 Found 999 hits with Last Name = 'ouyang' and Initial = 'l'
Found 999 hits with Last Name = 'ouyang' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513202
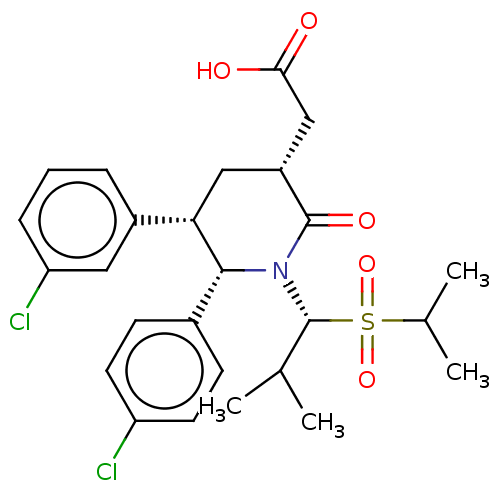
(CHEMBL4463050)Show SMILES CC(C)[C@H](N1[C@@H]([C@@H](C[C@H](CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)S(=O)(=O)C(C)C |r| Show InChI InChI=1S/C26H31Cl2NO5S/c1-15(2)26(35(33,34)16(3)4)29-24(17-8-10-20(27)11-9-17)22(18-6-5-7-21(28)12-18)13-19(25(29)32)14-23(30)31/h5-12,15-16,19,22,24,26H,13-14H2,1-4H3,(H,30,31)/t19-,22+,24-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 in human SJSA1 cells |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM370121
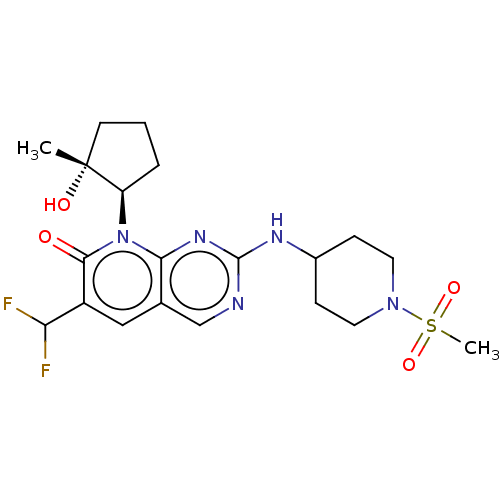
(6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...)Show SMILES C[C@@]1(O)CCC[C@H]1n1c2nc(NC3CCN(CC3)S(C)(=O)=O)ncc2cc(C(F)F)c1=O |r| Show InChI InChI=1S/C20H27F2N5O4S/c1-20(29)7-3-4-15(20)27-17-12(10-14(16(21)22)18(27)28)11-23-19(25-17)24-13-5-8-26(9-6-13)32(2,30)31/h10-11,13,15-16,29H,3-9H2,1-2H3,(H,23,24,25)/t15-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM370121

(6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...)Show SMILES C[C@@]1(O)CCC[C@H]1n1c2nc(NC3CCN(CC3)S(C)(=O)=O)ncc2cc(C(F)F)c1=O |r| Show InChI InChI=1S/C20H27F2N5O4S/c1-20(29)7-3-4-15(20)27-17-12(10-14(16(21)22)18(27)28)11-23-19(25-17)24-13-5-8-26(9-6-13)32(2,30)31/h10-11,13,15-16,29H,3-9H2,1-2H3,(H,23,24,25)/t15-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM370121

(6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...)Show SMILES C[C@@]1(O)CCC[C@H]1n1c2nc(NC3CCN(CC3)S(C)(=O)=O)ncc2cc(C(F)F)c1=O |r| Show InChI InChI=1S/C20H27F2N5O4S/c1-20(29)7-3-4-15(20)27-17-12(10-14(16(21)22)18(27)28)11-23-19(25-17)24-13-5-8-26(9-6-13)32(2,30)31/h10-11,13,15-16,29H,3-9H2,1-2H3,(H,23,24,25)/t15-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513204

(CHEMBL4537250)Show SMILES CC(C)(C)C[C@H]1NC([C@H](c2cccc(Cl)c2F)[C@@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@@](C)(O)C1 |r,wU:33.38,17.29,31.34,8.8,wD:5.4,(55.7,-19.72,;54.25,-19.21,;53.96,-17.7,;55.33,-18.11,;53.08,-20.22,;51.63,-19.71,;51.18,-18.23,;49.64,-18.2,;49.13,-19.66,;47.62,-19.35,;47.13,-17.89,;45.63,-17.58,;44.6,-18.74,;45.1,-20.2,;44.09,-21.36,;46.61,-20.5,;45.83,-21.82,;50.36,-20.59,;51.61,-21.5,;53.08,-21.03,;51.13,-22.97,;49.59,-22.97,;48.57,-24.1,;47.07,-23.79,;46.05,-24.94,;46.59,-22.32,;47.62,-21.19,;49.12,-21.51,;49.2,-16.72,;47.7,-16.36,;50.26,-15.6,;51.76,-15.96,;53.06,-15.15,;53.86,-16.46,;54.95,-15.36,;55.36,-16.85,;52.56,-17.27,)| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-26(2,3)13-20-28(17-9-8-14(29)10-19(17)33-25(28)36)21(16-6-5-7-18(30)22(16)31)23(34-20)24(35)32-15-11-27(4,37)12-15/h5-10,15,20-21,23,34,37H,11-13H2,1-4H3,(H,32,35)(H,33,36)/t15-,20-,21+,23?,27+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) by western blot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513204

