 Found 682 hits with Last Name = 'phuong' and Initial = 'vk'
Found 682 hits with Last Name = 'phuong' and Initial = 'vk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412863
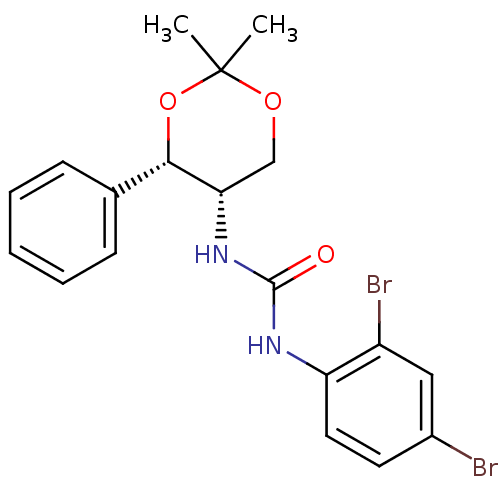
(CHEMBL359632 | JNJ-10397049)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccc(Br)cc2Br)[C@@H](O1)c1ccccc1 |r| Show InChI InChI=1S/C19H20Br2N2O3/c1-19(2)25-11-16(17(26-19)12-6-4-3-5-7-12)23-18(24)22-15-9-8-13(20)10-14(15)21/h3-10,16-17H,11H2,1-2H3,(H2,22,23,24)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415082

(CHEMBL583035)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(I)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15BrFIN4O5S2/c23-14-8-11(4-7-15(14)24)9-18(22(31)32)26-21(30)13-6-5-12(25)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415081
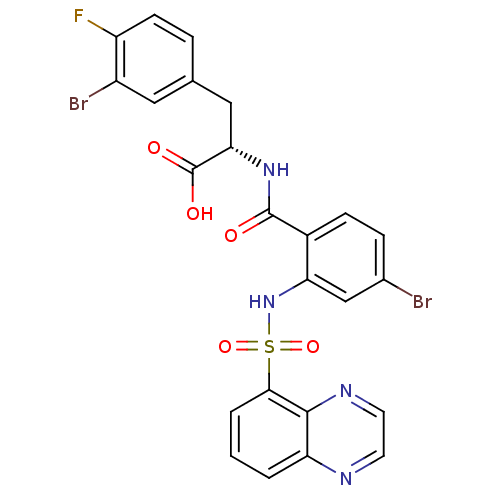
(CHEMBL571650)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H17Br2FN4O5S/c25-14-5-6-15(23(32)30-20(24(33)34)11-13-4-7-17(27)16(26)10-13)19(12-14)31-37(35,36)21-3-1-2-18-22(21)29-9-8-28-18/h1-10,12,20,31H,11H2,(H,30,32)(H,33,34)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412861
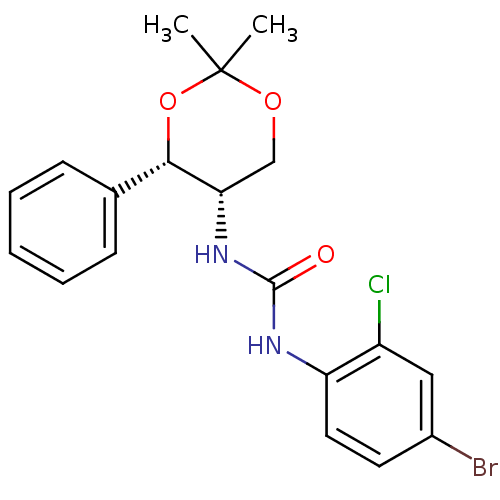
(CHEMBL185088)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccc(Br)cc2Cl)[C@@H](O1)c1ccccc1 |r| Show InChI InChI=1S/C19H20BrClN2O3/c1-19(2)25-11-16(17(26-19)12-6-4-3-5-7-12)23-18(24)22-15-9-8-13(20)10-14(15)21/h3-10,16-17H,11H2,1-2H3,(H2,22,23,24)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415083

