 Found 903 hits with Last Name = 'nadri' and Initial = 'h'
Found 903 hits with Last Name = 'nadri' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50059510

(8-Methoxy-3-phenyl-1,2,3,4-tetrahydro-chromeno[3,4...)Show InChI InChI=1S/C19H17NO3/c1-22-14-7-8-16-15-9-10-20(13-5-3-2-4-6-13)12-17(15)19(21)23-18(16)11-14/h2-8,11H,9-10,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50052204
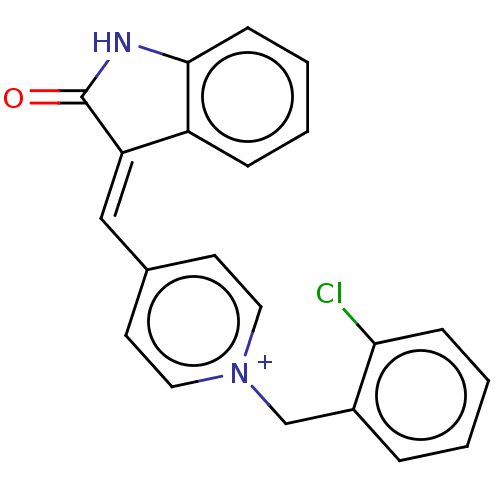
(CHEMBL3318392)Show SMILES [Cl-].Clc1ccccc1C[n+]1ccc(\C=C2\C(=O)Nc3ccccc23)cc1 Show InChI InChI=1S/C21H15ClN2O/c22-19-7-3-1-5-16(19)14-24-11-9-15(10-12-24)13-18-17-6-2-4-8-20(17)23-21(18)25/h1-13H,14H2/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of electric eel AChE using acetylthiocholine iodide substrate by Lineweaver-Burk plot analysis |
Eur J Med Chem 84: 375-81 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.017
BindingDB Entry DOI: 10.7270/Q2H996T1 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059503
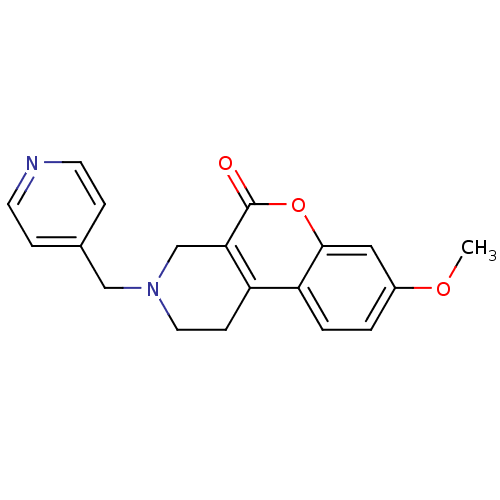
(8-Methoxy-3-pyridin-4-ylmethyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C19H18N2O3/c1-23-14-2-3-16-15-6-9-21(11-13-4-7-20-8-5-13)12-17(15)19(22)24-18(16)10-14/h2-5,7-8,10H,6,9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059507

(3-Benzyl-8-methoxy-1,2,3,4-tetrahydro-chromeno[3,4...)Show InChI InChI=1S/C20H19NO3/c1-23-15-7-8-17-16-9-10-21(12-14-5-3-2-4-6-14)13-18(16)20(22)24-19(17)11-15/h2-8,11H,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059505
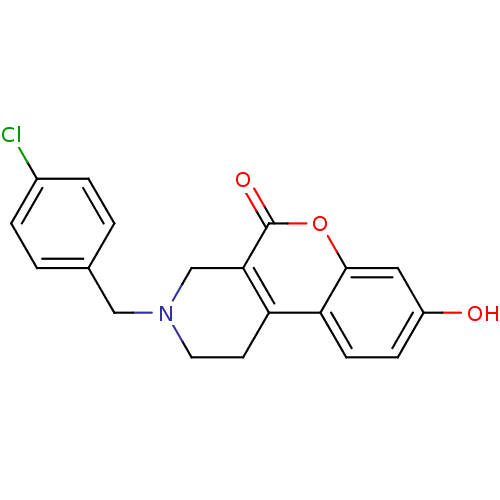
(3-(4-Chloro-benzyl)-8-hydroxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C19H16ClNO3/c20-13-3-1-12(2-4-13)10-21-8-7-15-16-6-5-14(22)9-18(16)24-19(23)17(15)11-21/h1-6,9,22H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059504