(CHEMBL4537250)Show SMILES CC(C)(C)C[C@H]1NC([C@H](c2cccc(Cl)c2F)[C@@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@@](C)(O)C1 |r,wU:33.38,17.29,31.34,8.8,wD:5.4,(55.7,-19.72,;54.25,-19.21,;53.96,-17.7,;55.33,-18.11,;53.08,-20.22,;51.63,-19.71,;51.18,-18.23,;49.64,-18.2,;49.13,-19.66,;47.62,-19.35,;47.13,-17.89,;45.63,-17.58,;44.6,-18.74,;45.1,-20.2,;44.09,-21.36,;46.61,-20.5,;45.83,-21.82,;50.36,-20.59,;51.61,-21.5,;53.08,-21.03,;51.13,-22.97,;49.59,-22.97,;48.57,-24.1,;47.07,-23.79,;46.05,-24.94,;46.59,-22.32,;47.62,-21.19,;49.12,-21.51,;49.2,-16.72,;47.7,-16.36,;50.26,-15.6,;51.76,-15.96,;53.06,-15.15,;53.86,-16.46,;54.95,-15.36,;55.36,-16.85,;52.56,-17.27,)| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-26(2,3)13-20-28(17-9-8-14(29)10-19(17)33-25(28)36)21(16-6-5-7-18(30)22(16)31)23(34-20)24(35)32-15-11-27(4,37)12-15/h5-10,15,20-21,23,34,37H,11-13H2,1-4H3,(H,32,35)(H,33,36)/t15-,20-,21+,23?,27+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of wild type p53 protein binding to MDM2 in human HCT116 cells by immunoblot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513202

(CHEMBL4463050)Show SMILES CC(C)[C@H](N1[C@@H]([C@@H](C[C@H](CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)S(=O)(=O)C(C)C |r| Show InChI InChI=1S/C26H31Cl2NO5S/c1-15(2)26(35(33,34)16(3)4)29-24(17-8-10-20(27)11-9-17)22(18-6-5-7-21(28)12-18)13-19(25(29)32)14-23(30)31/h5-12,15-16,19,22,24,26H,13-14H2,1-4H3,(H,30,31)/t19-,22+,24-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513209
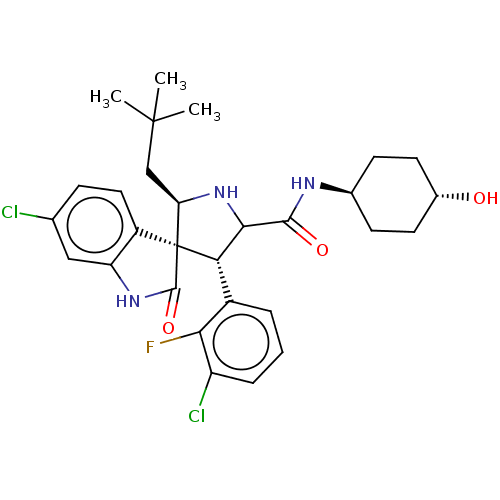
(CHEMBL4440151)Show SMILES CC(C)(C)C[C@H]1NC([C@H](c2cccc(Cl)c2F)[C@@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:17.29,8.8,31.34,wD:5.4,34.38,(26.42,-21.67,;26.71,-23.19,;27.79,-22.08,;28.16,-23.69,;25.54,-24.19,;24.09,-23.68,;23.64,-22.2,;22.09,-22.17,;21.58,-23.63,;20.07,-23.32,;19.59,-21.85,;18.08,-21.55,;17.05,-22.71,;17.55,-24.18,;16.53,-25.33,;19.06,-24.48,;18.28,-25.8,;22.81,-24.57,;24.07,-25.48,;25.53,-25,;23.58,-26.94,;22.04,-26.94,;21.02,-28.08,;19.52,-27.77,;18.49,-28.92,;19.04,-26.31,;20.07,-25.16,;21.57,-25.48,;21.65,-20.69,;20.15,-20.33,;22.71,-19.57,;22.28,-18.1,;20.79,-17.74,;20.37,-16.26,;21.43,-15.16,;21,-13.68,;22.92,-15.53,;23.35,-16.99,)| Show InChI InChI=1S/C29H34Cl2FN3O3/c1-28(2,3)14-22-29(19-12-7-15(30)13-21(19)34-27(29)38)23(18-5-4-6-20(31)24(18)32)25(35-22)26(37)33-16-8-10-17(36)11-9-16/h4-7,12-13,16-17,22-23,25,35-36H,8-11,14H2,1-3H3,(H,33,37)(H,34,38)/t16-,17-,22-,23+,25?,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) by western blot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24466
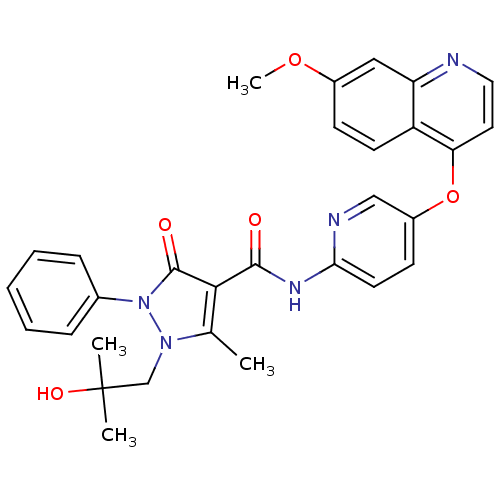
(1-(2-hydroxy-2-methylpropyl)-N-{5-[(7-methoxyquino...)Show SMILES COc1ccc2c(Oc3ccc(NC(=O)c4c(C)n(CC(C)(C)O)n(-c5ccccc5)c4=O)nc3)ccnc2c1 Show InChI InChI=1S/C30H29N5O5/c1-19-27(29(37)35(20-8-6-5-7-9-20)34(19)18-30(2,3)38)28(36)33-26-13-11-22(17-32-26)40-25-14-15-31-24-16-21(39-4)10-12-23(24)25/h5-17,38H,18H2,1-4H3,(H,32,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) |
Eur J Med Chem 108: 495-504 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.016
BindingDB Entry DOI: 10.7270/Q2G73GMC |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513206
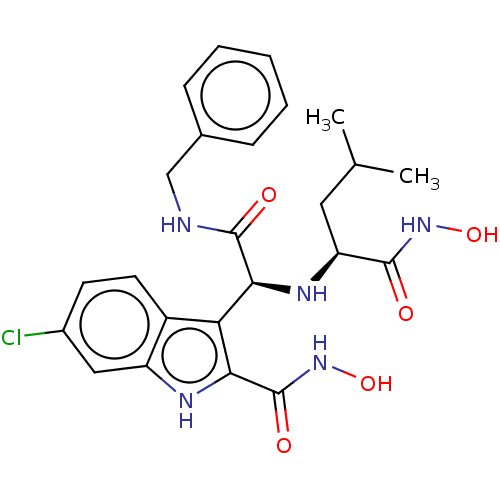
(CHEMBL4546186)Show SMILES CC(C)C[C@H](N[C@H](C(=O)NCc1ccccc1)c1c([nH]c2cc(Cl)ccc12)C(=O)NO)C(=O)NO |r| Show InChI InChI=1S/C24H28ClN5O5/c1-13(2)10-18(22(31)29-34)28-20(23(32)26-12-14-6-4-3-5-7-14)19-16-9-8-15(25)11-17(16)27-21(19)24(33)30-35/h3-9,11,13,18,20,27-28,34-35H,10,12H2,1-2H3,(H,26,32)(H,29,31)(H,30,33)/t18-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) by western blot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513205
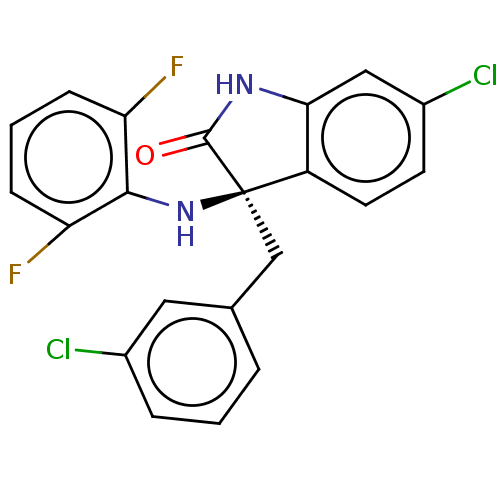
(CHEMBL4437666)Show SMILES Fc1cccc(F)c1N[C@@]1(Cc2cccc(Cl)c2)C(=O)Nc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C21H14Cl2F2N2O/c22-13-4-1-3-12(9-13)11-21(27-19-16(24)5-2-6-17(19)25)15-8-7-14(23)10-18(15)26-20(21)28/h1-10,27H,11H2,(H,26,28)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) by western blot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50593636

