 Found 211 hits with Last Name = 'nozoe' and Initial = 'a'
Found 211 hits with Last Name = 'nozoe' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606383
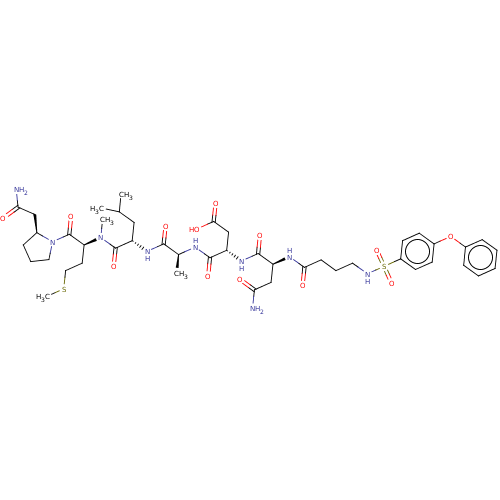
(CHEMBL5209489)Show SMILES CSCC[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606385
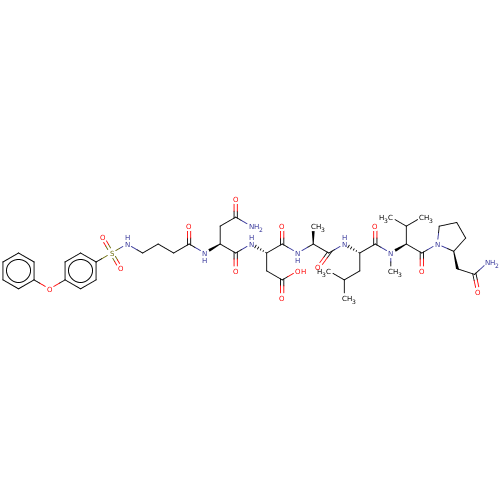
(CHEMBL5203315)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606395
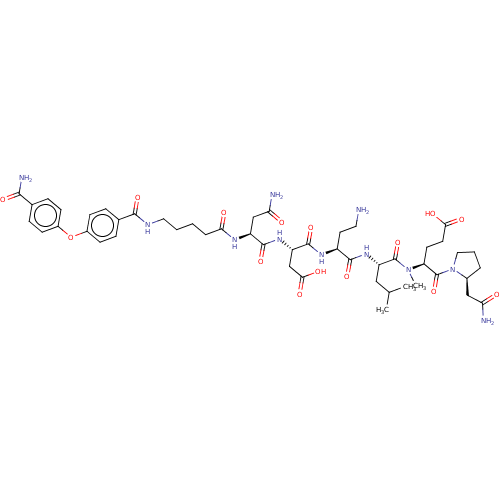
(CHEMBL5207094)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606386
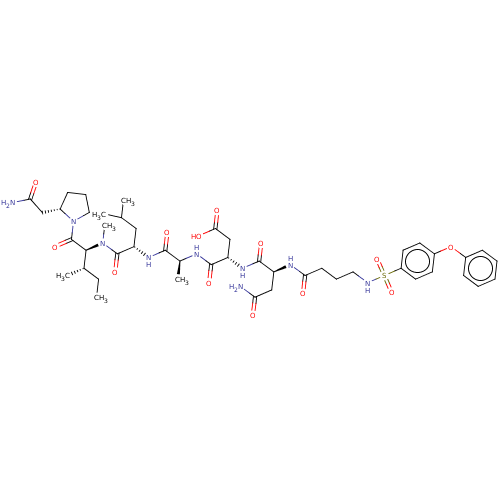
(CHEMBL5182207)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606384
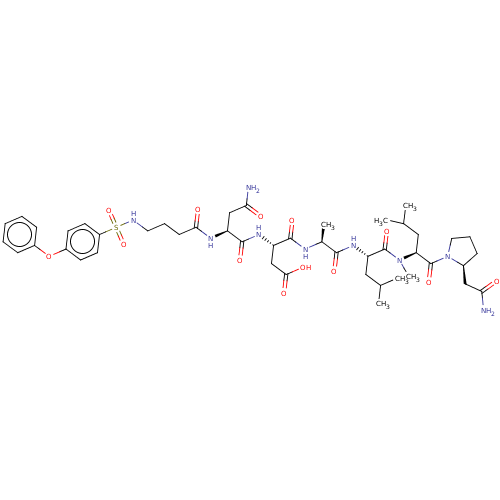
(CHEMBL5183245)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CC(C)C)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606380
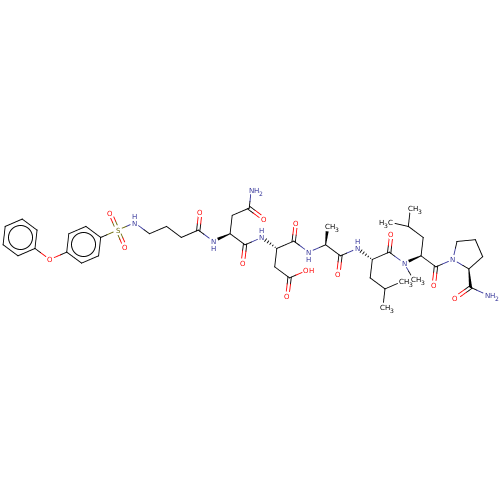
(CHEMBL5191373)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606390