(8-Methoxy-3-(4-methyl-benzyl)-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C21H21NO3/c1-14-3-5-15(6-4-14)12-22-10-9-17-18-8-7-16(24-2)11-20(18)25-21(23)19(17)13-22/h3-8,11H,9-10,12-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059496
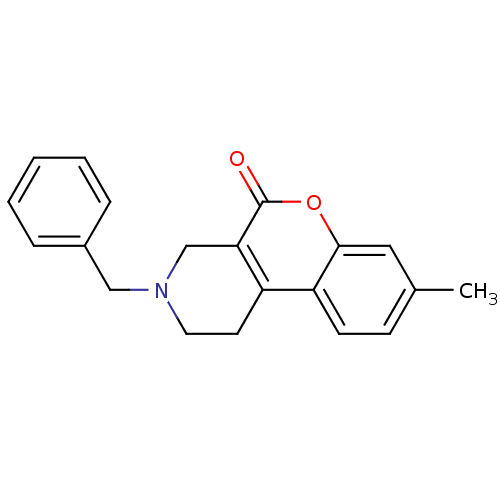
(3-Benzyl-8-methyl-1,2,3,4-tetrahydro-chromeno[3,4-...)Show InChI InChI=1S/C20H19NO2/c1-14-7-8-17-16-9-10-21(12-15-5-3-2-4-6-15)13-18(16)20(22)23-19(17)11-14/h2-8,11H,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medicinal Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE pre-incubated for 3 mins before acetylthiocholine substrate addition by Lineweaver-Burk plot |
Eur J Med Chem 97: 181-9 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.055
BindingDB Entry DOI: 10.7270/Q2P84DM4 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059491
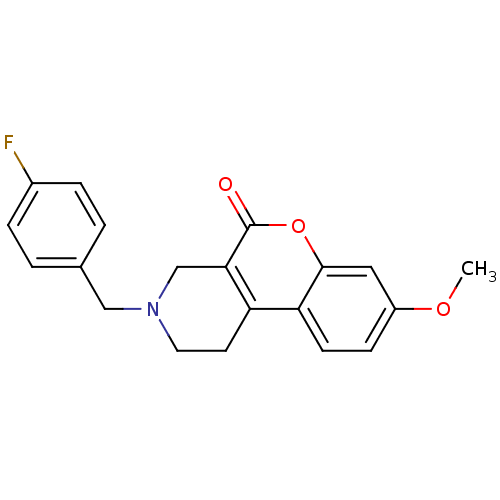
(3-(4-Fluoro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18FNO3/c1-24-15-6-7-17-16-8-9-22(11-13-2-4-14(21)5-3-13)12-18(16)20(23)25-19(17)10-15/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059492
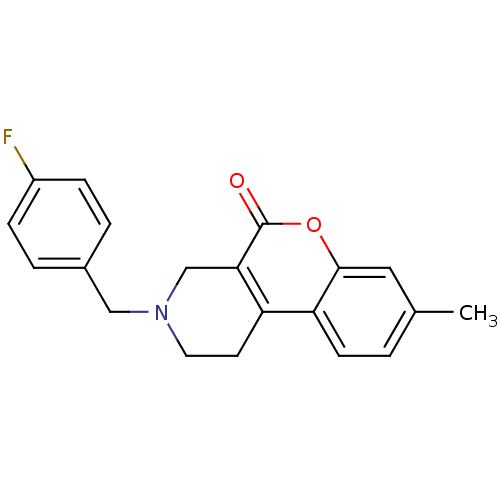
(3-(4-Fluoro-benzyl)-8-methyl-1,2,3,4-tetrahydro-ch...)Show InChI InChI=1S/C20H18FNO2/c1-13-2-7-17-16-8-9-22(11-14-3-5-15(21)6-4-14)12-18(16)20(23)24-19(17)10-13/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059497

(8-Methoxy-3-phenethyl-1,2,3,4-tetrahydro-chromeno[...)Show InChI InChI=1S/C21H21NO3/c1-24-16-7-8-18-17-10-12-22(11-9-15-5-3-2-4-6-15)14-19(17)21(23)25-20(18)13-16/h2-8,13H,9-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059501

