

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-K receptor (Homo sapiens (Human)) | BDBM50001447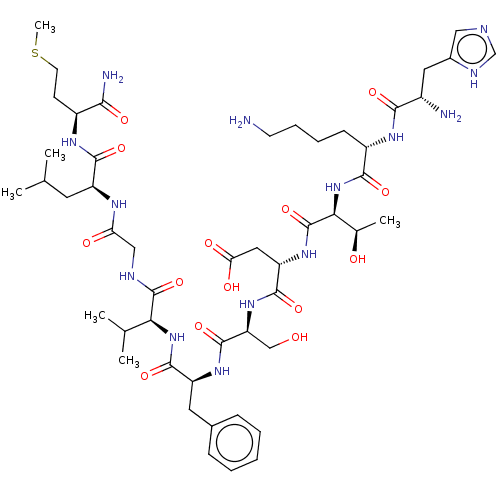 (CHEMBL217406 | His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for competition binding with [3H]NKA against the CHO cells from cloned human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079406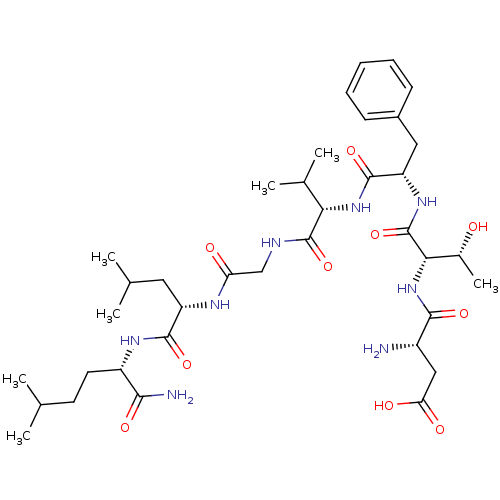 (Asp-Thr-D-Phe-Val-Gly-Leu-Nle-NH2 | Asp-Thr-Phe-Va...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for competition binding with [3H]NKA against the CHO cells from cloned human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079412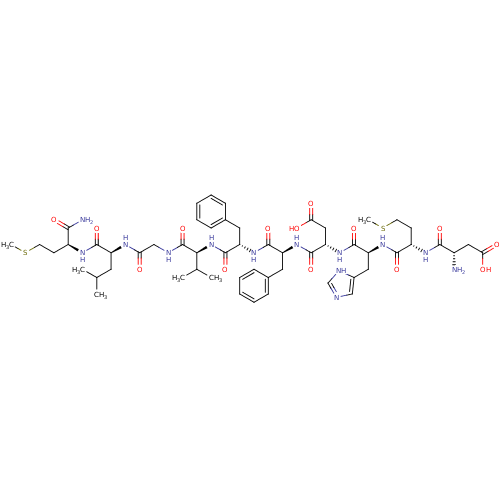 ((NKB)Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for competition binding with [3H]NKA against the CHO cells from cloned human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079409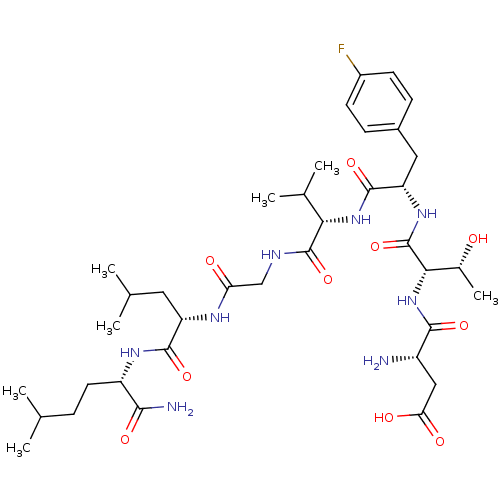 (Asp-Thr-p-F-Phe-Val-Gly-Leu-Nle-NH2 | CHEMBL103634) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for competition binding with [3H]NKA against the CHO cells from cloned human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for competition binding with [3H]NKA against the CHO cells from cloned human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079408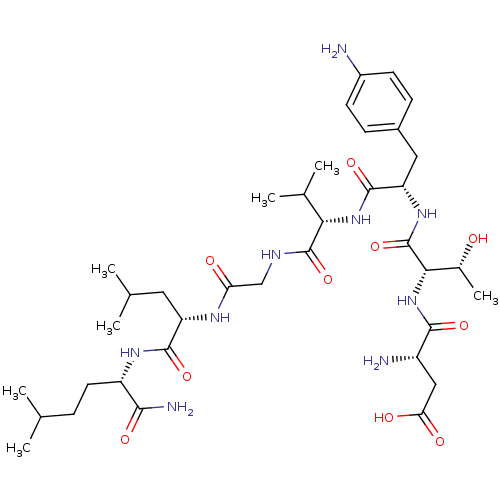 (Asp-Thr-p-NH2-Phe-Val-Gly-Leu-Nle-NH2 | CHEMBL1039...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for competition binding with [3H]NKA against the CHO cells from cloned human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079410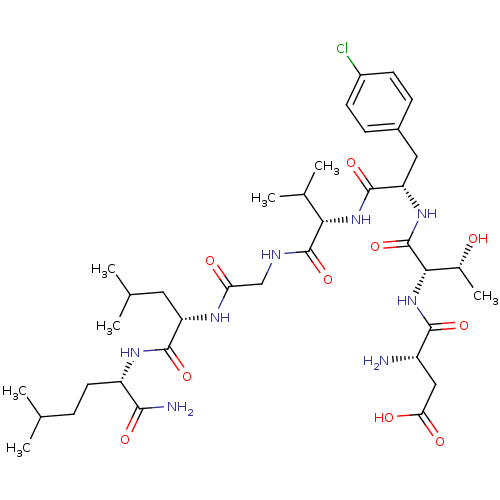 (Asp-Thr-p-Cl-Phe-Val-Gly-Leu-Nle-NH2 | CHEMBL10212...