(CHEMBL5192732)Show SMILES CCNC(=O)C1=CC2C(N1)C(=O)N(C)C=C2c1cc(ccc1Oc1c(C)cc(F)cc1C)S(=O)(=O)CC |c:15,t:5| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01835
BindingDB Entry DOI: 10.7270/Q2V98D2C |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50502237

(CHEMBL4470451)Show SMILES CCn1c2ccccc2c2cc(NC(=O)CSc3nc4ccccc4[nH]3)ccc12 Show InChI InChI=1S/C23H20N4OS/c1-2-27-20-10-6-3-7-16(20)17-13-15(11-12-21(17)27)24-22(28)14-29-23-25-18-8-4-5-9-19(18)26-23/h3-13H,2,14H2,1H3,(H,24,28)(H,25,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM439497
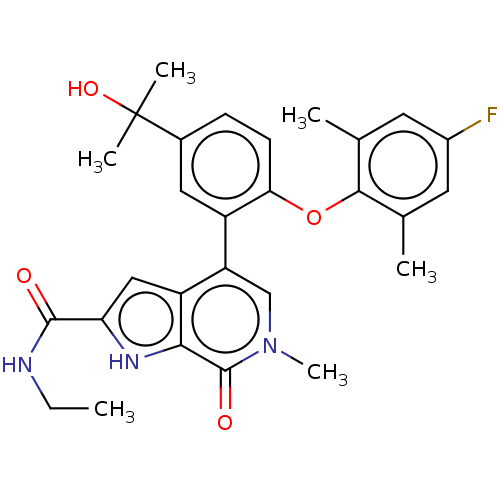
(US10633379, Example 35 | US10633379, Example 82)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(ccc1Oc1c(C)cc(F)cc1C)C(C)(C)O Show InChI InChI=1S/C28H30FN3O4/c1-7-30-26(33)22-13-20-21(14-32(6)27(34)24(20)31-22)19-12-17(28(4,5)35)8-9-23(19)36-25-15(2)10-18(29)11-16(25)3/h8-14,31,35H,7H2,1-6H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
pA2 for NK2 receptor of rabbit pulmonary artery |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50609374

(CHEMBL5274383) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM209932
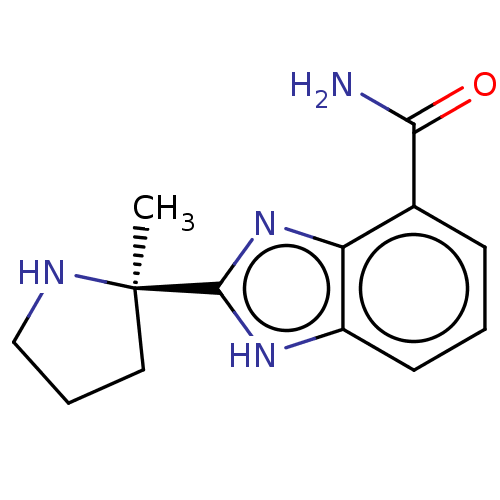
(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against histamine H3 receptor in an electrically stimulated guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against histamine H3 receptor in an electrically stimulated guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM190196
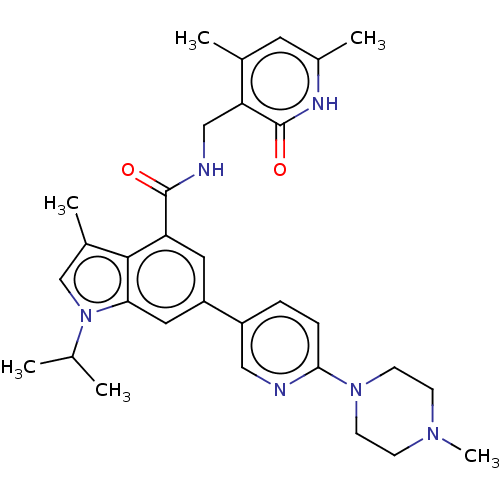
(EPZ008277 | US9175331, 25)Show SMILES CC(C)n1cc(C)c2c(cc(cc12)-c1ccc(nc1)N1CCN(C)CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C31H38N6O2/c1-19(2)37-18-21(4)29-25(30(38)33-17-26-20(3)13-22(5)34-31(26)39)14-24(15-27(29)37)23-7-8-28(32-16-23)36-11-9-35(6)10-12-36/h7-8,13-16,18-19H,9-12,17H2,1-6H3,(H,33,38)(H,34,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114419
BindingDB Entry DOI: 10.7270/Q2PR80Z0 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM190196