(8-Methoxy-3-(4-trifluoromethyl-benzyl)-1,2,3,4-tet...)Show SMILES COc1ccc2c3CCN(Cc4ccc(cc4)C(F)(F)F)Cc3c(=O)oc2c1 Show InChI InChI=1S/C21H18F3NO3/c1-27-15-6-7-17-16-8-9-25(12-18(16)20(26)28-19(17)10-15)11-13-2-4-14(5-3-13)21(22,23)24/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059493
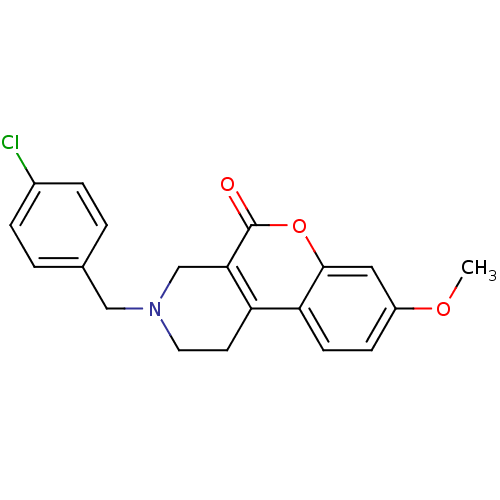
(3-(4-Chloro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18ClNO3/c1-24-15-6-7-17-16-8-9-22(11-13-2-4-14(21)5-3-13)12-18(16)20(23)25-19(17)10-15/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059499
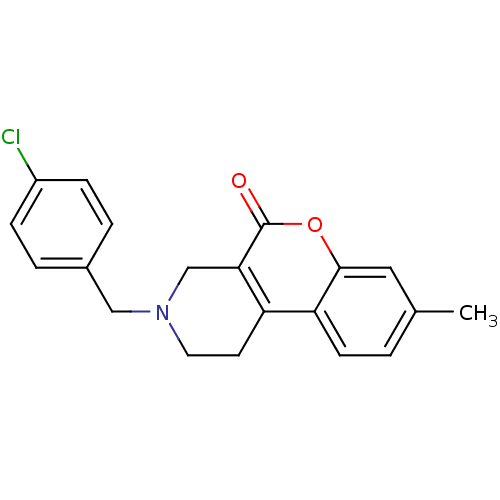
(3-(4-Chloro-benzyl)-8-methyl-1,2,3,4-tetrahydro-ch...)Show InChI InChI=1S/C20H18ClNO2/c1-13-2-7-17-16-8-9-22(11-14-3-5-15(21)6-4-14)12-18(16)20(23)24-19(17)10-13/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50457028

(CHEMBL4218303)Show SMILES COc1ccccc1C1c2c(nc3CCCCc3c2N)-n2n1c(=O)c1ccccc1c2=O Show InChI InChI=1S/C25H22N4O3/c1-32-19-13-7-5-11-17(19)22-20-21(26)16-10-4-6-12-18(16)27-23(20)29-25(31)15-9-3-2-8-14(15)24(30)28(22)29/h2-3,5,7-9,11,13,22H,4,6,10,12H2,1H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measur... |
Eur J Med Chem 139: 280-289 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.072
BindingDB Entry DOI: 10.7270/Q2SX6GVB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50510681
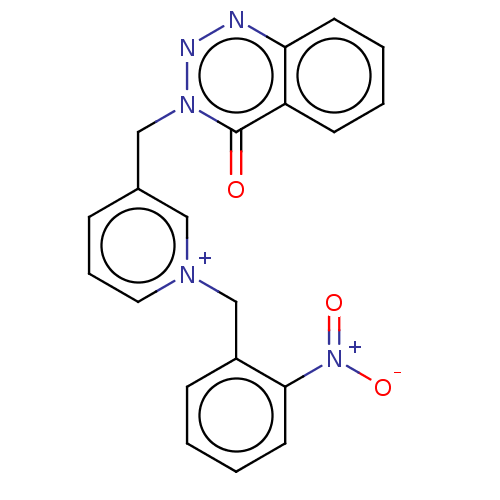
(CHEMBL4451509)Show SMILES [O-][N+](=O)c1ccccc1C[n+]1cccc(Cn2nnc3ccccc3c2=O)c1 Show InChI InChI=1S/C20H16N5O3/c26-20-17-8-2-3-9-18(17)21-22-24(20)13-15-6-5-11-23(12-15)14-16-7-1-4-10-19(16)25(27)28/h1-12H,13-14H2/q+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zanjan
Curated by ChEMBL
| Assay Description
Mixed type inhibition of electric eel AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition and measured after 15 mins by ... |
Bioorg Med Chem 27: 2914-2922 (2019)
Article DOI: 10.1016/j.bmc.2019.05.023
BindingDB Entry DOI: 10.7270/Q22F7RRB |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059494
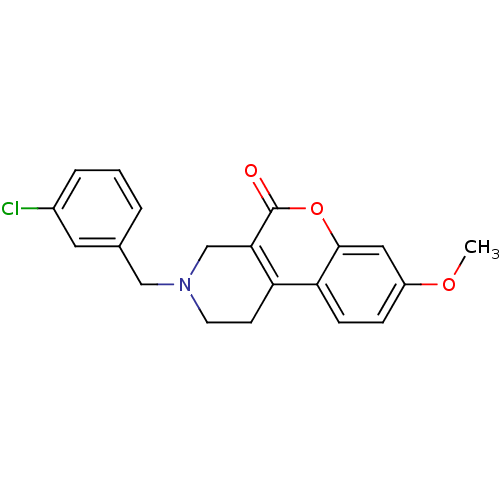
(3-(3-Chloro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18ClNO3/c1-24-15-5-6-17-16-7-8-22(11-13-3-2-4-14(21)9-13)12-18(16)20(23)25-19(17)10-15/h2-6,9-10H,7-8,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50440441
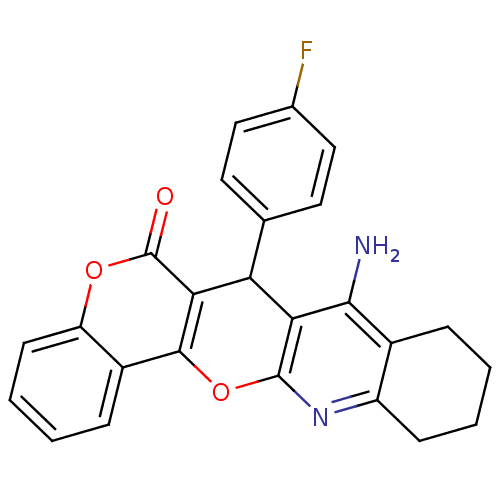
(CHEMBL2425846)Show SMILES Nc1c2CCCCc2nc2Oc3c(C(c4ccc(F)cc4)c12)c(=O)oc1ccccc31 Show InChI InChI=1S/C25H19FN2O3/c26-14-11-9-13(10-12-14)19-20-22(27)15-5-1-3-7-17(15)28-24(20)31-23-16-6-2-4-8-18(16)30-25(29)21(19)23/h2,4,6,8-12,19H,1,3,5,7H2,(H2,27,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis |
Eur J Med Chem 68: 291-300 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.045
BindingDB Entry DOI: 10.7270/Q2794637 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059511