(CHEMBL566574)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1cc(Cl)c(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H16BrCl2FN4O5S/c25-14-8-12(4-5-17(14)28)9-20(24(34)35)31-23(33)13-10-15(26)16(27)11-19(13)32-38(36,37)21-3-1-2-18-22(21)30-7-6-29-18/h1-8,10-11,20,32H,9H2,(H,31,33)(H,34,35)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415080
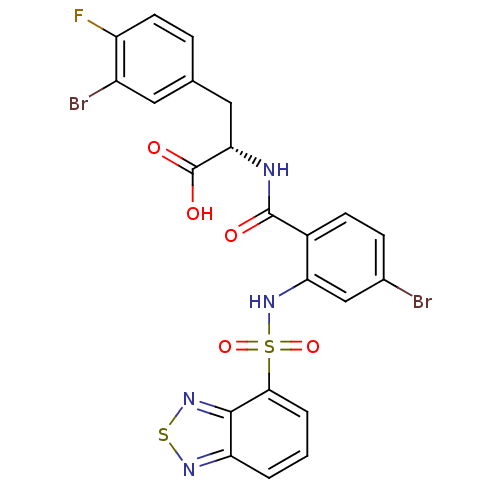
(CHEMBL585157)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Br2FN4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-15(25)14(24)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415077
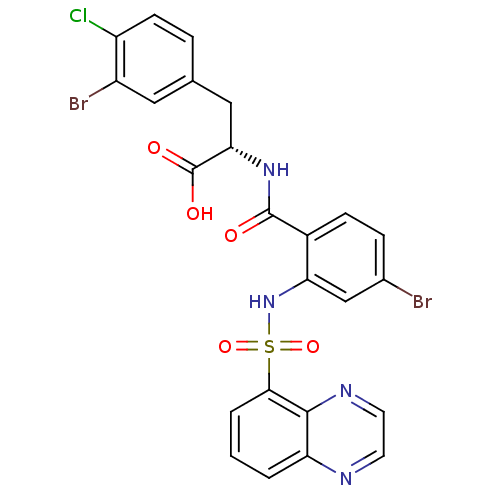
(CHEMBL584525)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H17Br2ClN4O5S/c25-14-5-6-15(23(32)30-20(24(33)34)11-13-4-7-17(27)16(26)10-13)19(12-14)31-37(35,36)21-3-1-2-18-22(21)29-9-8-28-18/h1-10,12,20,31H,11H2,(H,30,32)(H,33,34)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415084
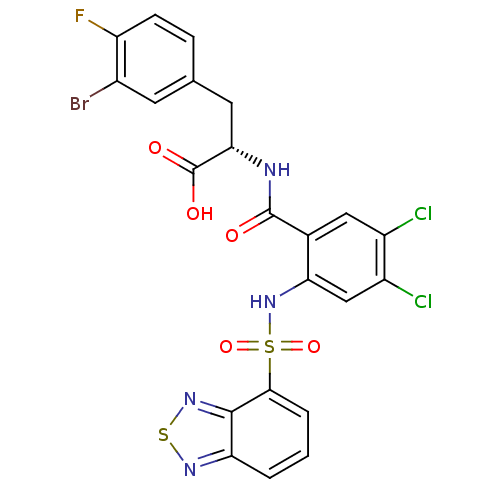
(CHEMBL565324)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1cc(Cl)c(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H14BrCl2FN4O5S2/c23-12-6-10(4-5-15(12)26)7-18(22(32)33)27-21(31)11-8-13(24)14(25)9-17(11)30-37(34,35)19-3-1-2-16-20(19)29-36-28-16/h1-6,8-9,18,30H,7H2,(H,27,31)(H,32,33)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415079
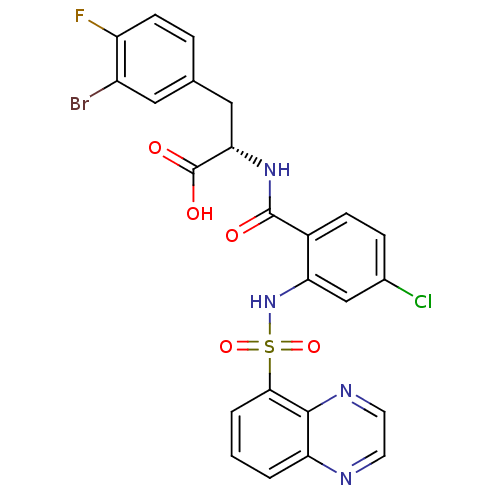
(CHEMBL569391)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H17BrClFN4O5S/c25-16-10-13(4-7-17(16)27)11-20(24(33)34)30-23(32)15-6-5-14(26)12-19(15)31-37(35,36)21-3-1-2-18-22(21)29-9-8-28-18/h1-10,12,20,31H,11H2,(H,30,32)(H,33,34)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415078
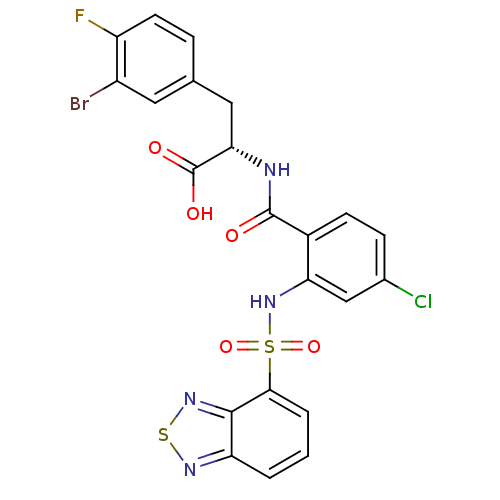
(CHEMBL565298)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15BrClFN4O5S2/c23-14-8-11(4-7-15(14)25)9-18(22(31)32)26-21(30)13-6-5-12(24)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415053
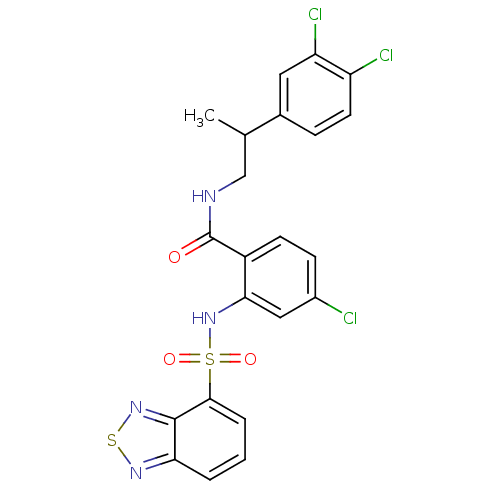
(CHEMBL583457)Show SMILES CC(CNC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H17Cl3N4O3S2/c1-12(13-5-8-16(24)17(25)9-13)11-26-22(30)15-7-6-14(23)10-19(15)29-34(31,32)20-4-2-3-18-21(20)28-33-27-18/h2-10,12,29H,11H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415085
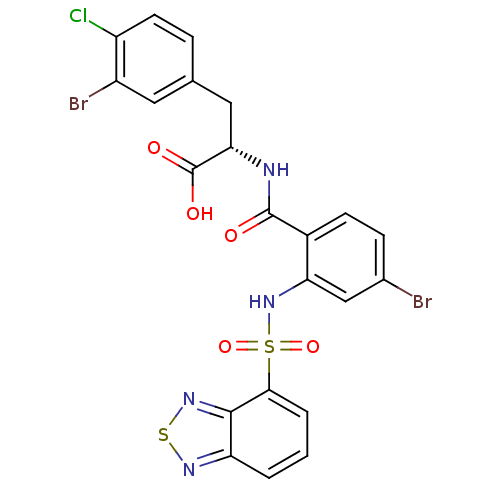
(CHEMBL570520)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Br2ClN4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-15(25)14(24)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415066

