-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure | J Med Chem 46: 2534-41 (2003) Article DOI: 10.1021/jm030110r BindingDB Entry DOI: 10.7270/Q2H41QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499346 (CHEMBL3736314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Rattus norvegicus) | BDBM50128847 (10-Oxime radicicol | 16-Chloro-17,19-dihydroxy-4-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure | J Med Chem 46: 2534-41 (2003) Article DOI: 10.1021/jm030110r BindingDB Entry DOI: 10.7270/Q2H41QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Rattus norvegicus) | BDBM50128857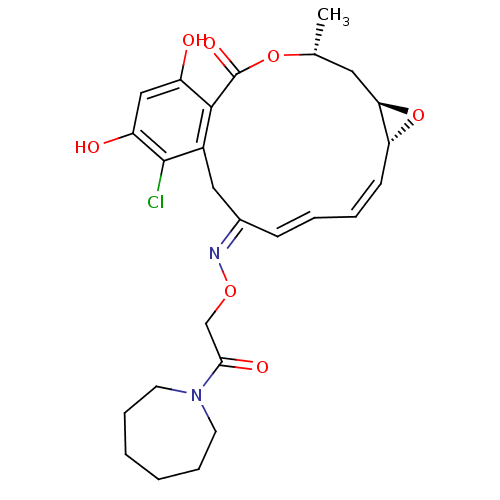 (16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxa-tricy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure | J Med Chem 46: 2534-41 (2003) Article DOI: 10.1021/jm030110r BindingDB Entry DOI: 10.7270/Q2H41QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499331 (CHEMBL4299417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Rattus norvegicus) | BDBM50128853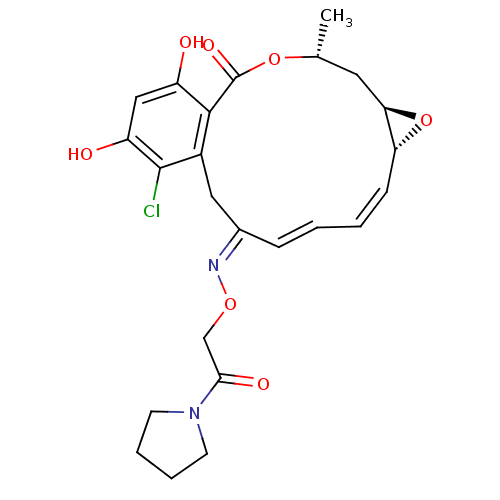 (16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxa-tricy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure | J Med Chem 46: 2534-41 (2003) Article DOI: 10.1021/jm030110r BindingDB Entry DOI: 10.7270/Q2H41QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499351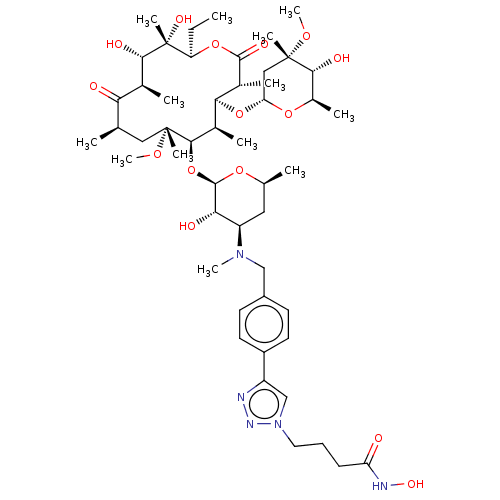 (CHEMBL4299406) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499320 (CHEMBL3735736) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499356 (CHEMBL3736170) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499360 (CHEMBL3734952) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499358 (CHEMBL4299467) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499342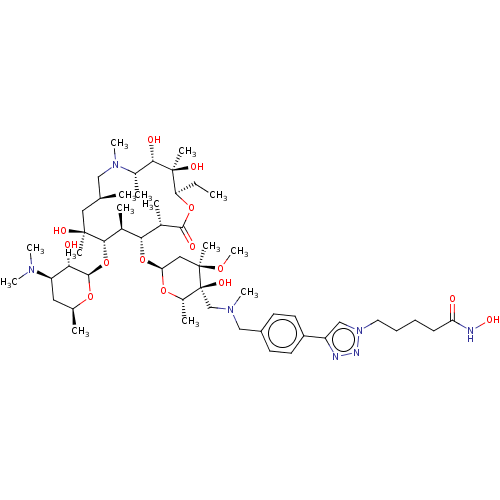 (CHEMBL4299456) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499321 (CHEMBL4299419) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499332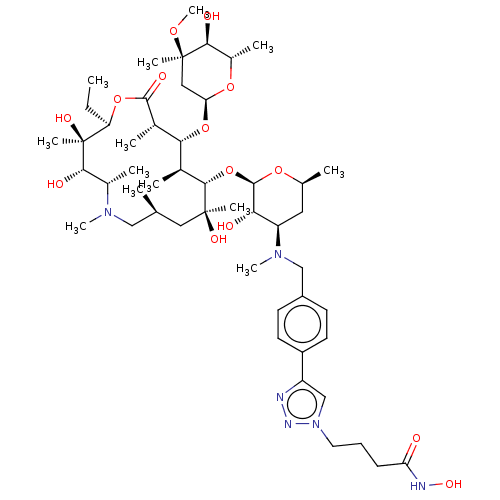 (CHEMBL4299391) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499338 (CHEMBL4299411) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499319 (CHEMBL3735212) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499333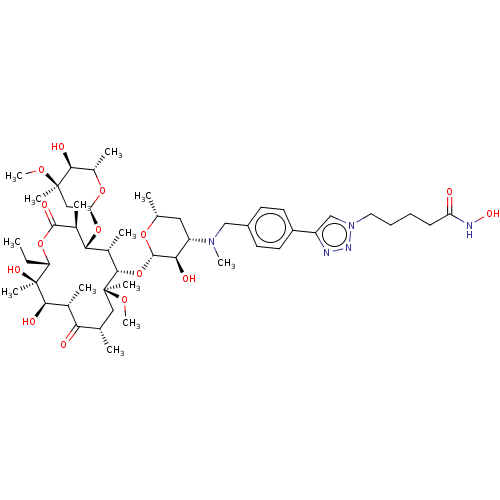 (CHEMBL4299380) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 165 total ) | Next | Last >> |