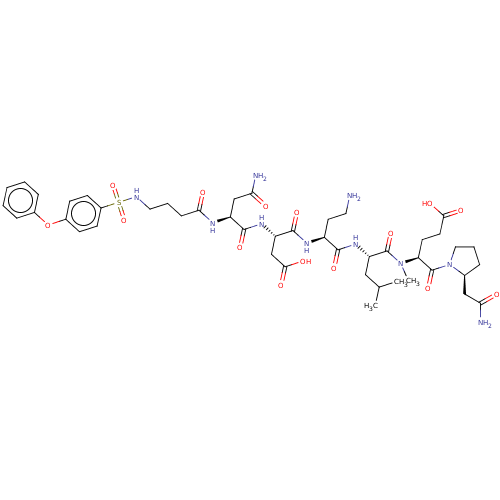
(CHEMBL5185612)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606381
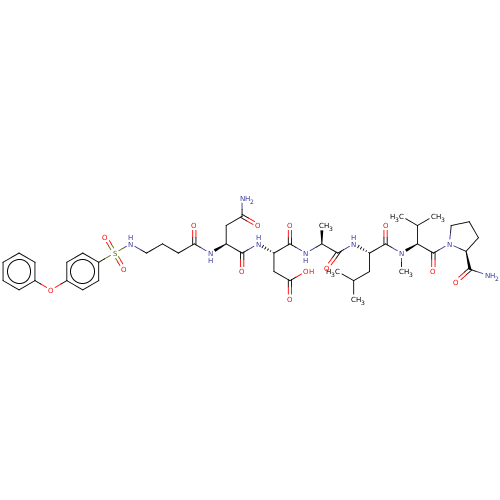
(CHEMBL5198135)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606377
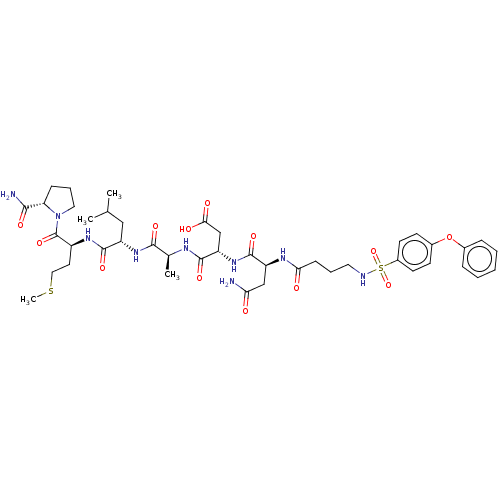
(CHEMBL5186594)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606378
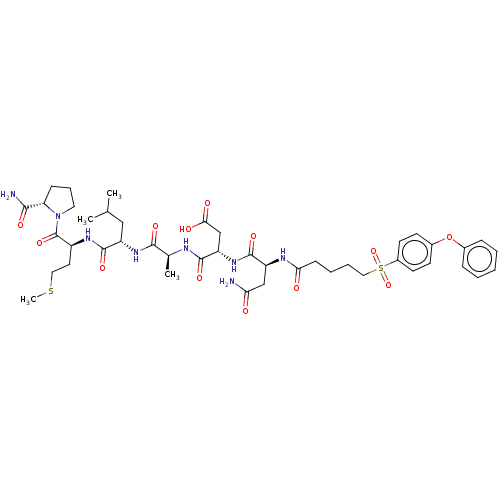
(CHEMBL5170274)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606388
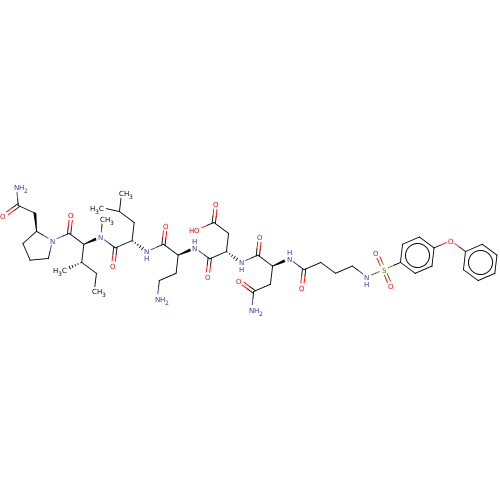
(CHEMBL5203492)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606382
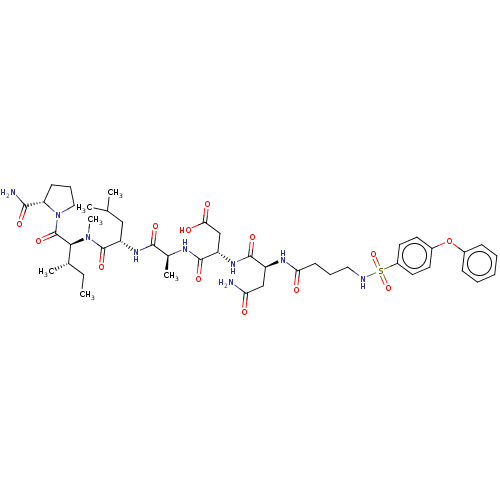
(CHEMBL5191820)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606392
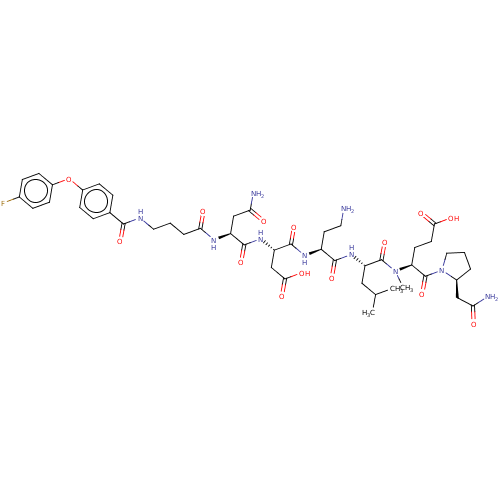
(CHEMBL5178802)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNC(=O)c1ccc(Oc2ccc(F)cc2)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606379