(CHEMBL585914)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Cl3N4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-14(24)15(25)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415066

(CHEMBL585914)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Cl3N4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-14(24)15(25)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412862
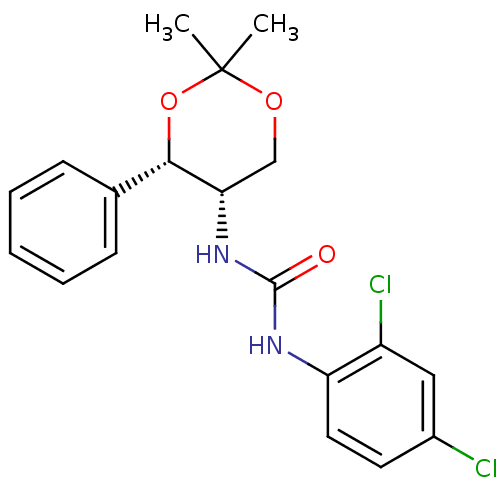
(CHEMBL185136)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccc(Cl)cc2Cl)[C@@H](O1)c1ccccc1 |r| Show InChI InChI=1S/C19H20Cl2N2O3/c1-19(2)25-11-16(17(26-19)12-6-4-3-5-7-12)23-18(24)22-15-9-8-13(20)10-14(15)21/h3-10,16-17H,11H2,1-2H3,(H2,22,23,24)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196190
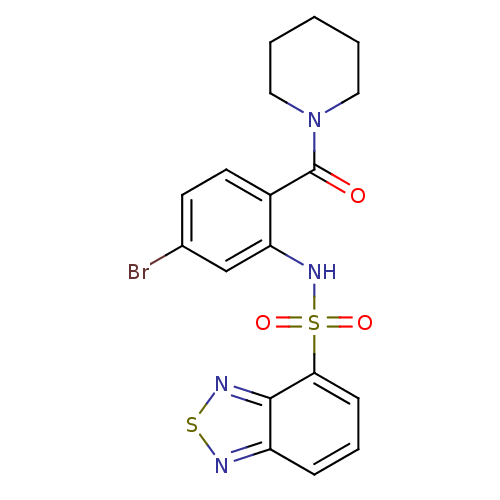
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C18H17BrN4O3S2/c19-12-7-8-13(18(24)23-9-2-1-3-10-23)15(11-12)22-28(25,26)16-6-4-5-14-17(16)21-27-20-14/h4-8,11,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from CCK2 receptor |
Bioorg Med Chem Lett 17: 6905-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.087
BindingDB Entry DOI: 10.7270/Q2G73HJW |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50474855

(CHEMBL364814)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccccc2-c2ccccc2Cl)[C@@H](O1)c1ccccc1 Show InChI InChI=1S/C25H25ClN2O3/c1-25(2)30-16-22(23(31-25)17-10-4-3-5-11-17)28-24(29)27-21-15-9-7-13-19(21)18-12-6-8-14-20(18)26/h3-15,22-23H,16H2,1-2H3,(H2,27,28,29)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196190

