

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (MOUSE) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-NLX from mouse mu opioid receptor expressed in CHO cells measured after 1.5 hrs by competitive-binding assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50583787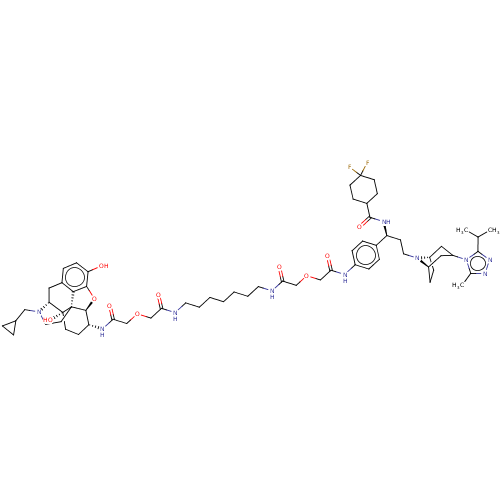 (CHEMBL5071060) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]1-alpha from recombinant rhesus macaque CCR5 expressed in Chem-1 cells incubated for 90 mins by competitive radioligand binding... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50561916 (CHEMBL4779258) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-NLX from mouse mu opioid receptor expressed in CHO cells measured after 1.5 hrs by competitive-binding assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50561915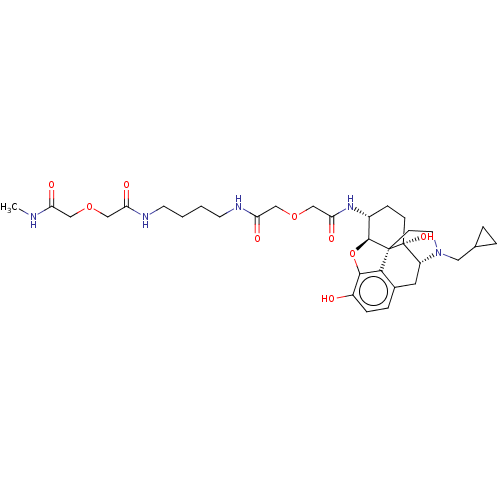 (CHEMBL4752423) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-NLX from mouse mu opioid receptor expressed in CHO cells measured after 1.5 hrs by competitive-binding assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50583784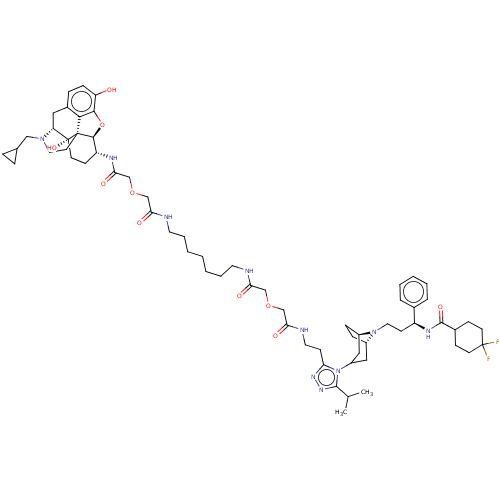 (CHEMBL5074744) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]1-alpha from recombinant rhesus macaque CCR5 expressed in Chem-1 cells incubated for 90 mins by competitive radioligand binding... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50561914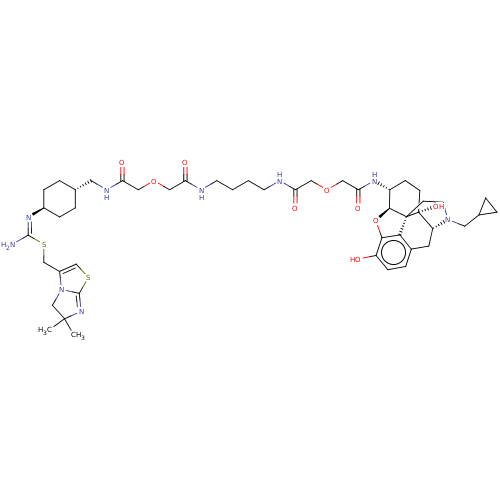 (CHEMBL4784104) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-NLX from mouse mu opioid receptor expressed in CHO cells measured after 1.5 hrs by competitive-binding assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50583786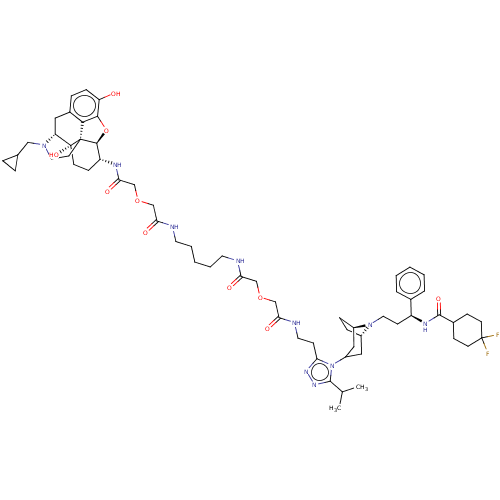 (CHEMBL5074037) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50583785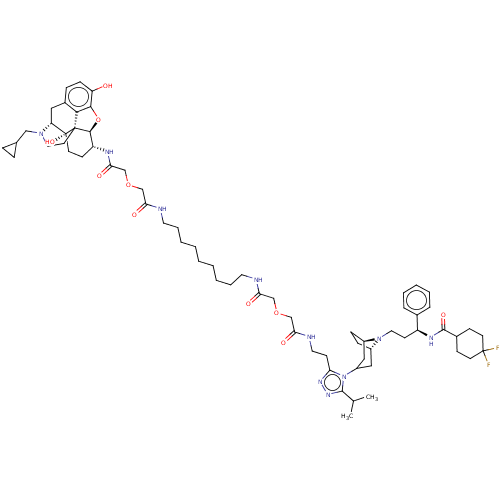 (CHEMBL5089300) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]1-alpha from recombinant rhesus macaque CCR5 expressed in Chem-1 cells incubated for 90 mins by competitive radioligand binding... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50583784 (CHEMBL5074744) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50583785 (CHEMBL5089300) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50583788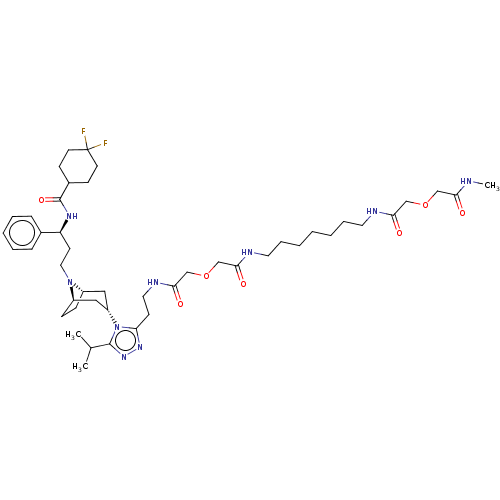 (CHEMBL5094690) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]1-alpha from recombinant rhesus macaque CCR5 expressed in Chem-1 cells by competitive radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50583787 (CHEMBL5071060) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50583789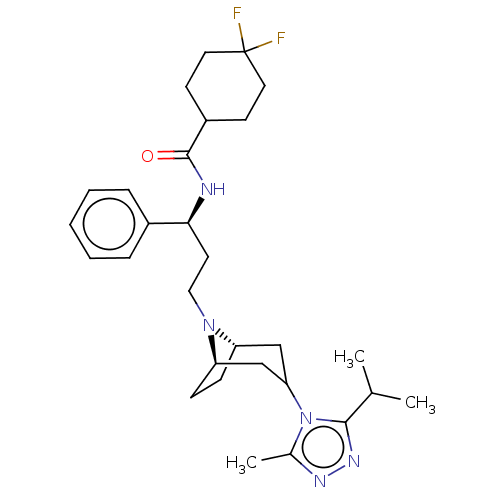 (CHEMBL584744) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HOS cells co-expressing Gqi-5 assessed as inhibition CCL5-stimulated Ca2+ mobilization by c... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse MOR expressed in CHO cells assessed as inhibition of DAMGO-induced calcium mobilization preincubated for 60 mins measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247128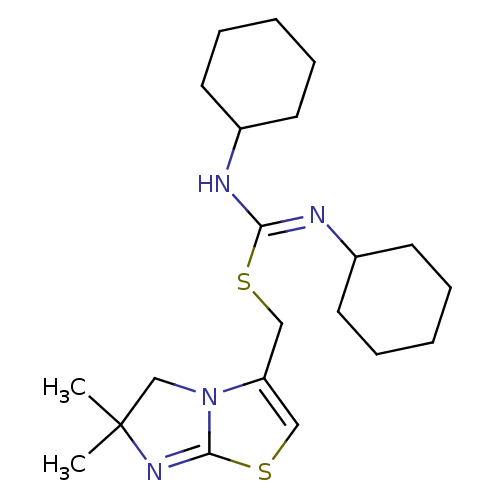 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 in human Chem-1 cells measured after 90 mins | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells co- expressing Gqi5 assessed as decrease in DAMGO-induced intracellular