 Found 803 hits with Last Name = 'moreno' and Initial = 'i'
Found 803 hits with Last Name = 'moreno' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556532
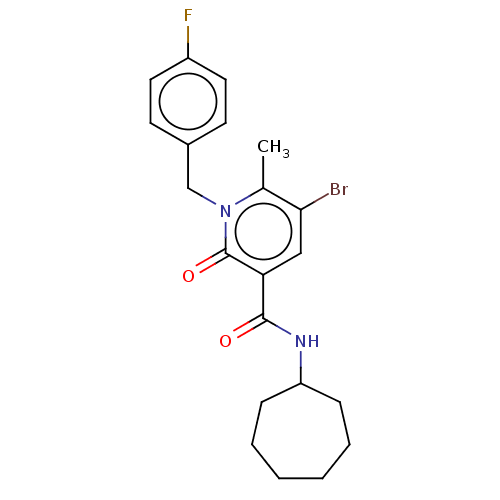
(CHEMBL4753891)Show SMILES Cc1c(Br)cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556542
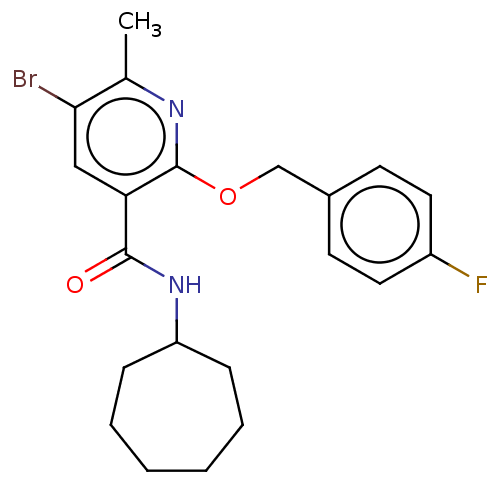
(CHEMBL4780771)Show SMILES Cc1nc(OCc2ccc(F)cc2)c(cc1Br)C(=O)NC1CCCCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556532

(CHEMBL4753891)Show SMILES Cc1c(Br)cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556533
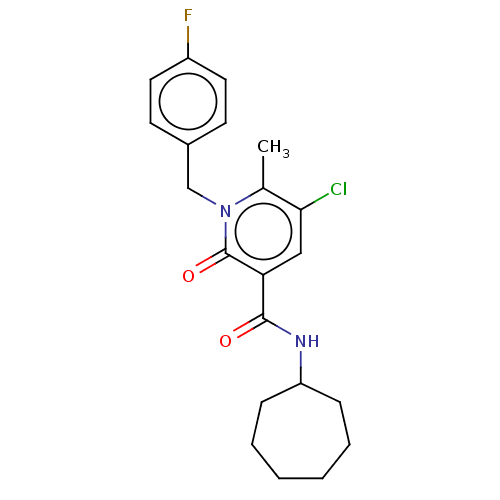
(CHEMBL4781952)Show SMILES Cc1c(Cl)cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556533

(CHEMBL4781952)Show SMILES Cc1c(Cl)cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556531

(CHEMBL4779298) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556534

(CHEMBL4760014)Show SMILES Cc1c(F)cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556535
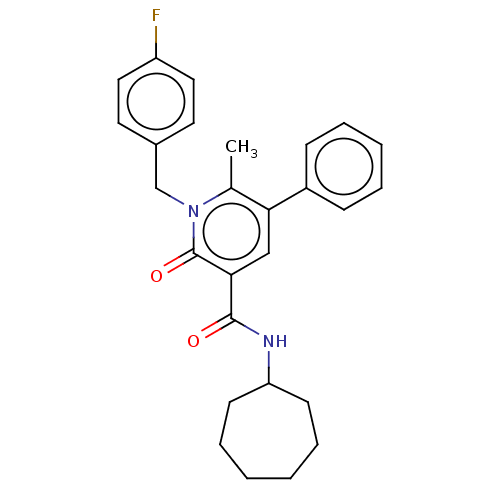
(CHEMBL4782654)Show SMILES Cc1c(cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1)-c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556534