(3-(2-Chloro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18ClNO3/c1-24-14-6-7-16-15-8-9-22(11-13-4-2-3-5-18(13)21)12-17(15)20(23)25-19(16)10-14/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50235419

(CHEMBL4077932)Show SMILES CCOC(=O)C1=C(Oc2nc3CCCCc3c(N)c2C1c1cccc(Br)c1)c1ccccc1 |t:5| Show InChI InChI=1S/C27H25BrN2O3/c1-2-32-27(31)23-21(17-11-8-12-18(28)15-17)22-24(29)19-13-6-7-14-20(19)30-26(22)33-25(23)16-9-4-3-5-10-16/h3-5,8-12,15,21H,2,6-7,13-14H2,1H3,(H2,29,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE preincubated for 5 mins followed by varying levels acetylthiocholine iodide substrate addition by Lineweav... |
Eur J Med Chem 128: 237-246 (2017)
Article DOI: 10.1016/j.ejmech.2017.01.042
BindingDB Entry DOI: 10.7270/Q2GM89J3 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50059507

(3-Benzyl-8-methoxy-1,2,3,4-tetrahydro-chromeno[3,4...)Show InChI InChI=1S/C20H19NO3/c1-23-15-7-8-17-16-9-10-21(12-14-5-3-2-4-6-14)13-18(16)20(22)24-19(17)11-15/h2-8,11H,9-10,12-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059498
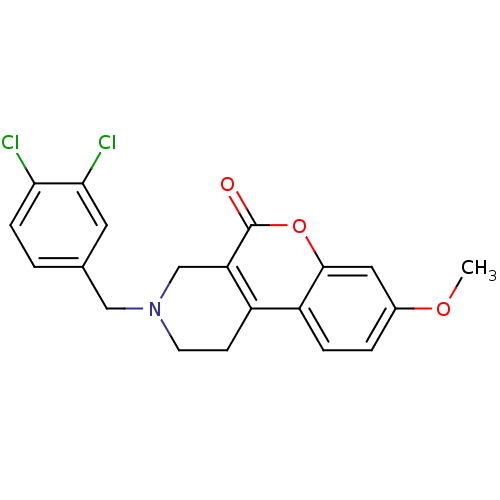
(3-(3,4-Dichloro-benzyl)-8-methoxy-1,2,3,4-tetrahyd...)Show SMILES COc1ccc2c3CCN(Cc4ccc(Cl)c(Cl)c4)Cc3c(=O)oc2c1 Show InChI InChI=1S/C20H17Cl2NO3/c1-25-13-3-4-15-14-6-7-23(10-12-2-5-17(21)18(22)8-12)11-16(14)20(24)26-19(15)9-13/h2-5,8-9H,6-7,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059503