(EPZ008277 | US9175331, 25)Show SMILES CC(C)n1cc(C)c2c(cc(cc12)-c1ccc(nc1)N1CCN(C)CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C31H38N6O2/c1-19(2)37-18-21(4)29-25(30(38)33-17-26-20(3)13-22(5)34-31(26)39)14-24(15-27(29)37)23-7-8-28(32-16-23)36-11-9-35(6)10-12-36/h7-8,13-16,18-19H,9-12,17H2,1-6H3,(H,33,38)(H,34,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114419
BindingDB Entry DOI: 10.7270/Q2PR80Z0 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) |
Eur J Med Chem 108: 495-504 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.016
BindingDB Entry DOI: 10.7270/Q2G73GMC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50610781
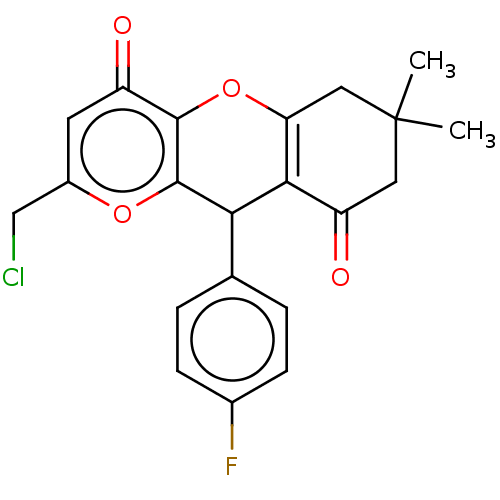
(CHEMBL5277261)Show SMILES CC1(C)CC(=O)C2=C(C1)Oc1c(oc(CCl)cc1=O)C2c1ccc(F)cc1 |c:6| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50031467

(5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...)Show InChI InChI=1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM209932

(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against histamine H3 receptor in an electrically stimulated guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM31196

(spiro-oxindole, 1)Show SMILES CN(C)C(=O)[C@@H]1N[C@H](CC(C)(C)C)[C@@]2([C@H]1c1cccc(Cl)c1)C(=O)Nc1cc(Cl)ccc21 |r| Show InChI InChI=1S/C25H29Cl2N3O2/c1-24(2,3)13-19-25(17-10-9-16(27)12-18(17)28-23(25)32)20(14-7-6-8-15(26)11-14)21(29-19)22(31)30(4)5/h6-12,19-21,29H,13H2,1-5H3,(H,28,32)/t19-,20+,21-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) by western blot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50300119
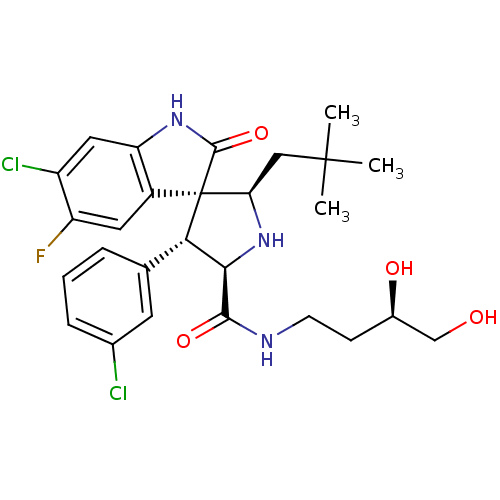
((2'R,3S,4'R,5'R)-6-chloro-4'-(3-chlorophenyl)-N-((...)Show SMILES CC(C)(C)C[C@H]1N[C@H]([C@H](c2cccc(Cl)c2)[C@@]11C(=O)Nc2cc(Cl)c(F)cc12)C(=O)NCC[C@@H](O)CO |r| Show InChI InChI=1S/C27H32Cl2FN3O4/c1-26(2,3)12-21-27(17-10-19(30)18(29)11-20(17)32-25(27)37)22(14-5-4-6-15(28)9-14)23(33-21)24(36)31-8-7-16(35)13-34/h4-6,9-11,16,21-23,33-35H,7-8,12-13H2,1-3H3,(H,31,36)(H,32,37)/t16-,21-,22+,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) by western blot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50300119

((2'R,3S,4'R,5'R)-6-chloro-4'-(3-chlorophenyl)-N-((...)Show SMILES CC(C)(C)C[C@H]1N[C@H]([C@H](c2cccc(Cl)c2)[C@@]11C(=O)Nc2cc(Cl)c(F)cc12)C(=O)NCC[C@@H](O)CO |r| Show InChI InChI=1S/C27H32Cl2FN3O4/c1-26(2,3)12-21-27(17-10-19(30)18(29)11-20(17)32-25(27)37)22(14-5-4-6-15(28)9-14)23(33-21)24(36)31-8-7-16(35)13-34/h4-6,9-11,16,21-23,33-35H,7-8,12-13H2,1-3H3,(H,31,36)(H,32,37)/t16-,21-,22+,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of wild type p53 protein binding to MDM2 in human HCT116 cells by immunoblot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50610790