(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C18H17BrN4O3S2/c19-12-7-8-13(18(24)23-9-2-1-3-10-23)15(11-12)22-28(25,26)16-6-4-5-14-17(16)21-27-20-14/h4-8,11,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415058
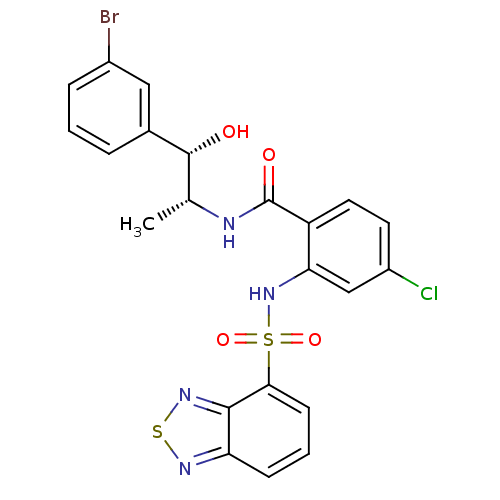
(CHEMBL571206)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12)[C@@H](O)c1cccc(Br)c1 |r| Show InChI InChI=1S/C22H18BrClN4O4S2/c1-12(21(29)13-4-2-5-14(23)10-13)25-22(30)16-9-8-15(24)11-18(16)28-34(31,32)19-7-3-6-17-20(19)27-33-26-17/h2-12,21,28-29H,1H3,(H,25,30)/t12-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415076
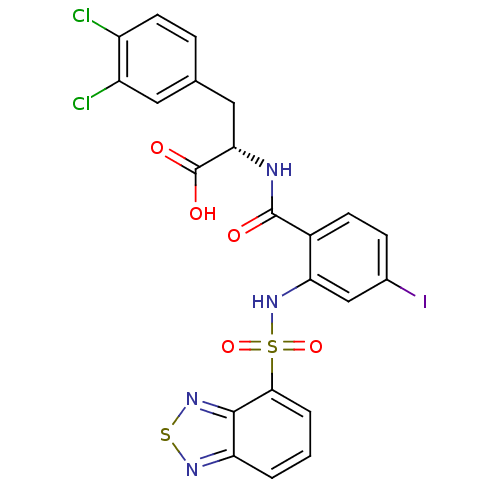
(CHEMBL583344)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)c1ccc(I)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Cl2IN4O5S2/c23-14-7-4-11(8-15(14)24)9-18(22(31)32)26-21(30)13-6-5-12(25)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415052
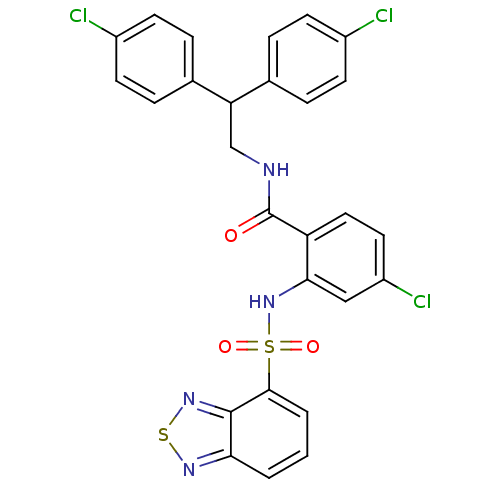
(CHEMBL583343)Show SMILES Clc1ccc(cc1)C(CNC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C27H19Cl3N4O3S2/c28-18-8-4-16(5-9-18)22(17-6-10-19(29)11-7-17)15-31-27(35)21-13-12-20(30)14-24(21)34-39(36,37)25-3-1-2-23-26(25)33-38-32-23/h1-14,22,34H,15H2,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196191
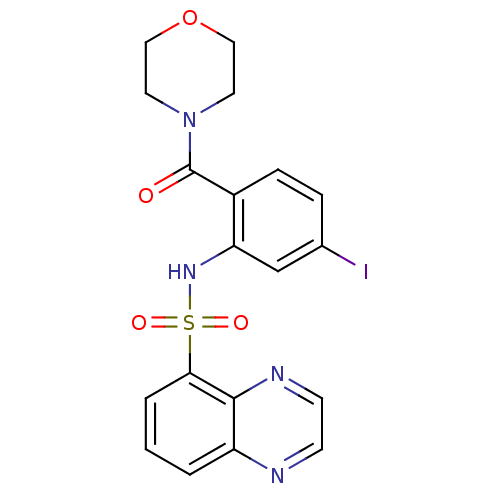
(4-[4-iodo-2-[(5-quinoxalinylsulfonyl)amino]benzoyl...)Show SMILES Ic1ccc(C(=O)N2CCOCC2)c(NS(=O)(=O)c2cccc3nccnc23)c1 Show InChI InChI=1S/C19H17IN4O4S/c20-13-4-5-14(19(25)24-8-10-28-11-9-24)16(12-13)23-29(26,27)17-3-1-2-15-18(17)22-7-6-21-15/h1-7,12,23H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415068
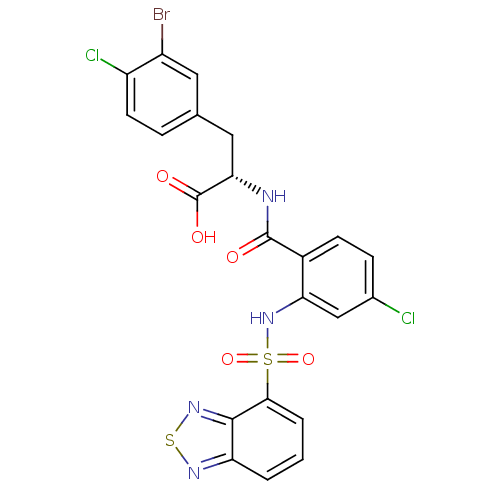
(CHEMBL569470)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15BrCl2N4O5S2/c23-14-8-11(4-7-15(14)25)9-18(22(31)32)26-21(30)13-6-5-12(24)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196157
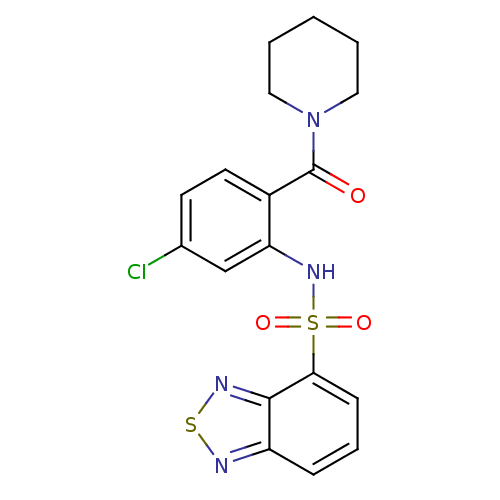
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Clc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C18H17ClN4O3S2/c19-12-7-8-13(18(24)23-9-2-1-3-10-23)15(11-12)22-28(25,26)16-6-4-5-14-17(16)21-27-20-14/h4-8,11,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412860