calciu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50553072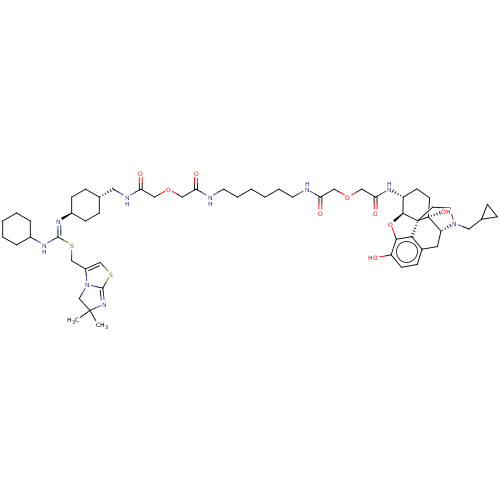 (CHEMBL4750948) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 in human Chem-1 cells measured after 90 mins | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50561914 (CHEMBL4784104) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells co- expressing Gqi5 assessed as decrease in DAMGO-induced intracellular calciu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247128 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 (unknown origin) expressed in CHO cells assessed as reduction in SDF1-induced intracellular calcium mobilization incubat... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50583787 (CHEMBL5071060) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse MOR expressed in CHO cells assessed as inhibition of DAMGO-induced calcium mobilization preincubated for 60 mins measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50583784 (CHEMBL5074744) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse MOR expressed in CHO cells assessed as inhibition of DAMGO-induced calcium mobilization preincubated for 60 mins measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50583784 (CHEMBL5074744) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HOS cells co-expressing Gqi-5 assessed as inhibition CCL5-stimulated Ca2+ mobilization by c... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50583785 (CHEMBL5089300) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse MOR expressed in CHO cells assessed as inhibition of DAMGO-induced calcium mobilization preincubated for 60 mins measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50583787 (CHEMBL5071060) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HOS cells co-expressing Gqi-5 assessed as inhibition CCL5-stimulated Ca2+ mobilization by c... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50561915 (CHEMBL4752423) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells co- expressing Gqi5 assessed as decrease in DAMGO-induced intracellular calciu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50583785 (CHEMBL5089300) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 965 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HOS cells co-expressing Gqi-5 assessed as inhibition CCL5-stimulated Ca2+ mobilization by c... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50583786 (CHEMBL5074037) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse MOR expressed in CHO cells assessed as inhibition of DAMGO-induced calcium mobilization preincubated for 60 mins measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50583786 (CHEMBL5074037) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HOS cells co-expressing Gqi-5 assessed as inhibition CCL5-stimulated Ca2+ mobilization by c... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50561914 (CHEMBL4784104) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 in human Chem-1 cells measured after 90 mins | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50561914 (CHEMBL4784104) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 (unknown origin) expressed in CHO cells assessed as reduction in SDF1-induced intracellular calcium mobilization incubat... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50561917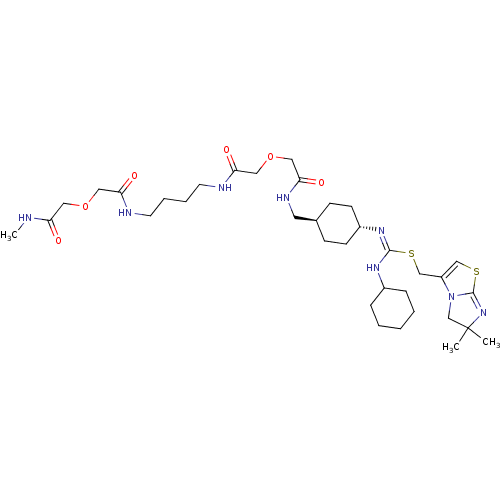 (CHEMBL4789939) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 (unknown origin) expressed in CHO cells assessed as reduction in SDF1-induced intracellular calcium mobilization incubat... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50561917 (CHEMBL4789939) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 in human Chem-1 cells measured after 90 mins | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50247128 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 3B reverse transcriptase infected in human GHOST CXCR4 cells assessed as reduction in viral replication incubated for 60 to 90 min... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50561917 (CHEMBL4789939) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 3B reverse transcriptase infected in human GHOST CXCR4 cells assessed as reduction in viral replication incubated for 60 to 90 min... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50561914 (CHEMBL4784104) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 960 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 3B reverse transcriptase infected in human GHOST CXCR4 cells assessed as reduction in viral replication incubated for 60 to 90 min... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50583784 (CHEMBL5074744) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mouse MOR expressed in CHO cells coexpressing Gqi5 assessed as inhibition of DAMGO-induced calcium mobilization preincubated for ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50583784 (CHEMBL5074744) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 247 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MOR-mediated HIV1 BaL01 infection in GFP-tagged human OPRM1 transfected TZM-bl cells co-expressing HIV1 - LTR assessed as inhibition of... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50583784 (CHEMBL5074744) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CCR5-mediated HIV-1 Bal entry in human GHOST CCR5 cells assessed as decrease in viral reverse transcriptase activity by measuring [3H]t... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50583787 (CHEMBL5071060) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CCR5-mediated HIV-1 Bal entry in human GHOST CCR5 cells assessed as decrease in viral reverse transcriptase activity by measuring [3H]t... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50583789 (CHEMBL584744) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CCR5-mediated HIV-1 Bal entry in human GHOST CCR5 cells assessed as decrease in viral reverse transcriptase activity by measuring [3H]t... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50583784 (CHEMBL5074744) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 368 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MOR-mediated HIV1 BaL01 infection in GFP-tagged human OPRM1 transfected TZM-bl cells co-expressing HIV1 - LTR assessed as inhibition of... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50561914 (CHEMBL4784104) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mu opioid receptor (unknown origin) by [35S]-GTPgammaS binding assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50561915 (CHEMBL4752423) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mu opioid receptor (unknown origin) by [35S]-GTPgammaS binding assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||