(8-Methoxy-3-pyridin-4-ylmethyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C19H18N2O3/c1-23-14-2-3-16-15-6-9-21(11-13-4-7-20-8-5-13)12-17(15)19(22)24-18(16)10-14/h2-5,7-8,10H,6,9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059502
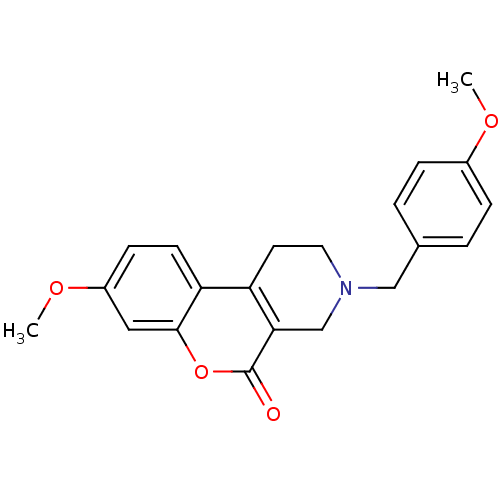
(8-Methoxy-3-(4-methoxy-benzyl)-1,2,3,4-tetrahydro-...)Show SMILES COc1ccc(CN2CCc3c(C2)c(=O)oc2cc(OC)ccc32)cc1 Show InChI InChI=1S/C21H21NO4/c1-24-15-5-3-14(4-6-15)12-22-10-9-17-18-8-7-16(25-2)11-20(18)26-21(23)19(17)13-22/h3-8,11H,9-10,12-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50464028
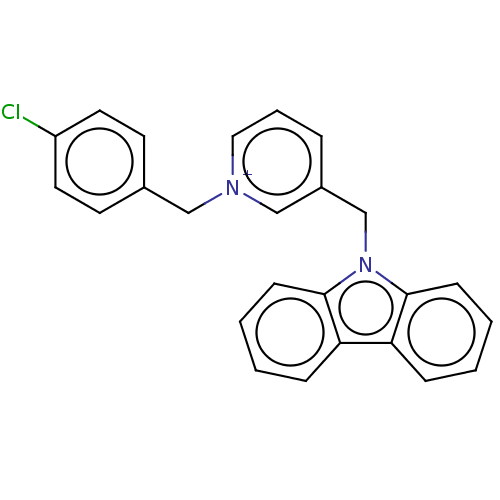
(CHEMBL4241164)Show SMILES [Cl-].Clc1ccc(C[n+]2cccc(Cn3c4ccccc4c4ccccc34)c2)cc1 Show InChI InChI=1S/C25H20ClN2/c26-21-13-11-19(12-14-21)16-27-15-5-6-20(17-27)18-28-24-9-3-1-7-22(24)23-8-2-4-10-25(23)28/h1-15,17H,16,18H2/q+1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of equine serum BuChE using varying levels of butyrylthiocholine iodide as substrate preincubated for 10 mins followed by subst... |
Bioorg Med Chem 26: 4952-4962 (2018)
Article DOI: 10.1016/j.bmc.2018.08.035
BindingDB Entry DOI: 10.7270/Q2NS0XJS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50422387
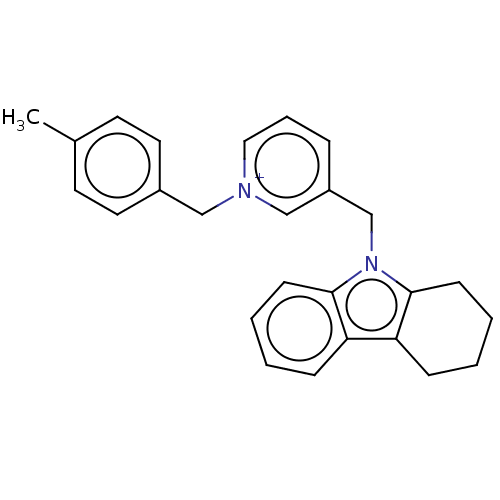
(CHEMBL4159171)Show SMILES [Cl-].Cc1ccc(C[n+]2cccc(Cn3c4CCCCc4c4ccccc34)c2)cc1 Show InChI InChI=1S/C52H76N4O2/c1-53-35-17-7-5-6-8-18-36-54(2)38-20-10-12-22-40-56(44-50-24-14-16-26-52(50)58-4)42-46-29-33-48(34-30-46)47-31-27-45(28-32-47)41-55(39-21-11-9-19-37-53)43-49-23-13-15-25-51(49)57-3/h13-16,23-34H,5-12,17-22,35-44H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of equine serum BChE using varying levels of butyrylthiocholine iodide as substrate by Lineweaver-burk plot analysis |
Eur J Med Chem 155: 49-60 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.031
BindingDB Entry DOI: 10.7270/Q2765HWM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50080392
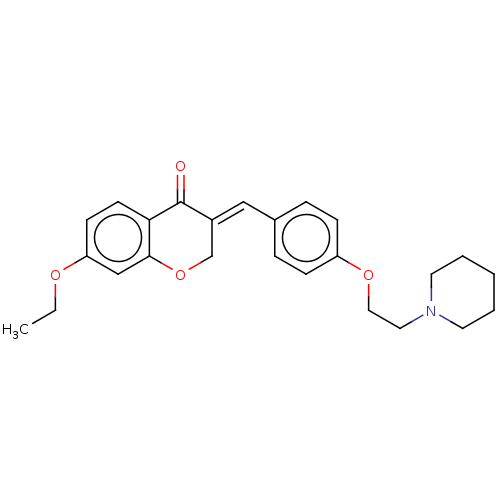
(CHEMBL3416513)Show SMILES Cl.CCOc1ccc2C(=O)\C(COc2c1)=C\c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C25H29NO4.ClH/c1-2-28-22-10-11-23-24(17-22)30-18-20(25(23)27)16-19-6-8-21(9-7-19)29-15-14-26-12-4-3-5-13-26;/h6-11,16-17H,2-5,12-15,18H2,1H3;1H/b20-16+; | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medicinal Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE pre-incubated for 3 mins before acetylthiocholine substrate addition by Lineweaver-Burk plot |
Eur J Med Chem 97: 181-9 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.055
BindingDB Entry DOI: 10.7270/Q2P84DM4 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50059505