(CHEMBL185093)Show SMILES Cc1cc(Br)ccc1NC(=O)N[C@H]1COC(C)(C)O[C@H]1c1ccccc1 |r| Show InChI InChI=1S/C20H23BrN2O3/c1-13-11-15(21)9-10-16(13)22-19(24)23-17-12-25-20(2,3)26-18(17)14-7-5-4-6-8-14/h4-11,17-18H,12H2,1-3H3,(H2,22,23,24)/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50474849
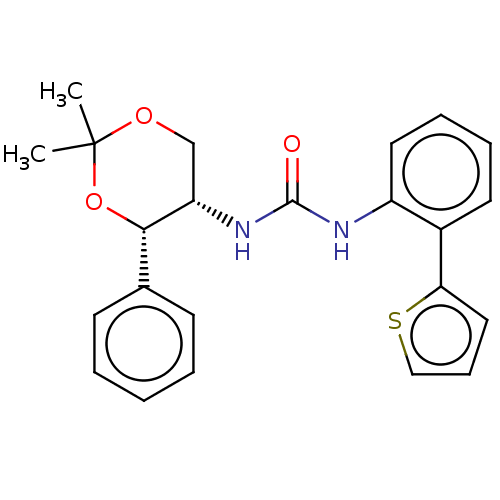
(CHEMBL185424)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccccc2-c2cccs2)[C@@H](O1)c1ccccc1 Show InChI InChI=1S/C23H24N2O3S/c1-23(2)27-15-19(21(28-23)16-9-4-3-5-10-16)25-22(26)24-18-12-7-6-11-17(18)20-13-8-14-29-20/h3-14,19,21H,15H2,1-2H3,(H2,24,25,26)/t19-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415072
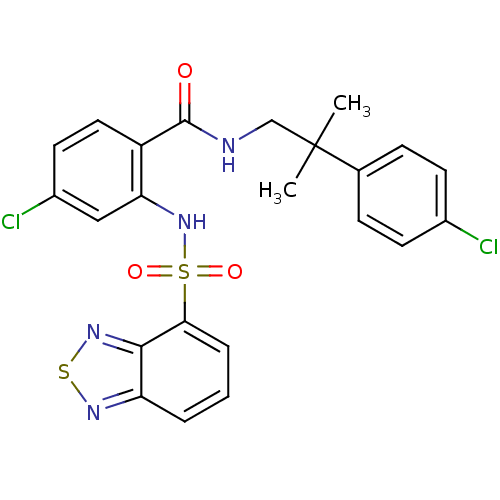
(CHEMBL565511)Show SMILES CC(C)(CNC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H20Cl2N4O3S2/c1-23(2,14-6-8-15(24)9-7-14)13-26-22(30)17-11-10-16(25)12-19(17)29-34(31,32)20-5-3-4-18-21(20)28-33-27-18/h3-12,29H,13H2,1-2H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412857

(CHEMBL361716)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2cc(Cl)ccc2Cl)[C@@H](O1)c1ccccc1 |r| Show InChI InChI=1S/C19H20Cl2N2O3/c1-19(2)25-11-16(17(26-19)12-6-4-3-5-7-12)23-18(24)22-15-10-13(20)8-9-14(15)21/h3-10,16-17H,11H2,1-2H3,(H2,22,23,24)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478086
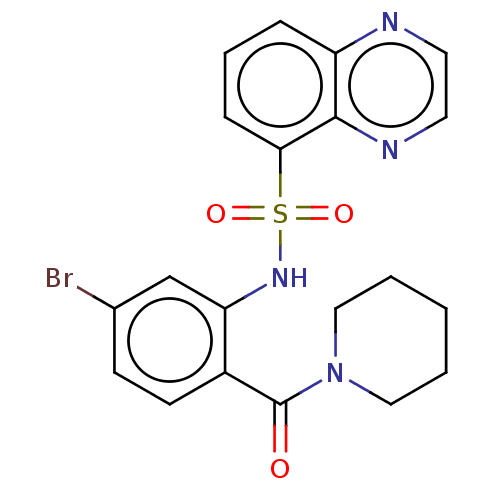
(CHEMBL261682)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nccnc23)c1 Show InChI InChI=1S/C20H19BrN4O3S/c21-14-7-8-15(20(26)25-11-2-1-3-12-25)17(13-14)24-29(27,28)18-6-4-5-16-19(18)23-10-9-22-16/h4-10,13,24H,1-3,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412859
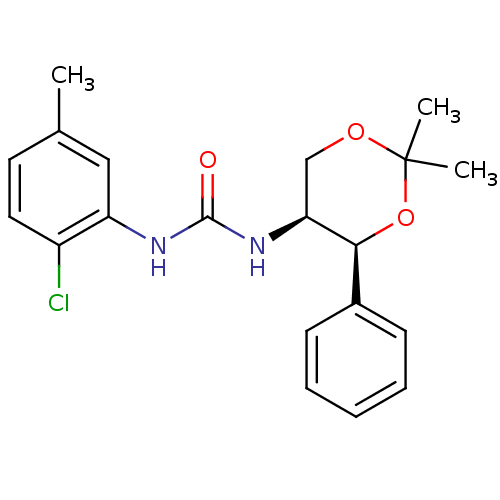
(CHEMBL182473)Show SMILES Cc1ccc(Cl)c(NC(=O)N[C@H]2COC(C)(C)O[C@H]2c2ccccc2)c1 |r| Show InChI InChI=1S/C20H23ClN2O3/c1-13-9-10-15(21)16(11-13)22-19(24)23-17-12-25-20(2,3)26-18(17)14-7-5-4-6-8-14/h4-11,17-18H,12H2,1-3H3,(H2,22,23,24)/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415064
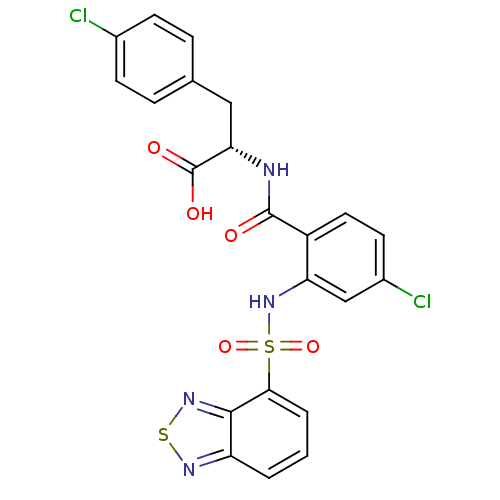
(CHEMBL584259)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H16Cl2N4O5S2/c23-13-6-4-12(5-7-13)10-18(22(30)31)25-21(29)15-9-8-14(24)11-17(15)28-35(32,33)19-3-1-2-16-20(19)27-34-26-16/h1-9,11,18,28H,10H2,(H,25,29)(H,30,31)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415074
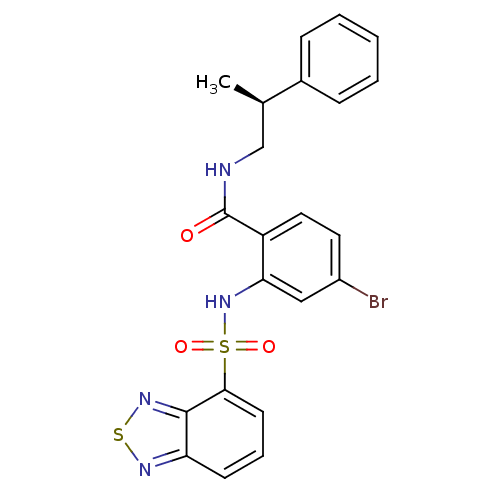
(CHEMBL566604)Show SMILES C[C@H](CNC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccccc1 |r| Show InChI InChI=1S/C22H19BrN4O3S2/c1-14(15-6-3-2-4-7-15)13-24-22(28)17-11-10-16(23)12-19(17)27-32(29,30)20-9-5-8-18-21(20)26-31-25-18/h2-12,14,27H,13H2,1H3,(H,24,28)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50474846
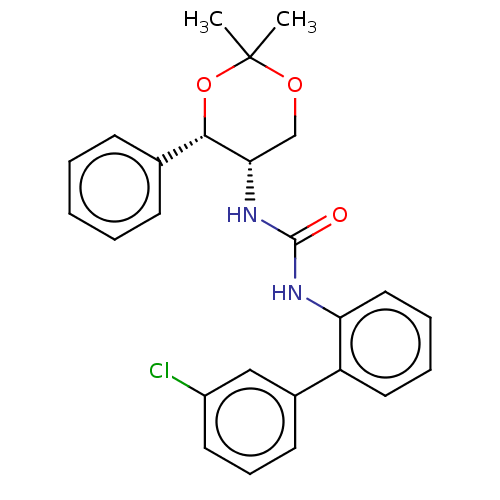
(CHEMBL185131)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccccc2-c2cccc(Cl)c2)[C@@H](O1)c1ccccc1 Show InChI InChI=1S/C25H25ClN2O3/c1-25(2)30-16-22(23(31-25)17-9-4-3-5-10-17)28-24(29)27-21-14-7-6-13-20(21)18-11-8-12-19(26)15-18/h3-15,22-23H,16H2,1-2H3,(H2,27,28,29)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50474848