(CHEMBL5180149)Show SMILES CSCC[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.128 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606387
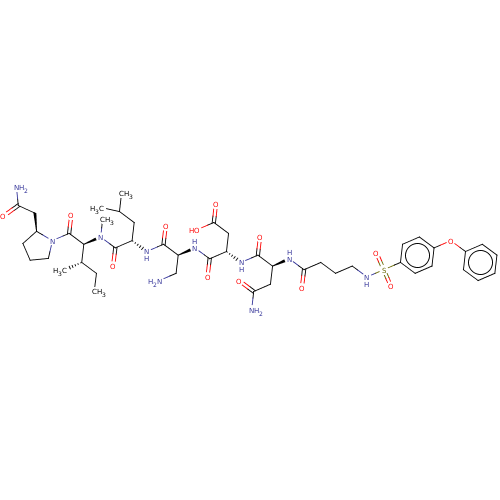
(CHEMBL5206852)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606396
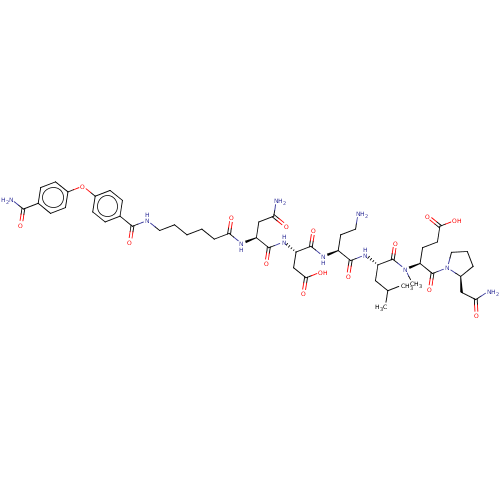
(CHEMBL5206743)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606393