(CHEMBL4760014)Show SMILES Cc1c(F)cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556536

(CHEMBL4781965)Show SMILES COc1ccc(cc1)-c1cc(C(=O)NC2CCCCCC2)c(=O)n(Cc2ccc(F)cc2)c1C | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556541
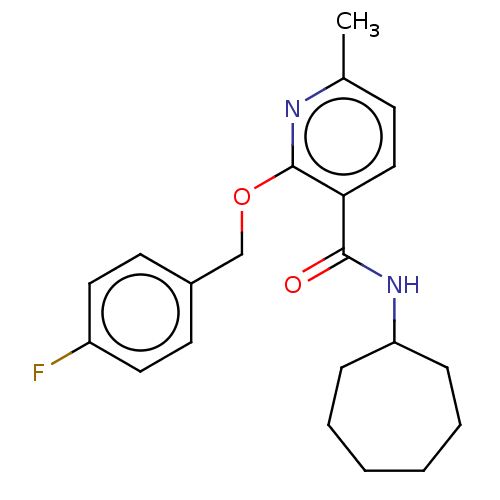
(CHEMBL4756103) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor transfected in CHO cells measured for 1.5 hrs by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00513
BindingDB Entry DOI: 10.7270/Q2251P0M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556536

(CHEMBL4781965)Show SMILES COc1ccc(cc1)-c1cc(C(=O)NC2CCCCCC2)c(=O)n(Cc2ccc(F)cc2)c1C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556537
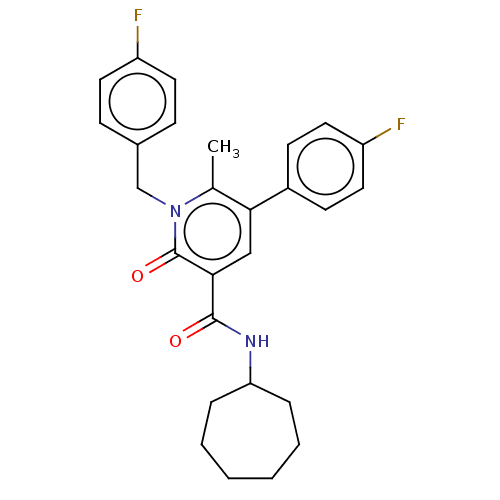
(CHEMBL4757488)Show SMILES Cc1c(cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1)-c1ccc(F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556541

(CHEMBL4756103) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556531

(CHEMBL4779298) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor transfected in CHO cells measured for 1.5 hrs by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00513
BindingDB Entry DOI: 10.7270/Q2251P0M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556537

(CHEMBL4757488)Show SMILES Cc1c(cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1)-c1ccc(F)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556542

(CHEMBL4780771)Show SMILES Cc1nc(OCc2ccc(F)cc2)c(cc1Br)C(=O)NC1CCCCCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236280
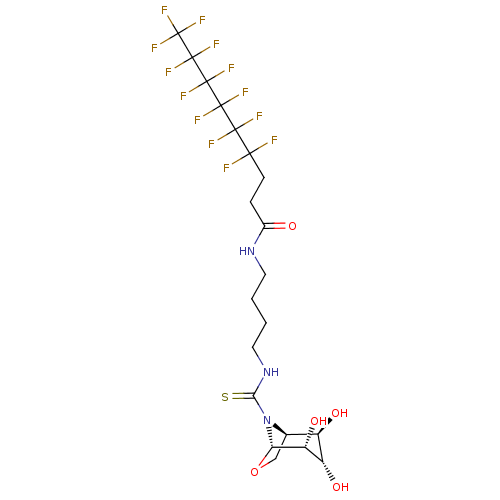
(CHEMBL4100971)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C20H24F13N3O5S/c21-15(22,16(23,24)17(25,26)18(27,28)19(29,30)20(31,32)33)4-3-9(37)34-5-1-2-6-35-14(42)36-8-7-41-13(36)12(40)11(39)10(8)38/h8,10-13,38-40H,1-7H2,(H,34,37)(H,35,42)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 2 |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50577909