(3-(4-Chloro-benzyl)-8-hydroxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C19H16ClNO3/c20-13-3-1-12(2-4-13)10-21-8-7-15-16-6-5-14(22)9-18(16)24-19(23)17(15)11-21/h1-6,9,22H,7-8,10-11H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 317 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50059496

(3-Benzyl-8-methyl-1,2,3,4-tetrahydro-chromeno[3,4-...)Show InChI InChI=1S/C20H19NO2/c1-14-7-8-17-16-9-10-21(12-15-5-3-2-4-6-15)13-18(16)20(22)23-19(17)11-14/h2-8,11H,9-10,12-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 338 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50059507

(3-Benzyl-8-methoxy-1,2,3,4-tetrahydro-chromeno[3,4...)Show InChI InChI=1S/C20H19NO3/c1-23-15-7-8-17-16-9-10-21(12-14-5-3-2-4-6-14)13-18(16)20(22)24-19(17)11-15/h2-8,11H,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50038390
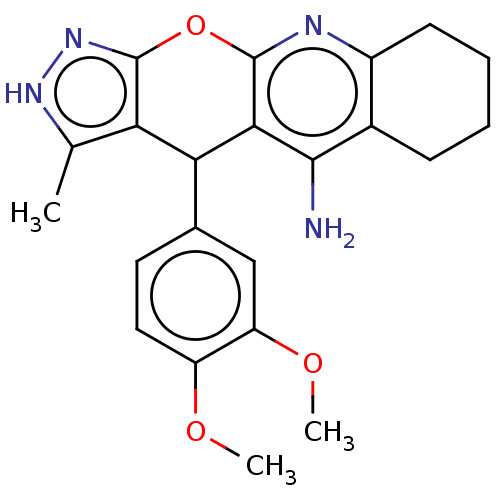
(CHEMBL3352904)Show SMILES COc1ccc(cc1OC)C1c2c(C)[nH]nc2Oc2nc3CCCCc3c(N)c12 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Lineweaver-Burk plot |
Eur J Med Chem 89: 296-303 (2014)
Article DOI: 10.1016/j.ejmech.2014.10.049
BindingDB Entry DOI: 10.7270/Q2T72K1Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50204859
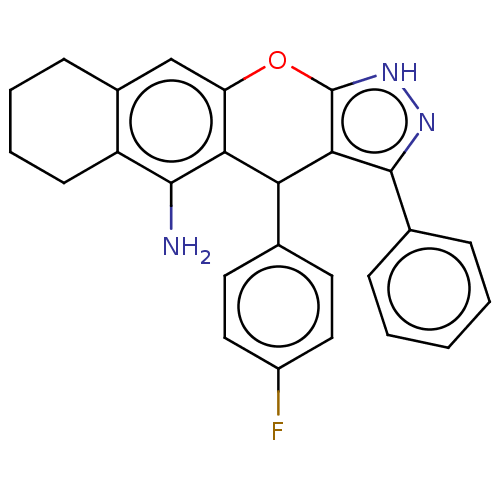
(CHEMBL3939483)Show SMILES Nc1c2CCCCc2cc2Oc3[nH]nc(c3C(c3ccc(F)cc3)c12)-c1ccccc1 Show InChI InChI=1S/C26H22FN3O/c27-18-12-10-15(11-13-18)21-22-20(14-17-8-4-5-9-19(17)24(22)28)31-26-23(21)25(29-30-26)16-6-2-1-3-7-16/h1-3,6-7,10-14,21H,4-5,8-9,28H2,(H,29,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Mixed type inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured f... |
Eur J Med Chem 123: 298-308 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.043
BindingDB Entry DOI: 10.7270/Q2V40X55 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50197934
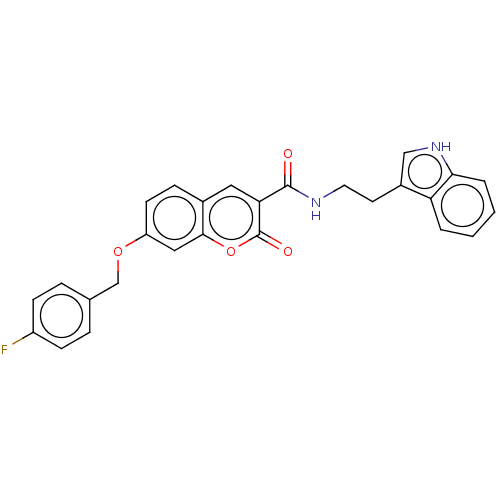
(CHEMBL3889532)Show SMILES Fc1ccc(COc2ccc3cc(C(=O)NCCc4c[nH]c5ccccc45)c(=O)oc3c2)cc1 Show InChI InChI=1S/C27H21FN2O4/c28-20-8-5-17(6-9-20)16-33-21-10-7-18-13-23(27(32)34-25(18)14-21)26(31)29-12-11-19-15-30-24-4-2-1-3-22(19)24/h1-10,13-15,30H,11-12,16H2,(H,29,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured for 2 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 121: 40-46 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.014
BindingDB Entry DOI: 10.7270/Q2XS5XBG |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50059504