(CHEMBL5190160)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606391
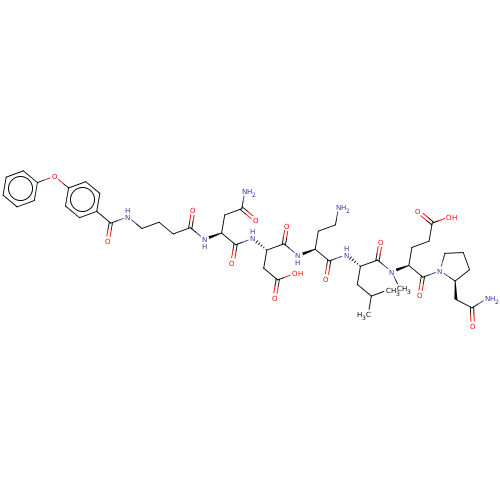
(CHEMBL5207603)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNC(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606397

(CHEMBL5169565)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606376
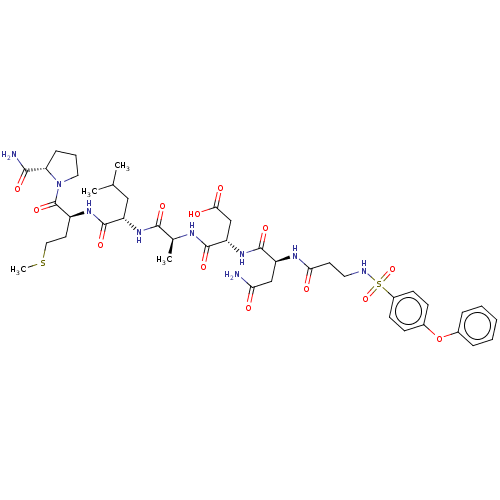
(CHEMBL5202634)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606394

(CHEMBL5177058)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606389

(CHEMBL5209121)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50606384

(CHEMBL5183245)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CC(C)C)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50606383

(CHEMBL5209489)Show SMILES CSCC[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50402166
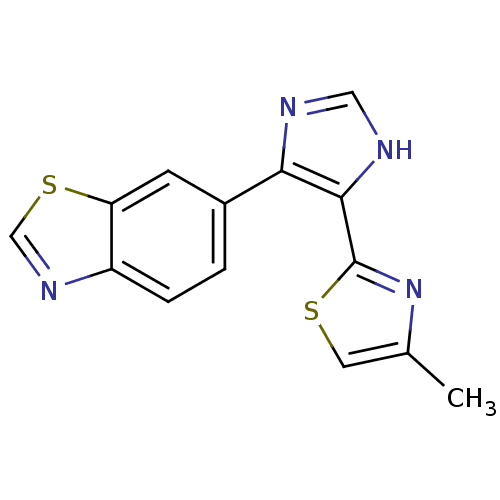
(CHEMBL2207988)Show InChI InChI=1S/C14H10N4S2/c1-8-5-19-14(18-8)13-12(15-6-16-13)9-2-3-10-11(4-9)20-7-17-10/h2-7H,1H3,(H,15,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ALK5 using GST-tagged Smad3 as substrate assessed as substrate phosphorylation after 30 mins by ELISA |
Bioorg Med Chem 20: 7128-38 (2012)
Article DOI: 10.1016/j.bmc.2012.09.066
BindingDB Entry DOI: 10.7270/Q2542PQ0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606373
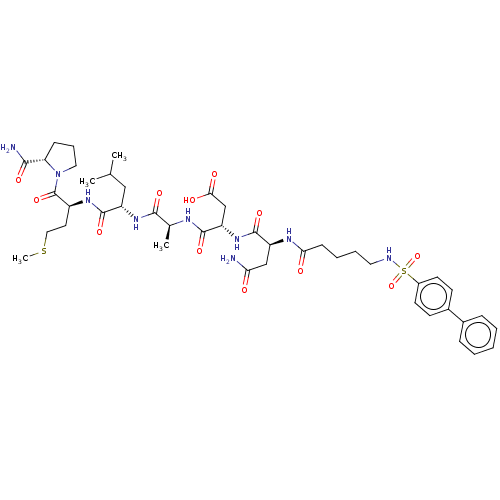
(CHEMBL5185823)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCNS(=O)(=O)c1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50606380