(CHEMBL4845760)Show SMILES [H][C@@]12C=C(C)CC[C@]1([H])C(C)(C)Oc1cc(CCCCC)cc(O)c21 |r,t:2| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor transfected in CHO cells measured for 1.5 hrs by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00513
BindingDB Entry DOI: 10.7270/Q2251P0M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556535

(CHEMBL4782654)Show SMILES Cc1c(cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1)-c1ccccc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50577909

(CHEMBL4845760)Show SMILES [H][C@@]12C=C(C)CC[C@]1([H])C(C)(C)Oc1cc(CCCCC)cc(O)c21 |r,t:2| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor transfected in CHO cells measured for 1.5 hrs by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00513
BindingDB Entry DOI: 10.7270/Q2251P0M |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236278
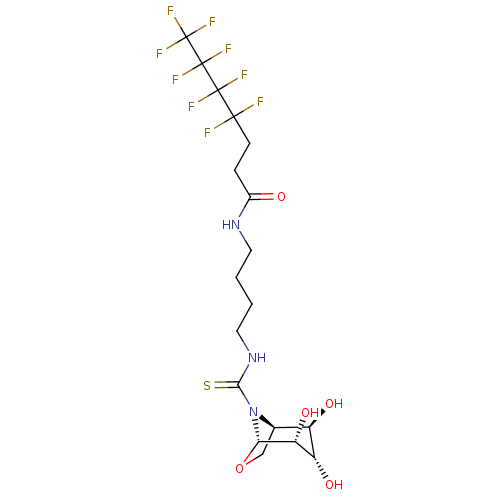
(CHEMBL4077472)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C18H24F9N3O5S/c19-15(20,16(21,22)17(23,24)18(25,26)27)4-3-9(31)28-5-1-2-6-29-14(36)30-8-7-35-13(30)12(34)11(33)10(8)32/h8,10-13,32-34H,1-7H2,(H,28,31)(H,29,36)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 in presence of bet... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556544
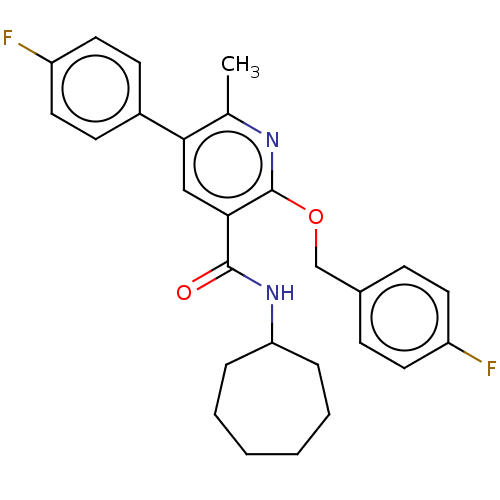
(CHEMBL4792271)Show SMILES Cc1nc(OCc2ccc(F)cc2)c(cc1-c1ccc(F)cc1)C(=O)NC1CCCCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 531 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236279

(CHEMBL4093241)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C25H34F13N3O5S/c26-20(27,21(28,29)22(30,31)23(32,33)24(34,35)25(36,37)38)9-8-14(42)39-10-6-4-2-1-3-5-7-11-40-19(47)41-13-12-46-18(41)17(45)16(44)15(13)43/h13,15-18,43-45H,1-12H2,(H,39,42)(H,40,47)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 in presence of bet... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236281