(8-Methoxy-3-(4-methyl-benzyl)-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C21H21NO3/c1-14-3-5-15(6-4-14)12-22-10-9-17-18-8-7-16(24-2)11-20(18)25-21(23)19(17)13-22/h3-8,11H,9-10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 491 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50059491

(3-(4-Fluoro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18FNO3/c1-24-15-6-7-17-16-8-9-22(11-13-2-4-14(21)5-3-13)12-18(16)20(23)25-19(17)10-15/h2-7,10H,8-9,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 548 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50059497

(8-Methoxy-3-phenethyl-1,2,3,4-tetrahydro-chromeno[...)Show InChI InChI=1S/C21H21NO3/c1-24-16-7-8-18-17-10-12-22(11-9-15-5-3-2-4-6-15)14-19(17)21(23)25-20(18)13-16/h2-8,13H,9-12,14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 591 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50059496

(3-Benzyl-8-methyl-1,2,3,4-tetrahydro-chromeno[3,4-...)Show InChI InChI=1S/C20H19NO2/c1-14-7-8-17-16-9-10-21(12-15-5-3-2-4-6-15)13-18(16)20(22)23-19(17)11-14/h2-8,11H,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 774 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50059501

(8-Methoxy-3-(4-trifluoromethyl-benzyl)-1,2,3,4-tet...)Show SMILES COc1ccc2c3CCN(Cc4ccc(cc4)C(F)(F)F)Cc3c(=O)oc2c1 Show InChI InChI=1S/C21H18F3NO3/c1-27-15-6-7-17-16-8-9-25(12-18(16)20(26)28-19(17)10-15)11-13-2-4-14(5-3-13)21(22,23)24/h2-7,10H,8-9,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 966 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50059499

(3-(4-Chloro-benzyl)-8-methyl-1,2,3,4-tetrahydro-ch...)Show InChI InChI=1S/C20H18ClNO2/c1-13-2-7-17-16-8-9-22(11-14-3-5-15(21)6-4-14)12-18(16)20(23)24-19(17)10-13/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50059504

(8-Methoxy-3-(4-methyl-benzyl)-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C21H21NO3/c1-14-3-5-15(6-4-14)12-22-10-9-17-18-8-7-16(24-2)11-20(18)25-21(23)19(17)13-22/h3-8,11H,9-10,12-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50020730
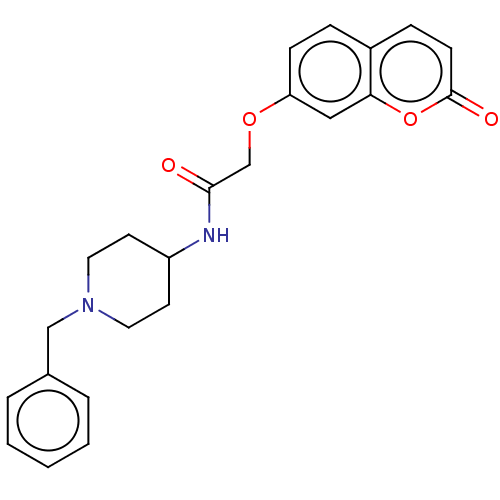
(CHEMBL1469070)Show SMILES O=C(COc1ccc2ccc(=O)oc2c1)NC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C23H24N2O4/c26-22(16-28-20-8-6-18-7-9-23(27)29-21(18)14-20)24-19-10-12-25(13-11-19)15-17-4-2-1-3-5-17/h1-9,14,19H,10-13,15-16H2,(H,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweavere-Burk plot |
Eur J Med Chem 82: 536-44 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.056
BindingDB Entry DOI: 10.7270/Q27D2WQV |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50059492