(CHEMBL5191373)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50402175
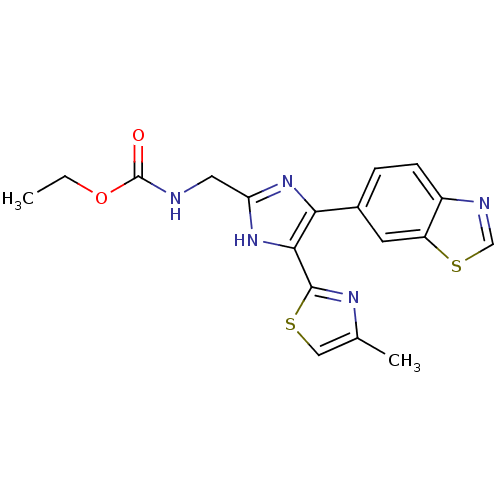
(CHEMBL2208016)Show SMILES CCOC(=O)NCc1nc(c([nH]1)-c1nc(C)cs1)-c1ccc2ncsc2c1 Show InChI InChI=1S/C18H17N5O2S2/c1-3-25-18(24)19-7-14-22-15(16(23-14)17-21-10(2)8-26-17)11-4-5-12-13(6-11)27-9-20-12/h4-6,8-9H,3,7H2,1-2H3,(H,19,24)(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ALK5 using GST-tagged Smad3 as substrate assessed as substrate phosphorylation after 30 mins by ELISA |
Bioorg Med Chem 20: 7128-38 (2012)
Article DOI: 10.1016/j.bmc.2012.09.066
BindingDB Entry DOI: 10.7270/Q2542PQ0 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50606386

(CHEMBL5182207)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50402168
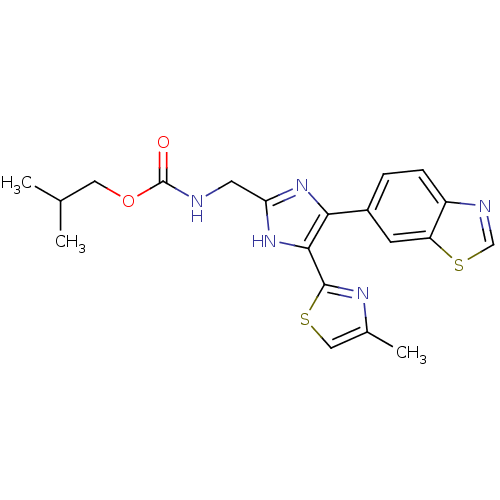
(CHEMBL2208237)Show SMILES CC(C)COC(=O)NCc1nc(c([nH]1)-c1nc(C)cs1)-c1ccc2ncsc2c1 Show InChI InChI=1S/C20H21N5O2S2/c1-11(2)8-27-20(26)21-7-16-24-17(18(25-16)19-23-12(3)9-28-19)13-4-5-14-15(6-13)29-10-22-14/h4-6,9-11H,7-8H2,1-3H3,(H,21,26)(H,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ALK5 using GST-tagged Smad3 as substrate assessed as substrate phosphorylation after 30 mins by ELISA |
Bioorg Med Chem 20: 7128-38 (2012)
Article DOI: 10.1016/j.bmc.2012.09.066
BindingDB Entry DOI: 10.7270/Q2542PQ0 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50606385

(CHEMBL5203315)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50606383

(CHEMBL5209489)Show SMILES CSCC[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50402158