(CHEMBL4076141)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C23H34F9N3O5S/c24-20(25,21(26,27)22(28,29)23(30,31)32)9-8-14(36)33-10-6-4-2-1-3-5-7-11-34-19(41)35-13-12-40-18(35)17(39)16(38)15(13)37/h13,15-18,37-39H,1-12H2,(H,33,36)(H,34,41)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 in presence of bet... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556543
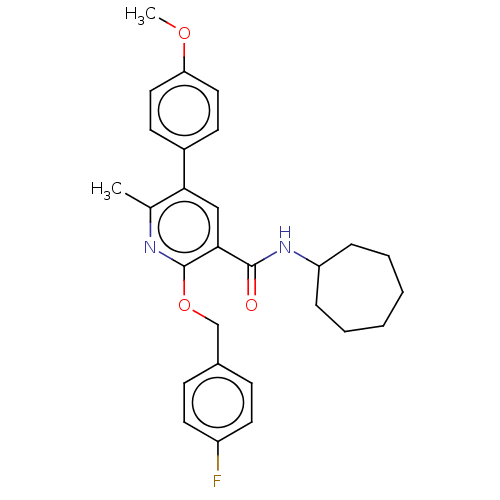
(CHEMBL4753784)Show SMILES COc1ccc(cc1)-c1cc(C(=O)NC2CCCCCC2)c(OCc2ccc(F)cc2)nc1C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 919 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236280

(CHEMBL4100971)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C20H24F13N3O5S/c21-15(22,16(23,24)17(25,26)18(27,28)19(29,30)20(31,32)33)4-3-9(37)34-5-1-2-6-35-14(42)36-8-7-41-13(36)12(40)11(39)10(8)38/h8,10-13,38-40H,1-7H2,(H,34,37)(H,35,42)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 1 |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase
(Coffea arabica (Coffee beans)) | BDBM50375511
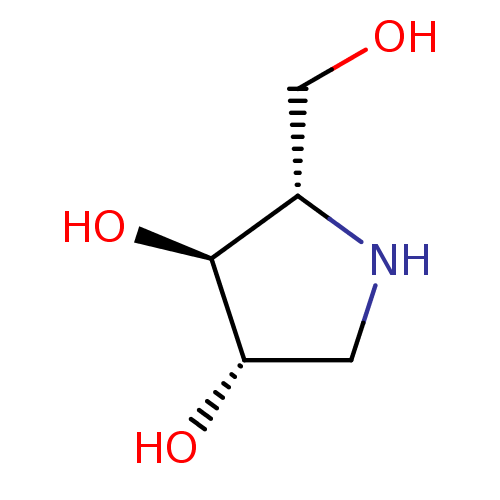
(CHEMBL406973)Show InChI InChI=1S/C5H11NO3/c7-2-3-5(9)4(8)1-6-3/h3-9H,1-2H2/t3-,4-,5-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Seville
Curated by ChEMBL
| Assay Description
Inhibition of green coffee bean alpha-galactosidase using o-nitrophenyl alpha-D-galactopyranoside after 10 to 30 mins by spectrophotometric method |
Eur J Med Chem 121: 880-891 (2016)
Article DOI: 10.1016/j.ejmech.2015.08.038
BindingDB Entry DOI: 10.7270/Q2FT8P07 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50577908

(CHEMBL4862731)Show SMILES [H][C@]12C=C(C)CC[C@@]1([H])C(C)(C)Oc1cc(CCCCC)cc(O)c21 |r,t:2| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor transfected in CHO cells measured for 1.5 hrs by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00513
BindingDB Entry DOI: 10.7270/Q2251P0M |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Bos taurus) | BDBM50329782
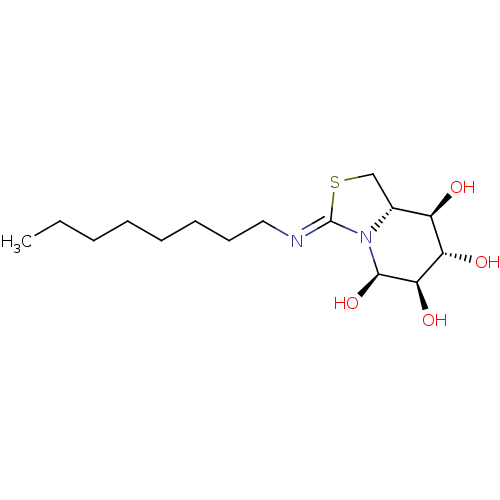
((5R,6R,7S,8R,8aS)-3-(octylimino)hexahydro-1H-thiaz...)Show SMILES CCCCCCCC\N=C1/SC[C@@H]2[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O)N12 |r| Show InChI InChI=1S/C15H28N2O4S/c1-2-3-4-5-6-7-8-16-15-17-10(9-22-15)11(18)12(19)13(20)14(17)21/h10-14,18-21H,2-9H2,1H3/b16-15-/t10-,11-,12+,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver beta-glucosidase after 10 to 30 mins |
Bioorg Med Chem 18: 7439-45 (2010)
Article DOI: 10.1016/j.bmc.2010.09.003
BindingDB Entry DOI: 10.7270/Q2JS9QPK |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236279