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for competition binding with [3H]NKA against the CHO cells from cloned human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079411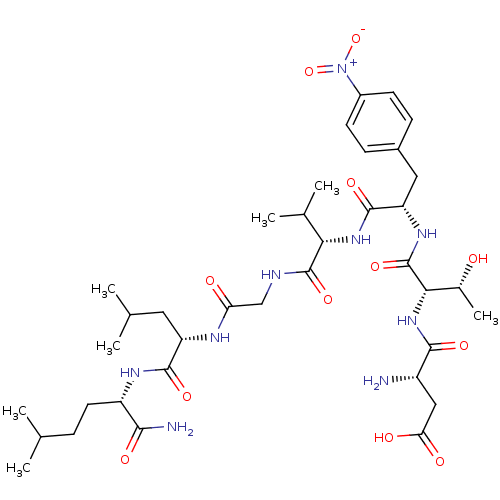 (Asp-Thr-p-NO2-Phe-Val-Gly-Leu-Nle-NH2 | CHEMBL4306...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for competition binding with [3H]NKA against the CHO cells from cloned human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079407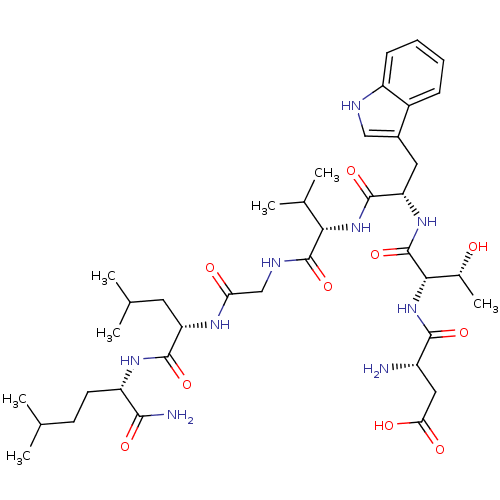 (Asp-Thr-Trp-Val-Gly-Leu-Nle-NH2 | CHEMBL319256) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for competition binding with [3H]NKA against the CHO cells from cloned human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50408715 (CHEMBL2111616) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards NK-2 receptors using [3H]NKA as radioligand in cloned human CHO cells | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50001447 (CHEMBL217406 | His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079406 (Asp-Thr-D-Phe-Val-Gly-Leu-Nle-NH2 | Asp-Thr-Phe-Va...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079412 ((NKB)Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079409 (Asp-Thr-p-F-Phe-Val-Gly-Leu-Nle-NH2 | CHEMBL103634) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079408 (Asp-Thr-p-NH2-Phe-Val-Gly-Leu-Nle-NH2 | CHEMBL1039...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50079410 (Asp-Thr-p-Cl-Phe-Val-Gly-Leu-Nle-NH2 | CHEMBL10212...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||