(3-(4-Fluoro-benzyl)-8-methyl-1,2,3,4-tetrahydro-ch...)Show InChI InChI=1S/C20H18FNO2/c1-13-2-7-17-16-8-9-22(11-14-3-5-15(21)6-4-14)12-18(16)20(23)24-19(17)10-13/h2-7,10H,8-9,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50059511

(3-(2-Chloro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18ClNO3/c1-24-14-6-7-16-15-8-9-22(11-13-4-2-3-5-18(13)21)12-17(15)20(23)25-19(16)10-14/h2-7,10H,8-9,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50059494

(3-(3-Chloro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18ClNO3/c1-24-15-5-6-17-16-7-8-22(11-13-3-2-4-14(21)9-13)12-18(16)20(23)25-19(17)10-15/h2-6,9-10H,7-8,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50059493

(3-(4-Chloro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18ClNO3/c1-24-15-6-7-17-16-8-9-22(11-13-2-4-14(21)5-3-13)12-18(16)20(23)25-19(17)10-15/h2-7,10H,8-9,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50059505

(3-(4-Chloro-benzyl)-8-hydroxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C19H16ClNO3/c20-13-3-1-12(2-4-13)10-21-8-7-15-16-6-5-14(22)9-18(16)24-19(23)17(15)11-21/h1-6,9,22H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
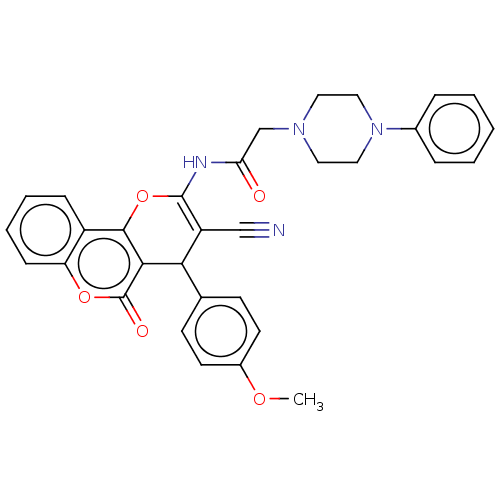
(Electrophorus electricus (Electric eel)) | BDBM50268048
(CHEMBL4098491)Show SMILES COc1ccc(cc1)C1C(C#N)=C(NC(=O)CN2CCN(CC2)c2ccccc2)Oc2c1c(=O)oc1ccccc21 |t:12| Show InChI InChI=1S/C32H28N4O5/c1-39-23-13-11-21(12-14-23)28-25(19-33)31(41-30-24-9-5-6-10-26(24)40-32(38)29(28)30)34-27(37)20-35-15-17-36(18-16-35)22-7-3-2-4-8-22/h2-14,28H,15-18,20H2,1H3,(H,34,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Faculty of Pharmacy, International Campus (TUMS-IC), Tehran University of Medical Sciences, Tehran, Iran.
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE using varying levels of acetylthiocholine iodide as substrate measured for 2 mins by Lineweaver-Burk plot ... |
Bioorg Med Chem 25: 3980-3988 (2017)
Article DOI: 10.1016/j.bmc.2017.05.043
BindingDB Entry DOI: 10.7270/Q25Q4ZK4 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059510

(8-Methoxy-3-phenyl-1,2,3,4-tetrahydro-chromeno[3,4...)Show InChI InChI=1S/C19H17NO3/c1-22-14-7-8-16-15-9-10-20(13-5-3-2-4-6-13)12-17(15)19(21)23-18(16)11-14/h2-8,11H,9-10,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | >3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50059501

(8-Methoxy-3-(4-trifluoromethyl-benzyl)-1,2,3,4-tet...)Show SMILES COc1ccc2c3CCN(Cc4ccc(cc4)C(F)(F)F)Cc3c(=O)oc2c1 Show InChI InChI=1S/C21H18F3NO3/c1-27-15-6-7-17-16-8-9-25(12-18(16)20(26)28-19(17)10-15)11-13-2-4-14(5-3-13)21(22,23)24/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50059494

(3-(3-Chloro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18ClNO3/c1-24-15-5-6-17-16-7-8-22(11-13-3-2-4-14(21)9-13)12-18(16)20(23)25-19(17)10-15/h2-6,9-10H,7-8,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data