(CHEMBL4093241)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C25H34F13N3O5S/c26-20(27,21(28,29)22(30,31)23(32,33)24(34,35)25(36,37)38)9-8-14(42)39-10-6-4-2-1-3-5-7-11-40-19(47)41-13-12-46-18(41)17(45)16(44)15(13)43/h13,15-18,43-45H,1-12H2,(H,39,42)(H,40,47)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 by luminescence sp... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Escherichia coli (Enterobacteria)) | BDBM50375511

(CHEMBL406973)Show InChI InChI=1S/C5H11NO3/c7-2-3-5(9)4(8)1-6-3/h3-9H,1-2H2/t3-,4-,5-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Seville
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-galactosidase using o-nitrophenyl beta-D-galactopyranoside after 10 to 30 mins by spectrophotometric method |
Eur J Med Chem 121: 880-891 (2016)
Article DOI: 10.1016/j.ejmech.2015.08.038
BindingDB Entry DOI: 10.7270/Q2FT8P07 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236278

(CHEMBL4077472)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C18H24F9N3O5S/c19-15(20,16(21,22)17(23,24)18(25,26)27)4-3-9(31)28-5-1-2-6-29-14(36)30-8-7-35-13(30)12(34)11(33)10(8)32/h8,10-13,32-34H,1-7H2,(H,28,31)(H,29,36)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 in presence of bet... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556544

(CHEMBL4792271)Show SMILES Cc1nc(OCc2ccc(F)cc2)c(cc1-c1ccc(F)cc1)C(=O)NC1CCCCCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50577908

(CHEMBL4862731)Show SMILES [H][C@]12C=C(C)CC[C@@]1([H])C(C)(C)Oc1cc(CCCCC)cc(O)c21 |r,t:2| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor transfected in CHO cells measured for 1.5 hrs by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00513
BindingDB Entry DOI: 10.7270/Q2251P0M |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236279

(CHEMBL4093241)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C25H34F13N3O5S/c26-20(27,21(28,29)22(30,31)23(32,33)24(34,35)25(36,37)38)9-8-14(42)39-10-6-4-2-1-3-5-7-11-40-19(47)41-13-12-46-18(41)17(45)16(44)15(13)43/h13,15-18,43-45H,1-12H2,(H,39,42)(H,40,47)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 2 |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236281

(CHEMBL4076141)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C23H34F9N3O5S/c24-20(25,21(26,27)22(28,29)23(30,31)32)9-8-14(36)33-10-6-4-2-1-3-5-7-11-34-19(41)35-13-12-40-18(35)17(39)16(38)15(13)37/h13,15-18,37-39H,1-12H2,(H,33,36)(H,34,41)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 in presence of bet... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236281