(CHEMBL5287587) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50593636

(CHEMBL5192732)Show SMILES CCNC(=O)C1=CC2C(N1)C(=O)N(C)C=C2c1cc(ccc1Oc1c(C)cc(F)cc1C)S(=O)(=O)CC |c:15,t:5| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01835
BindingDB Entry DOI: 10.7270/Q2V98D2C |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50420273

(CHEMBL2089208)Show SMILES COC(=O)C(N1C(c2ccc(Cl)cc2)C(=S)Nc2ccc(F)cc2C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H17Cl2FN2O3S/c1-32-24(31)21(14-4-8-16(26)9-5-14)29-20(13-2-6-15(25)7-3-13)22(33)28-19-11-10-17(27)12-18(19)23(29)30/h2-12,20-21H,1H3,(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50420268
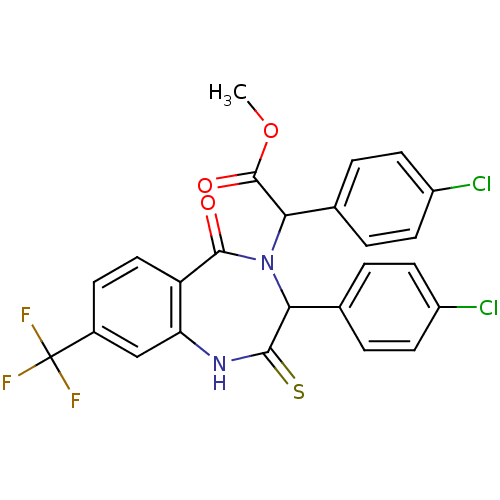
(CHEMBL2089203)Show SMILES COC(=O)C(N1C(c2ccc(Cl)cc2)C(=S)Nc2cc(ccc2C1=O)C(F)(F)F)c1ccc(Cl)cc1 Show InChI InChI=1S/C25H17Cl2F3N2O3S/c1-35-24(34)21(14-4-9-17(27)10-5-14)32-20(13-2-7-16(26)8-3-13)22(36)31-19-12-15(25(28,29)30)6-11-18(19)23(32)33/h2-12,20-21H,1H3,(H,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50339398
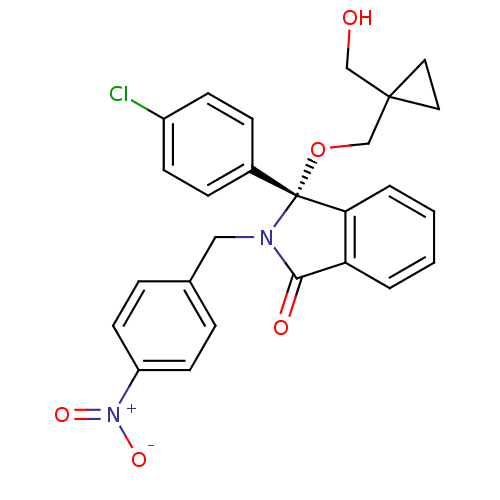
((+)-R-3-(4-Chlorophenyl)-3-(1-hydroxymethylcyclopr...)Show SMILES OCC1(CO[C@]2(N(Cc3ccc(cc3)[N+]([O-])=O)C(=O)c3ccccc23)c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C26H23ClN2O5/c27-20-9-7-19(8-10-20)26(34-17-25(16-30)13-14-25)23-4-2-1-3-22(23)24(31)28(26)15-18-5-11-21(12-6-18)29(32)33/h1-12,30H,13-17H2/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) by western blot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50146168
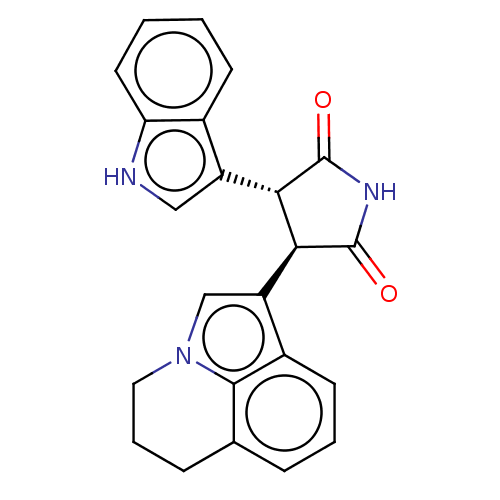
(ARQ-197 | Tivantinib)Show SMILES O=C1NC(=O)[C@H]([C@@H]1c1c[nH]c2ccccc12)c1cn2CCCc3cccc1c23 |r| Show InChI InChI=1S/C23H19N3O2/c27-22-19(16-11-24-18-9-2-1-7-14(16)18)20(23(28)25-22)17-12-26-10-4-6-13-5-3-8-15(17)21(13)26/h1-3,5,7-9,11-12,19-20,24H,4,6,10H2,(H,25,27,28)/t19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) |
Eur J Med Chem 108: 495-504 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.016
BindingDB Entry DOI: 10.7270/Q2G73GMC |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513207
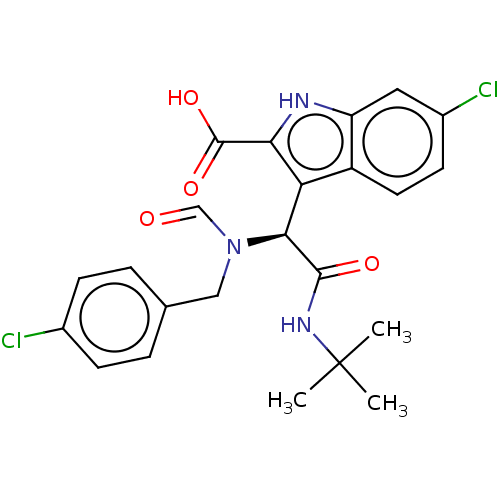
(CHEMBL4566706)Show SMILES CC(C)(C)NC(=O)[C@@H](N(Cc1ccc(Cl)cc1)C=O)c1c([nH]c2cc(Cl)ccc12)C(O)=O |r| Show InChI InChI=1S/C23H23Cl2N3O4/c1-23(2,3)27-21(30)20(28(12-29)11-13-4-6-14(24)7-5-13)18-16-9-8-15(25)10-17(16)26-19(18)22(31)32/h4-10,12,20,26H,11H2,1-3H3,(H,27,30)(H,31,32)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) by western blot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM439497