(CHEMBL2207996)Show SMILES Cc1csc(n1)-c1[nH]c(nc1-c1ccc2ncsc2c1)C(F)(F)F Show InChI InChI=1S/C15H9F3N4S2/c1-7-5-23-13(20-7)12-11(21-14(22-12)15(16,17)18)8-2-3-9-10(4-8)24-6-19-9/h2-6H,1H3,(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ALK5 using GST-tagged Smad3 as substrate assessed as substrate phosphorylation after 30 mins by ELISA |
Bioorg Med Chem 20: 7128-38 (2012)
Article DOI: 10.1016/j.bmc.2012.09.066
BindingDB Entry DOI: 10.7270/Q2542PQ0 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50402165
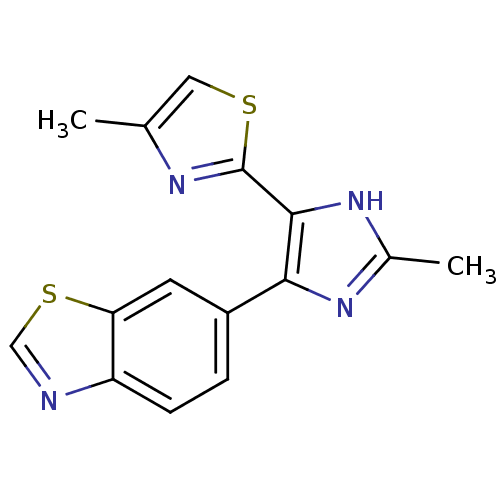
(CHEMBL2207989)Show InChI InChI=1S/C15H12N4S2/c1-8-6-20-15(17-8)14-13(18-9(2)19-14)10-3-4-11-12(5-10)21-7-16-11/h3-7H,1-2H3,(H,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ALK5 using GST-tagged Smad3 as substrate assessed as substrate phosphorylation after 30 mins by ELISA |
Bioorg Med Chem 20: 7128-38 (2012)
Article DOI: 10.1016/j.bmc.2012.09.066
BindingDB Entry DOI: 10.7270/Q2542PQ0 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50606378

(CHEMBL5170274)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606371
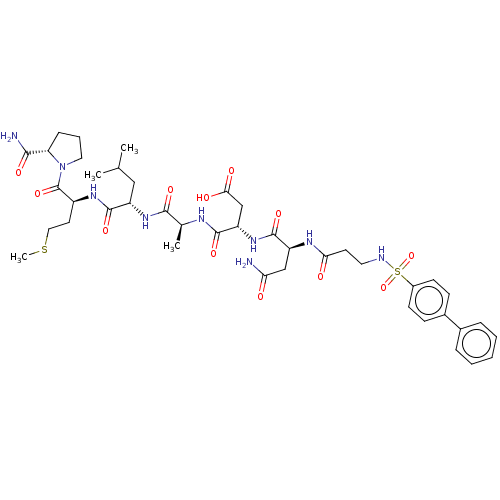
(CHEMBL5209314)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCNS(=O)(=O)c1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50606379