(CHEMBL184596)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccccc2-c2ccsc2)[C@@H](O1)c1ccccc1 Show InChI InChI=1S/C23H24N2O3S/c1-23(2)27-14-20(21(28-23)16-8-4-3-5-9-16)25-22(26)24-19-11-7-6-10-18(19)17-12-13-29-15-17/h3-13,15,20-21H,14H2,1-2H3,(H2,24,25,26)/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412855
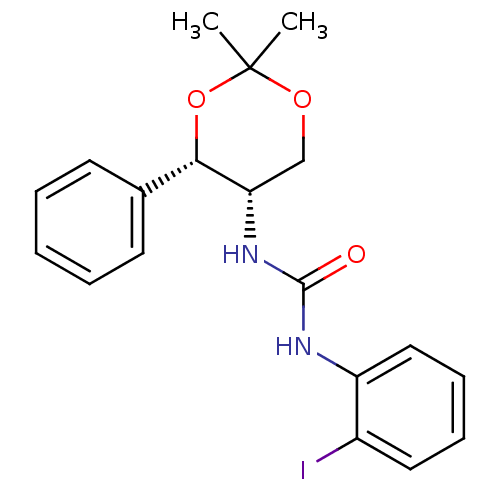
(CHEMBL185382)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccccc2I)[C@@H](O1)c1ccccc1 |r| Show InChI InChI=1S/C19H21IN2O3/c1-19(2)24-12-16(17(25-19)13-8-4-3-5-9-13)22-18(23)21-15-11-7-6-10-14(15)20/h3-11,16-17H,12H2,1-2H3,(H2,21,22,23)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478090
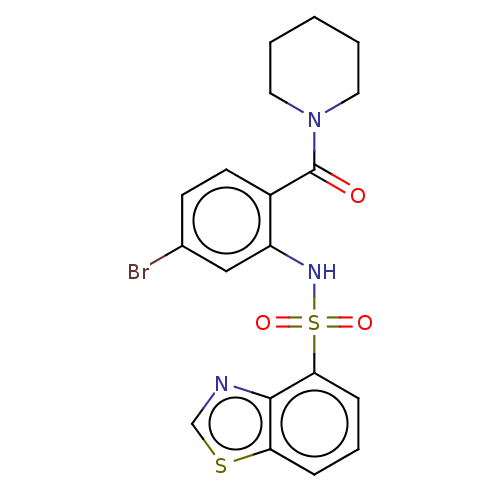
(CHEMBL259762)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3scnc23)c1 Show InChI InChI=1S/C19H18BrN3O3S2/c20-13-7-8-14(19(24)23-9-2-1-3-10-23)15(11-13)22-28(25,26)17-6-4-5-16-18(17)21-12-27-16/h4-8,11-12,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412854