(US10633379, Example 35 | US10633379, Example 82)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(ccc1Oc1c(C)cc(F)cc1C)C(C)(C)O Show InChI InChI=1S/C28H30FN3O4/c1-7-30-26(33)22-13-20-21(14-32(6)27(34)24(20)31-22)19-12-17(28(4,5)35)8-9-23(19)36-25-15(2)10-18(29)11-16(25)3/h8-14,31,35H,7H2,1-6H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
pA2 for NK2 receptor of rabbit pulmonary artery |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513201
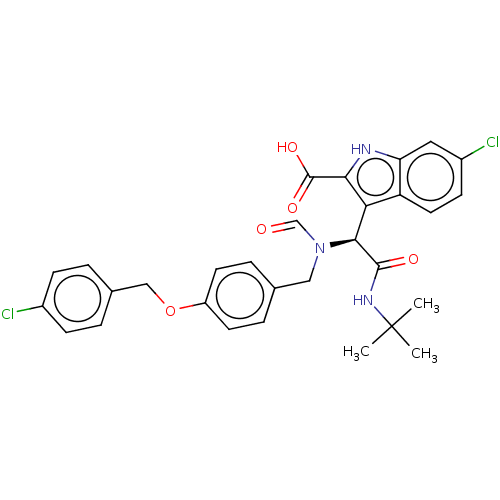
(CHEMBL4447592)Show SMILES CC(C)(C)NC(=O)[C@@H](N(Cc1ccc(OCc2ccc(Cl)cc2)cc1)C=O)c1c([nH]c2cc(Cl)ccc12)C(O)=O |r| Show InChI InChI=1S/C30H29Cl2N3O5/c1-30(2,3)34-28(37)27(25-23-13-10-21(32)14-24(23)33-26(25)29(38)39)35(17-36)15-18-6-11-22(12-7-18)40-16-19-4-8-20(31)9-5-19/h4-14,17,27,33H,15-16H2,1-3H3,(H,34,37)(H,38,39)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) by western blot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50148573
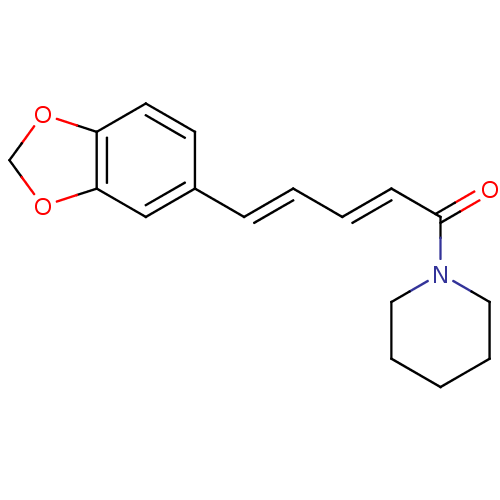
((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...)Show InChI InChI=1S/C17H19NO3/c19-17(18-10-4-1-5-11-18)7-3-2-6-14-8-9-15-16(12-14)21-13-20-15/h2-3,6-9,12H,1,4-5,10-11,13H2/b6-2+,7-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114176
BindingDB Entry DOI: 10.7270/Q2N58RDR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM31196

(spiro-oxindole, 1)Show SMILES CN(C)C(=O)[C@@H]1N[C@H](CC(C)(C)C)[C@@]2([C@H]1c1cccc(Cl)c1)C(=O)Nc1cc(Cl)ccc21 |r| Show InChI InChI=1S/C25H29Cl2N3O2/c1-24(2,3)13-19-25(17-10-9-16(27)12-18(17)28-23(25)32)20(14-7-6-8-15(26)11-14)21(29-19)22(31)30(4)5/h6-12,19-21,29H,13H2,1-5H3,(H,28,32)/t19-,20+,21-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of wild type p53 protein binding to MDM2 in human HCT116 cells by immunoblot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM110191