(CHEMBL5180149)Show SMILES CSCC[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50402159
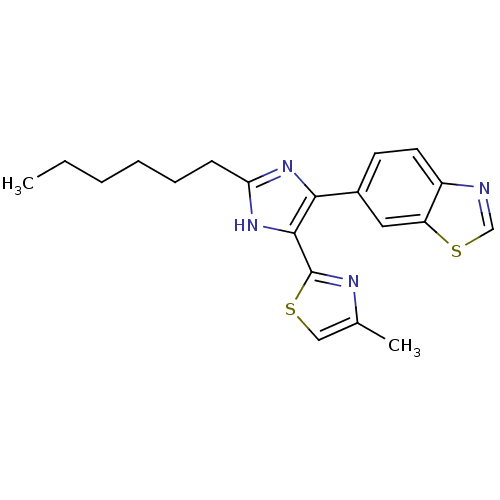
(CHEMBL2207995)Show SMILES CCCCCCc1nc(c([nH]1)-c1nc(C)cs1)-c1ccc2ncsc2c1 Show InChI InChI=1S/C20H22N4S2/c1-3-4-5-6-7-17-23-18(19(24-17)20-22-13(2)11-25-20)14-8-9-15-16(10-14)26-12-21-15/h8-12H,3-7H2,1-2H3,(H,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ALK5 using GST-tagged Smad3 as substrate assessed as substrate phosphorylation after 30 mins by ELISA |
Bioorg Med Chem 20: 7128-38 (2012)
Article DOI: 10.1016/j.bmc.2012.09.066
BindingDB Entry DOI: 10.7270/Q2542PQ0 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50402144
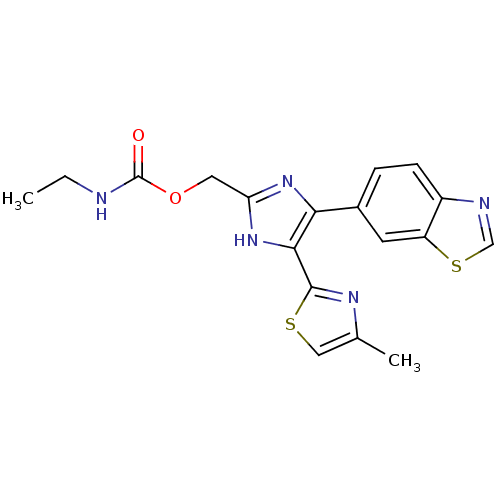
(CHEMBL2208011)Show SMILES CCNC(=O)OCc1nc(c([nH]1)-c1nc(C)cs1)-c1ccc2ncsc2c1 Show InChI InChI=1S/C18H17N5O2S2/c1-3-19-18(24)25-7-14-22-15(16(23-14)17-21-10(2)8-26-17)11-4-5-12-13(6-11)27-9-20-12/h4-6,8-9H,3,7H2,1-2H3,(H,19,24)(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ALK5 using GST-tagged Smad3 as substrate assessed as substrate phosphorylation after 30 mins by ELISA |
Bioorg Med Chem 20: 7128-38 (2012)
Article DOI: 10.1016/j.bmc.2012.09.066
BindingDB Entry DOI: 10.7270/Q2542PQ0 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50402172
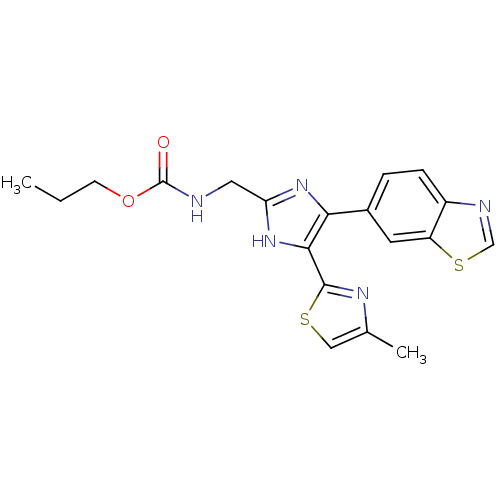
(CHEMBL2208019)Show SMILES CCCOC(=O)NCc1nc(c([nH]1)-c1nc(C)cs1)-c1ccc2ncsc2c1 Show InChI InChI=1S/C19H19N5O2S2/c1-3-6-26-19(25)20-8-15-23-16(17(24-15)18-22-11(2)9-27-18)12-4-5-13-14(7-12)28-10-21-13/h4-5,7,9-10H,3,6,8H2,1-2H3,(H,20,25)(H,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ALK5 using GST-tagged Smad3 as substrate assessed as substrate phosphorylation after 30 mins by ELISA |
Bioorg Med Chem 20: 7128-38 (2012)
Article DOI: 10.1016/j.bmc.2012.09.066
BindingDB Entry DOI: 10.7270/Q2542PQ0 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50606379

(CHEMBL5180149)Show SMILES CSCC[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50402173
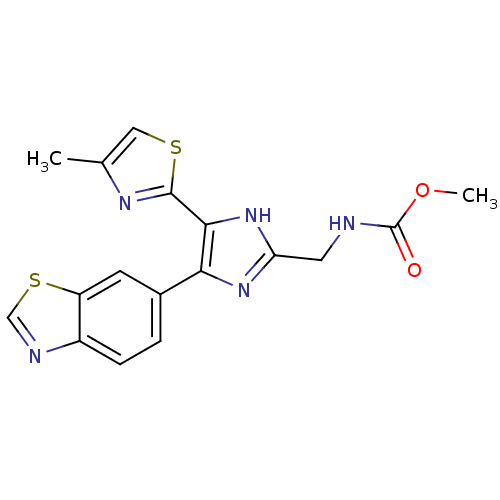
(CHEMBL2208018)Show SMILES COC(=O)NCc1nc(c([nH]1)-c1nc(C)cs1)-c1ccc2ncsc2c1 Show InChI InChI=1S/C17H15N5O2S2/c1-9-7-25-16(20-9)15-14(21-13(22-15)6-18-17(23)24-2)10-3-4-11-12(5-10)26-8-19-11/h3-5,7-8H,6H2,1-2H3,(H,18,23)(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ALK5 using GST-tagged Smad3 as substrate assessed as substrate phosphorylation after 30 mins by ELISA |
Bioorg Med Chem 20: 7128-38 (2012)
Article DOI: 10.1016/j.bmc.2012.09.066
BindingDB Entry DOI: 10.7270/Q2542PQ0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data