(CHEMBL185435)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccccc2Cl)[C@@H](O1)c1ccccc1 |r| Show InChI InChI=1S/C19H21ClN2O3/c1-19(2)24-12-16(17(25-19)13-8-4-3-5-9-13)22-18(23)21-15-11-7-6-10-14(15)20/h3-11,16-17H,12H2,1-2H3,(H2,21,22,23)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415061
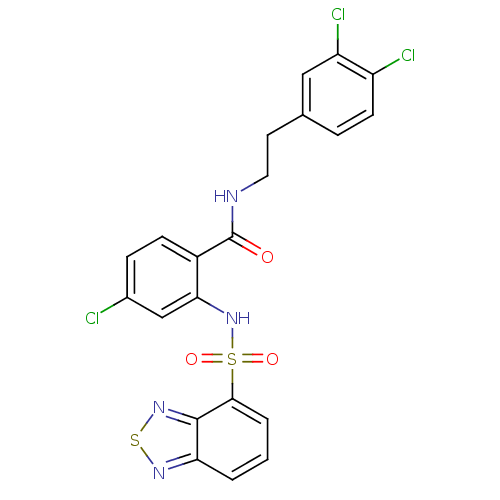
(CHEMBL570521)Show SMILES Clc1ccc(C(=O)NCCc2ccc(Cl)c(Cl)c2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C21H15Cl3N4O3S2/c22-13-5-6-14(21(29)25-9-8-12-4-7-15(23)16(24)10-12)18(11-13)28-33(30,31)19-3-1-2-17-20(19)27-32-26-17/h1-7,10-11,28H,8-9H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415062
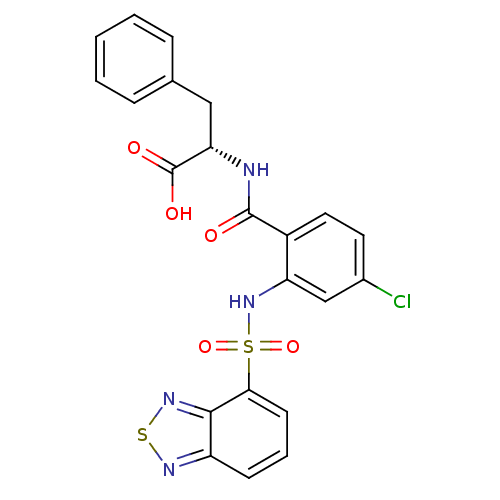
(CHEMBL566390)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H17ClN4O5S2/c23-14-9-10-15(21(28)24-18(22(29)30)11-13-5-2-1-3-6-13)17(12-14)27-34(31,32)19-8-4-7-16-20(19)26-33-25-16/h1-10,12,18,27H,11H2,(H,24,28)(H,29,30)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415051
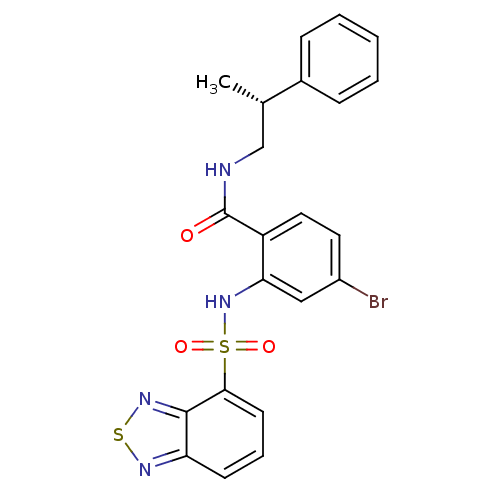
(CHEMBL584063)Show SMILES C[C@@H](CNC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccccc1 |r| Show InChI InChI=1S/C22H19BrN4O3S2/c1-14(15-6-3-2-4-7-15)13-24-22(28)17-11-10-16(23)12-19(17)27-32(29,30)20-9-5-8-18-21(20)26-31-25-18/h2-12,14,27H,13H2,1H3,(H,24,28)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415051

(CHEMBL584063)Show SMILES C[C@@H](CNC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccccc1 |r| Show InChI InChI=1S/C22H19BrN4O3S2/c1-14(15-6-3-2-4-7-15)13-24-22(28)17-11-10-16(23)12-19(17)27-32(29,30)20-9-5-8-18-21(20)26-31-25-18/h2-12,14,27H,13H2,1H3,(H,24,28)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415067
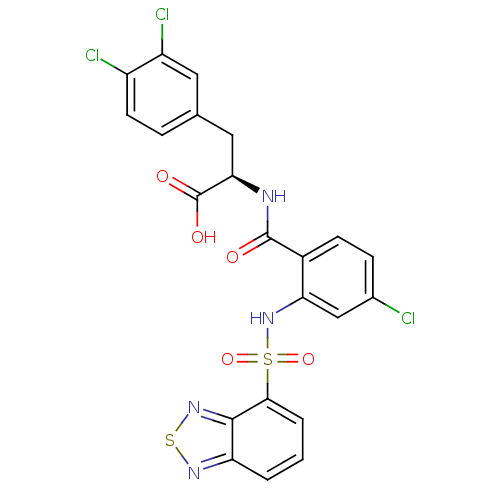
(CHEMBL566359)Show SMILES OC(=O)[C@@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Cl3N4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-14(24)15(25)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415071
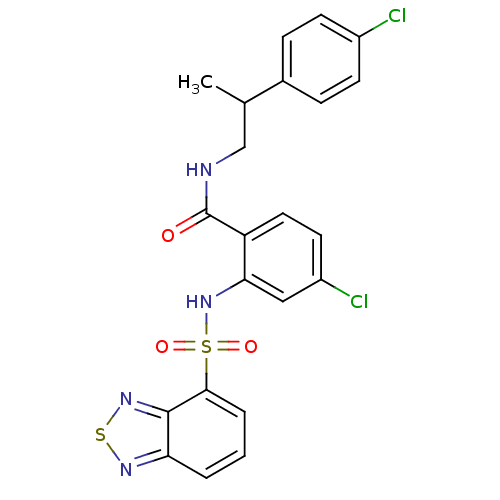
(CHEMBL566360)Show SMILES CC(CNC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H18Cl2N4O3S2/c1-13(14-5-7-15(23)8-6-14)12-25-22(29)17-10-9-16(24)11-19(17)28-33(30,31)20-4-2-3-18-21(20)27-32-26-18/h2-11,13,28H,12H2,1H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50474847
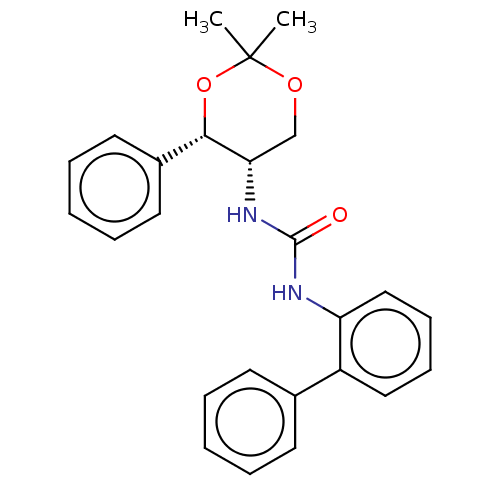
(CHEMBL183421)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccccc2-c2ccccc2)[C@@H](O1)c1ccccc1 Show InChI InChI=1S/C25H26N2O3/c1-25(2)29-17-22(23(30-25)19-13-7-4-8-14-19)27-24(28)26-21-16-10-9-15-20(21)18-11-5-3-6-12-18/h3-16,22-23H,17H2,1-2H3,(H2,26,27,28)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412849