(3-(2,4-Dihydroxyphenyl)-1-(thiophen-2-yl)propenone...)Show InChI InChI=1S/C13H10O3S/c14-10-5-3-9(12(16)8-10)4-6-11(15)13-2-1-7-17-13/h1-8,14,16H/b6-4+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50021574
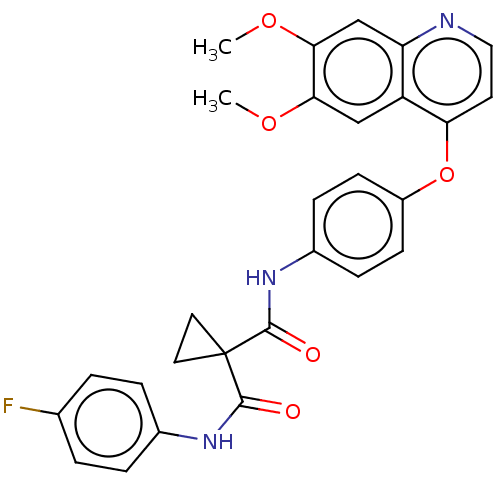
(BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3)c2cc1OC Show InChI InChI=1S/C28H24FN3O5/c1-35-24-15-21-22(16-25(24)36-2)30-14-11-23(21)37-20-9-7-19(8-10-20)32-27(34)28(12-13-28)26(33)31-18-5-3-17(29)4-6-18/h3-11,14-16H,12-13H2,1-2H3,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR-2 using poly(Glu, Tyr) as substrate by AlphaScreen assay |
Eur J Med Chem 108: 495-504 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.016
BindingDB Entry DOI: 10.7270/Q2G73GMC |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM110192
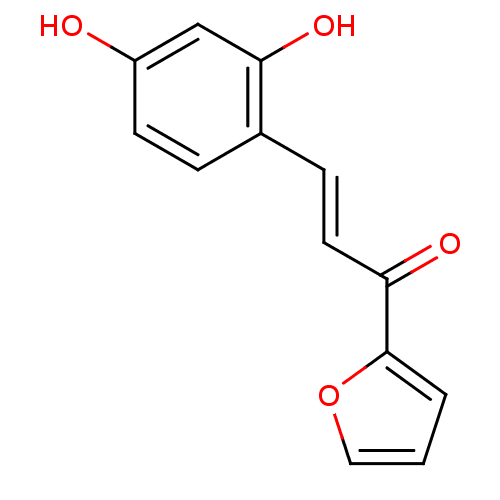
(3-(2,4-Dihydroxyphenyl)-1-(furan-2-yl)propenone (1...)Show InChI InChI=1S/C13H10O4/c14-10-5-3-9(12(16)8-10)4-6-11(15)13-2-1-7-17-13/h1-8,14,16H/b6-4+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0433 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50356191
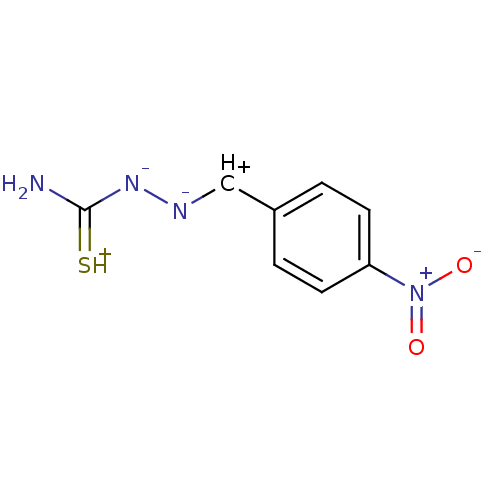
(CHEMBL193804)Show SMILES NC(=[SH+])[N-][N-][CH+]c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C8H7N4O2S/c9-8(15)11-10-5-6-1-3-7(4-2-6)12(13)14/h1-5H,(H2-,9,11,15)/q-1/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50610779
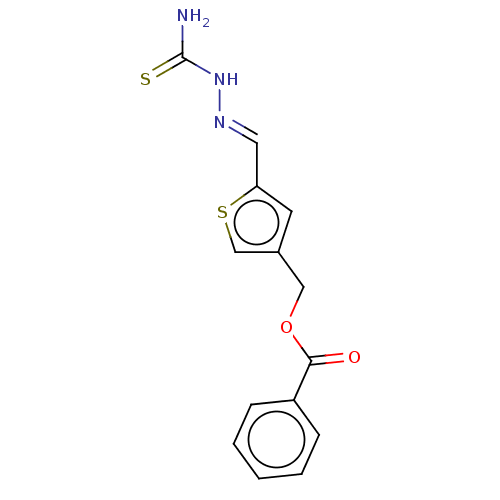
(CHEMBL4475728) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
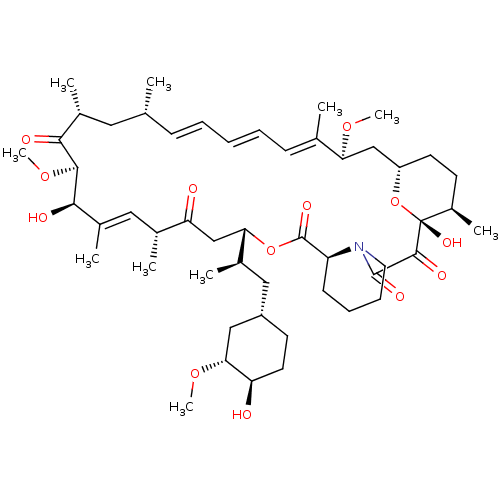
(Homo sapiens (Human)) | BDBM36609
(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyphenol oxidase 2
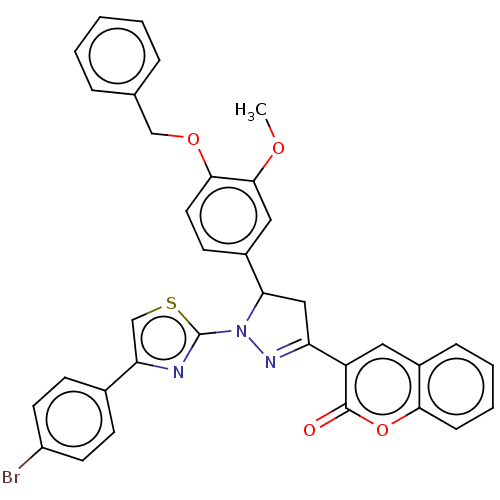
(Agaricus bisporus (Common mushroom)) | BDBM50276368