(CHEMBL4076141)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C23H34F9N3O5S/c24-20(25,21(26,27)22(28,29)23(30,31)32)9-8-14(36)33-10-6-4-2-1-3-5-7-11-34-19(41)35-13-12-40-18(35)17(39)16(38)15(13)37/h13,15-18,37-39H,1-12H2,(H,33,36)(H,34,41)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 1 |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556539
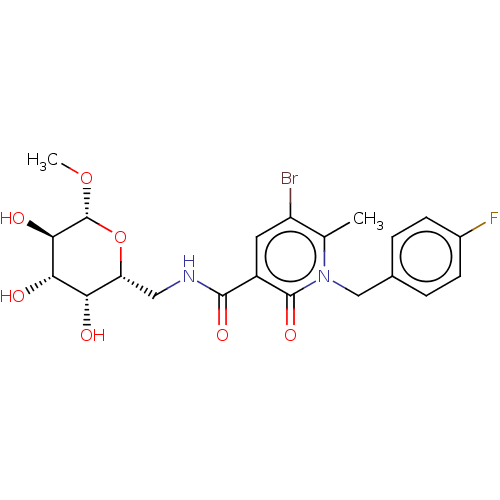
(CHEMBL4750323)Show SMILES CO[C@@H]1O[C@H](CNC(=O)c2cc(Br)c(C)n(Cc3ccc(F)cc3)c2=O)[C@H](O)[C@H](O)[C@H]1O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556540
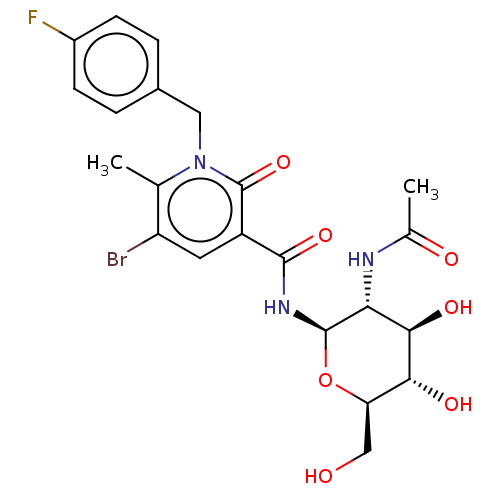
(CHEMBL4740428)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1NC(=O)c1cc(Br)c(C)n(Cc2ccc(F)cc2)c1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556538
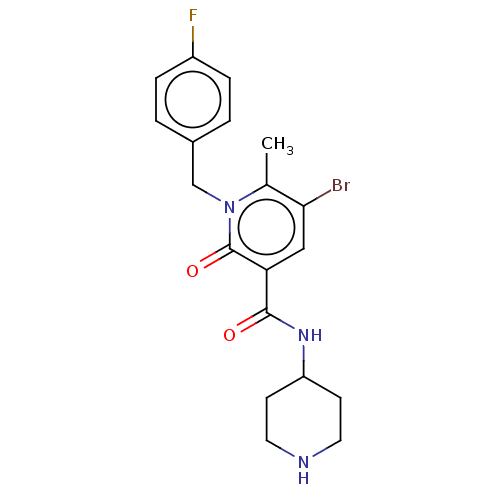
(CHEMBL4740330)Show SMILES Cc1c(Br)cc(C(=O)NC2CCNCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556543

(CHEMBL4753784)Show SMILES COc1ccc(cc1)-c1cc(C(=O)NC2CCCCCC2)c(OCc2ccc(F)cc2)nc1C | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556540

(CHEMBL4740428)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1NC(=O)c1cc(Br)c(C)n(Cc2ccc(F)cc2)c1=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556539

(CHEMBL4750323)Show SMILES CO[C@@H]1O[C@H](CNC(=O)c2cc(Br)c(C)n(Cc3ccc(F)cc3)c2=O)[C@H](O)[C@H](O)[C@H]1O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556538

(CHEMBL4740330)Show SMILES Cc1c(Br)cc(C(=O)NC2CCNCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1R expressed in CHO cell membranes incubated for 90 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112858
BindingDB Entry DOI: 10.7270/Q2SQ943D |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236280

(CHEMBL4100971)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C20H24F13N3O5S/c21-15(22,16(23,24)17(25,26)18(27,28)19(29,30)20(31,32)33)4-3-9(37)34-5-1-2-6-35-14(42)36-8-7-41-13(36)12(40)11(39)10(8)38/h8,10-13,38-40H,1-7H2,(H,34,37)(H,35,42)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 by luminescence sp... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data