

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-acetyllactosaminide alpha-1,3-galactosyltransferase (Bos taurus) | BDBM50389789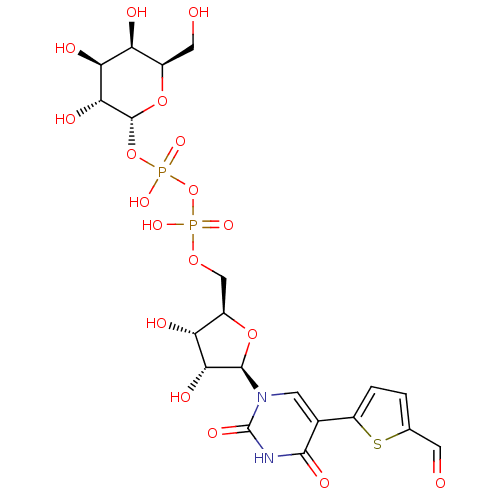 (CHEMBL1229737) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of East Anglia Curated by ChEMBL | Assay Description Inhibition of alpha-1,3-GalT in bovine after 15 mins by Dixon plot analysis | J Med Chem 55: 2015-24 (2012) Article DOI: 10.1021/jm201154p BindingDB Entry DOI: 10.7270/Q28W3FCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetyllactosaminide alpha-1,3-galactosyltransferase (Bos taurus) | BDBM50389786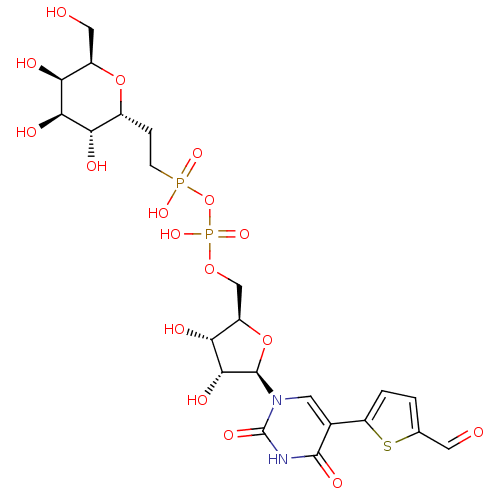 (CHEMBL2070378) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of East Anglia Curated by ChEMBL | Assay Description Inhibition of alpha-1,3-GalT in bovine after 15 mins by Dixon plot analysis | J Med Chem 55: 2015-24 (2012) Article DOI: 10.1021/jm201154p BindingDB Entry DOI: 10.7270/Q28W3FCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetyllactosaminide alpha-1,3-galactosyltransferase (Bos taurus) | BDBM50389788 (CHEMBL2070375) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of East Anglia Curated by ChEMBL | Assay Description Inhibition of alpha-1,3-GalT in bovine after 15 mins by Dixon plot analysis | J Med Chem 55: 2015-24 (2012) Article DOI: 10.1021/jm201154p BindingDB Entry DOI: 10.7270/Q28W3FCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetyllactosaminide alpha-1,3-galactosyltransferase (Bos taurus) | BDBM50389787 (CHEMBL2070376) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of East Anglia Curated by ChEMBL | Assay Description Inhibition of alpha-1,3-GalT in bovine after 15 mins by Dixon plot analysis | J Med Chem 55: 2015-24 (2012) Article DOI: 10.1021/jm201154p BindingDB Entry DOI: 10.7270/Q28W3FCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427444 (CHEMBL2326313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human blood group B Galactosyltransferase using UDP-Gal as substrate by STD NMR analysis in presence of magnesium ions | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427446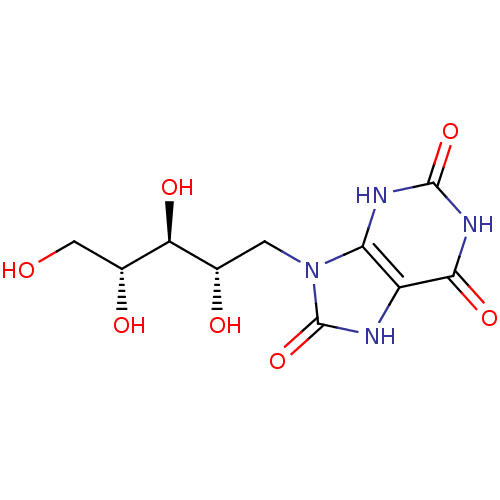 (CHEMBL477507) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human blood group B Galactosyltransferase using UDP-Gal as substrate by STD NMR analysis in presence of magnesium ions | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427444 (CHEMBL2326313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Competitive inhibition of human blood group B Galactosyltransferase using UDP-Gal as substrate by Michaelis-Menten equation analysis in presence of m... | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427445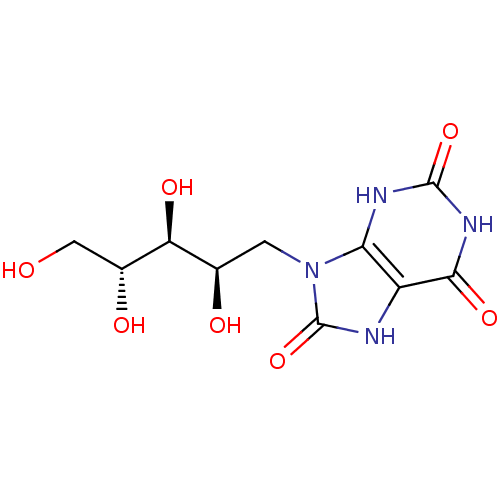 (CHEMBL1235497) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human blood group B Galactosyltransferase using UDP-Gal as substrate by STD NMR analysis in presence of magnesium ions | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427446 (CHEMBL477507) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human blood group B Galactosyltransferase using radiolabeled UDP-Gal as substrate in presence of manganese ions | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427444 (CHEMBL2326313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human blood group B Galactosyltransferase using radiolabeled UDP-Gal as substrate in presence of manganese ions | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427445 (CHEMBL1235497) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human blood group B Galactosyltransferase using radiolabeled UDP-Gal as substrate in presence of manganese ions | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427444 (CHEMBL2326313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human blood group B Galactosyltransferase using UDP-Gal as substrate by STD NMR analysis in presence of magnesium ions | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427444 (CHEMBL2326313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of human blood group B Galactosyltransferase using radiolabeled UDP-Gal as substrate in presence of manganese ions | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427444 (CHEMBL2326313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.82E+5 | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Binding affinity to human blood group B Galactosyltransferase by STD NMR analysis in presence of magnesium ions | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427445 (CHEMBL1235497) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.30E+6 | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Binding affinity to human blood group B Galactosyltransferase after 300 secs by SPR analysis | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histo-blood group ABO system transferase (Homo sapiens (Human)) | BDBM50427444 (CHEMBL2326313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.54E+5 | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Binding affinity to human blood group B Galactosyltransferase after 300 secs by SPR analysis | J Med Chem 56: 2150-4 (2013) Article DOI: 10.1021/jm300642a BindingDB Entry DOI: 10.7270/Q26T0P01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||