(CHEMBL4128371)Show SMILES COc1cc(ccc1OCc1ccccc1)C1CC(=NN1c1nc(cs1)-c1ccc(Br)cc1)c1cc2ccccc2oc1=O |c:20| Show InChI InChI=1S/C35H26BrN3O4S/c1-41-33-18-24(13-16-32(33)42-20-22-7-3-2-4-8-22)30-19-28(27-17-25-9-5-6-10-31(25)43-34(27)40)38-39(30)35-37-29(21-44-35)23-11-14-26(36)15-12-23/h2-18,21,30H,19-20H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50610793
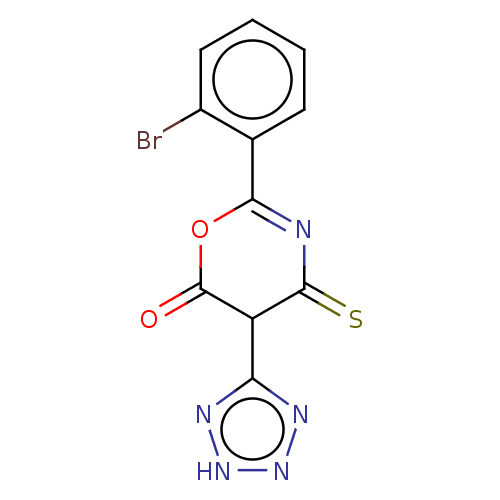
(CHEMBL5286240)Show SMILES Brc1ccccc1C1=NC(=S)C(c2nn[nH]n2)C(=O)O1 |t:8| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50610794
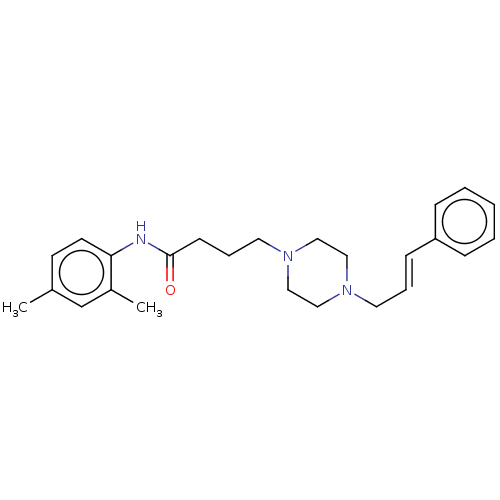
(CHEMBL5286802)Show SMILES Cc1ccc(NC(=O)CCCN2CCN(C\C=C\c3ccccc3)CC2)c(C)c1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50610795
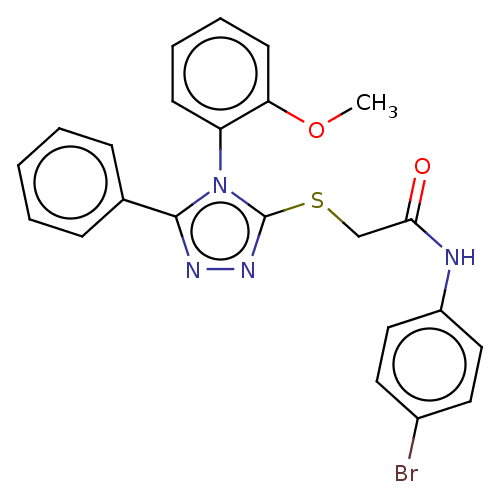
(CHEMBL5289108)Show SMILES COc1ccccc1-n1c(SCC(=O)Nc2ccc(Br)cc2)nnc1-c1ccccc1 |(.69,-1.48,;-.64,-.71,;-1.97,-1.48,;-1.97,-3.01,;-3.31,-3.78,;-4.64,-3.02,;-4.65,-1.48,;-3.31,-.71,;-3.31,.83,;-2.09,1.71,;-.6,1.31,;.49,2.4,;1.98,2,;2.38,.52,;3.07,3.09,;4.55,2.69,;5.64,3.78,;7.13,3.38,;7.52,1.9,;9.01,1.5,;6.44,.81,;4.95,1.2,;-2.55,3.21,;-4.09,3.21,;-4.55,1.75,;-6.04,1.35,;-6.44,-.13,;-7.92,-.53,;-9.01,.56,;-8.62,2.04,;-7.13,2.45,)| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50146167
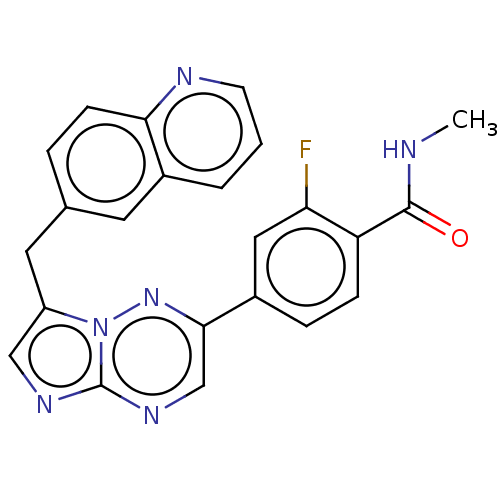
(Capmatinib | INC-280 | INCB-28060 | NVP-INC280 | U...)Show SMILES CNC(=O)c1ccc(cc1F)-c1cnc2ncc(Cc3ccc4ncccc4c3)n2n1 Show InChI InChI=1S/C23H17FN6O/c1-25-22(31)18-6-5-16(11-19(18)24)21-13-28-23-27-12-17(30(23)29-21)10-14-4-7-20-15(9-14)3-2-8-26-20/h2-9,11-13H,10H2,1H3,(H,25,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) |
Eur J Med Chem 108: 495-504 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.016
BindingDB Entry DOI: 10.7270/Q2G73GMC |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50610775
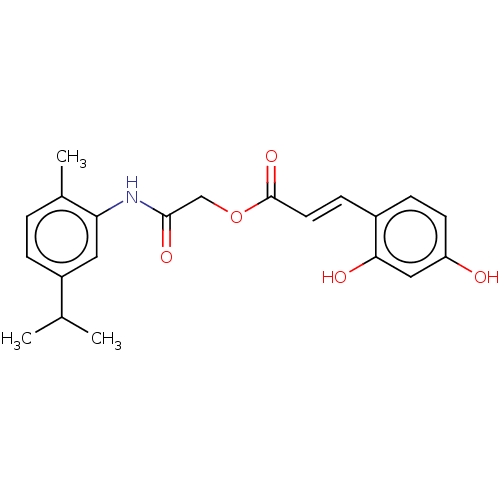
(CHEMBL5277673)Show SMILES CC(C)c1ccc(C)c(NC(=O)COC(=O)\C=C\c2ccc(O)cc2O)c1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data