(CHEMBL363743)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccccc2Br)[C@@H](O1)c1ccccc1 |r| Show InChI InChI=1S/C19H21BrN2O3/c1-19(2)24-12-16(17(25-19)13-8-4-3-5-9-13)22-18(23)21-15-11-7-6-10-14(15)20/h3-11,16-17H,12H2,1-2H3,(H2,21,22,23)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415077

(CHEMBL584525)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H17Br2ClN4O5S/c25-14-5-6-15(23(32)30-20(24(33)34)11-13-4-7-17(27)16(26)10-13)19(12-14)31-37(35,36)21-3-1-2-18-22(21)29-9-8-28-18/h1-10,12,20,31H,11H2,(H,30,32)(H,33,34)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412851
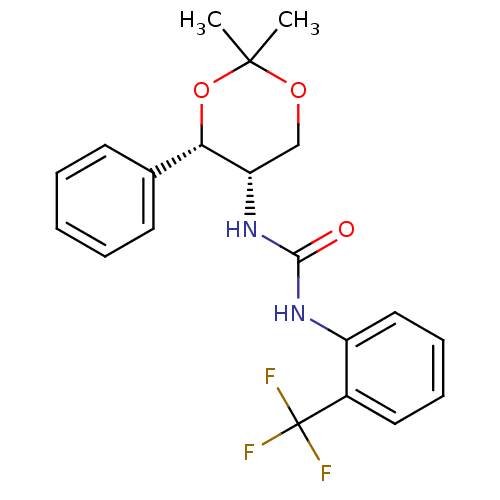
(CHEMBL363951)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccccc2C(F)(F)F)[C@@H](O1)c1ccccc1 |r| Show InChI InChI=1S/C20H21F3N2O3/c1-19(2)27-12-16(17(28-19)13-8-4-3-5-9-13)25-18(26)24-15-11-7-6-10-14(15)20(21,22)23/h3-11,16-17H,12H2,1-2H3,(H2,24,25,26)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415085

(CHEMBL570520)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Br2ClN4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-15(25)14(24)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412858
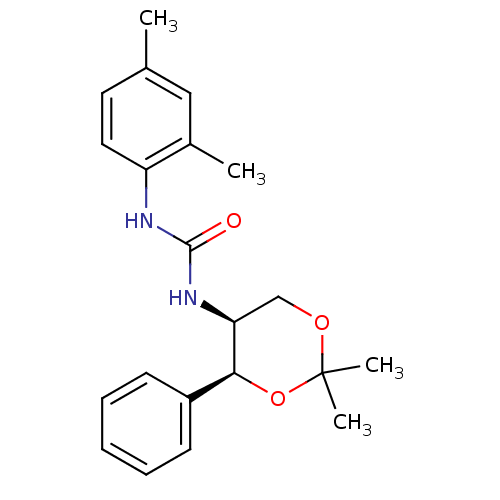
(CHEMBL183576)Show SMILES Cc1ccc(NC(=O)N[C@H]2COC(C)(C)O[C@H]2c2ccccc2)c(C)c1 |r| Show InChI InChI=1S/C21H26N2O3/c1-14-10-11-17(15(2)12-14)22-20(24)23-18-13-25-21(3,4)26-19(18)16-8-6-5-7-9-16/h5-12,18-19H,13H2,1-4H3,(H2,22,23,24)/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50474855

(CHEMBL364814)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccccc2-c2ccccc2Cl)[C@@H](O1)c1ccccc1 Show InChI InChI=1S/C25H25ClN2O3/c1-25(2)30-16-22(23(31-25)17-10-4-3-5-11-17)28-24(29)27-21-15-9-7-13-19(21)18-12-6-8-14-20(18)26/h3-15,22-23H,16H2,1-2H3,(H2,27,28,29)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human orexin receptor type 1 was determined using [125I]-Orexin A as radio ligand |
Bioorg Med Chem Lett 14: 4225-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.032
BindingDB Entry DOI: 10.7270/Q2K64MV4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data