 Found 13 hits with Last Name = 'pipe' and Initial = 'a'
Found 13 hits with Last Name = 'pipe' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.338 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome Tor Vergata
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 3B reverse transcriptase activity |
Bioorg Med Chem 19: 2084-9 (2011)
Article DOI: 10.1016/j.bmc.2011.01.024
BindingDB Entry DOI: 10.7270/Q2CN76Q4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.413 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome Tor Vergata
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 3B reverse transcriptase activity infected in human H9 cells assessed as level of p24 antigen |
Bioorg Med Chem 19: 2084-9 (2011)
Article DOI: 10.1016/j.bmc.2011.01.024
BindingDB Entry DOI: 10.7270/Q2CN76Q4 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50100381
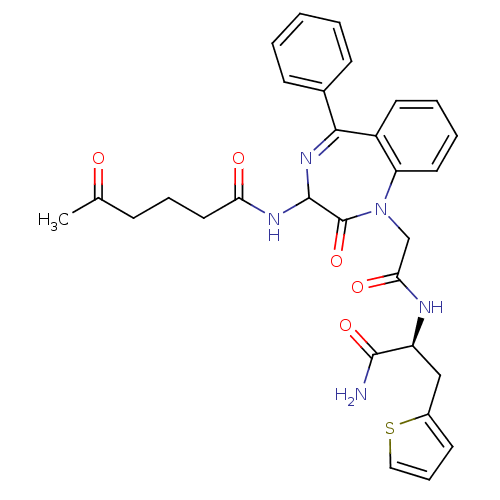
(5-Oxo-hexanoic acid {1-[((S)-1-carbamoyl-2-thiophe...)Show SMILES CC(=O)CCCC(=O)NC1N=C(c2ccccc2)c2ccccc2N(CC(=O)N[C@@H](Cc2cccs2)C(N)=O)C1=O |t:10| Show InChI InChI=1S/C30H31N5O5S/c1-19(36)9-7-15-25(37)33-29-30(40)35(18-26(38)32-23(28(31)39)17-21-12-8-16-41-21)24-14-6-5-13-22(24)27(34-29)20-10-3-2-4-11-20/h2-6,8,10-14,16,23,29H,7,9,15,17-18H2,1H3,(H2,31,39)(H,32,38)(H,33,37)/t23-,29?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for antagonist activity against oxytocin receptor |
Bioorg Med Chem Lett 11: 1297-300 (2001)
BindingDB Entry DOI: 10.7270/Q2T72GQF |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50418089
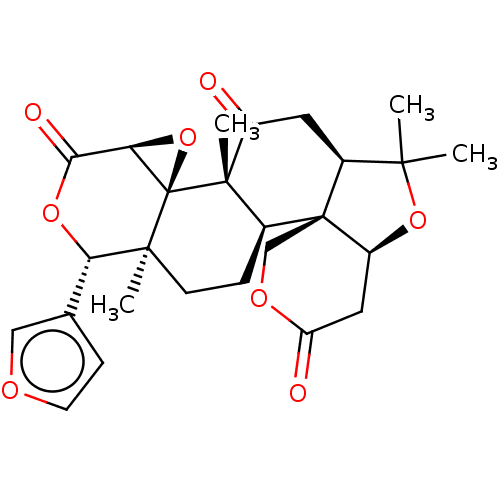
(Limonin)Show SMILES [H][C@]12O[C@@]11[C@@](C)(CC[C@]3([H])[C@@]45COC(=O)C[C@]4([H])OC(C)(C)[C@]5([H])CC(=O)[C@@]13C)[C@@H](OC2=O)c1ccoc1 |r| Show InChI InChI=1S/C27H32O7/c1-23(2)18-11-19(29)25(4)17(26(18)13-32-21(30)12-20(26)33-23)7-8-24(3)14(16-6-5-9-31-16)10-15(28)22-27(24,25)34-22/h5-6,9,14,17-18,20,22H,7-8,10-13H2,1-4H3/t14-,17-,18-,20+,22+,24-,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome Tor Vergata
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 3B reverse transcriptase activity infected in human H9 cells assessed as level of p24 antigen |
Bioorg Med Chem 19: 2084-9 (2011)
Article DOI: 10.1016/j.bmc.2011.01.024
BindingDB Entry DOI: 10.7270/Q2CN76Q4 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50100380

(5-Oxo-hexanoic acid (1-{[(S)-1-carbamoyl-2-(3,4-di...)Show SMILES CC(=O)CCCC(=O)NC1N=C(c2ccccc2)c2ccccc2N(CC(=O)N[C@@H](Cc2ccc(Cl)c(Cl)c2)C(N)=O)C1=O |t:10| Show InChI InChI=1S/C32H31Cl2N5O5/c1-19(40)8-7-13-27(41)37-31-32(44)39(26-12-6-5-11-22(26)29(38-31)21-9-3-2-4-10-21)18-28(42)36-25(30(35)43)17-20-14-15-23(33)24(34)16-20/h2-6,9-12,14-16,25,31H,7-8,13,17-18H2,1H3,(H2,35,43)(H,36,42)(H,37,41)/t25-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for antagonist activity against oxytocin receptor |
Bioorg Med Chem Lett 11: 1297-300 (2001)
BindingDB Entry DOI: 10.7270/Q2T72GQF |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480319
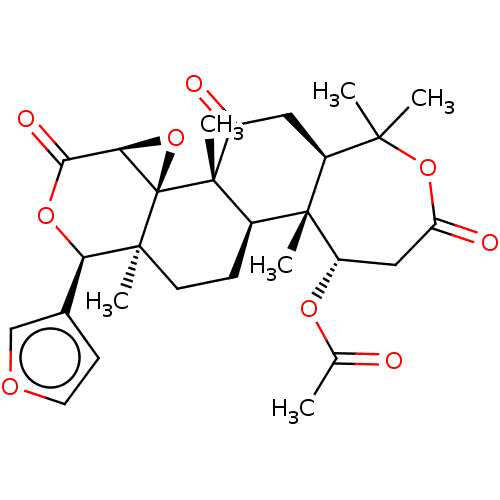
(Nomilin)Show SMILES [H][C@]12O[C@@]11[C@@](C)(CC[C@]3([H])[C@@]4(C)[C@H](CC(=O)OC(C)(C)[C@]4([H])CC(=O)[C@@]13C)OC(C)=O)[C@H](OC2=O)c1ccoc1 |r| Show InChI InChI=1S/C28H34O9/c1-14(29)34-19-12-20(31)36-24(2,3)17-11-18(30)27(6)16(26(17,19)5)7-9-25(4)21(15-8-10-33-13-15)35-23(32)22-28(25,27)37-22/h8,10,13,16-17,19,21-22H,7,9,11-12H2,1-6H3/t16-,17+,19+,21-,22-,25+,26-,27+,28-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome Tor Vergata
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 3B reverse transcriptase activity |
Bioorg Med Chem 19: 2084-9 (2011)
Article DOI: 10.1016/j.bmc.2011.01.024
BindingDB Entry DOI: 10.7270/Q2CN76Q4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480319

(Nomilin)Show SMILES [H][C@]12O[C@@]11[C@@](C)(CC[C@]3([H])[C@@]4(C)[C@H](CC(=O)OC(C)(C)[C@]4([H])CC(=O)[C@@]13C)OC(C)=O)[C@H](OC2=O)c1ccoc1 |r| Show InChI InChI=1S/C28H34O9/c1-14(29)34-19-12-20(31)36-24(2,3)17-11-18(30)27(6)16(26(17,19)5)7-9-25(4)21(15-8-10-33-13-15)35-23(32)22-28(25,27)37-22/h8,10,13,16-17,19,21-22H,7,9,11-12H2,1-6H3/t16-,17+,19+,21-,22-,25+,26-,27+,28-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.92 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome Tor Vergata
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 3B reverse transcriptase activity infected in human H9 cells assessed as level of p24 antigen |
Bioorg Med Chem 19: 2084-9 (2011)
Article DOI: 10.1016/j.bmc.2011.01.024
BindingDB Entry DOI: 10.7270/Q2CN76Q4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50418089

(Limonin)Show SMILES [H][C@]12O[C@@]11[C@@](C)(CC[C@]3([H])[C@@]45COC(=O)C[C@]4([H])OC(C)(C)[C@]5([H])CC(=O)[C@@]13C)[C@@H](OC2=O)c1ccoc1 |r| Show InChI InChI=1S/C27H32O7/c1-23(2)18-11-19(29)25(4)17(26(18)13-32-21(30)12-20(26)33-23)7-8-24(3)14(16-6-5-9-31-16)10-15(28)22-27(24,25)34-22/h5-6,9,14,17-18,20,22H,7-8,10-13H2,1-4H3/t14-,17-,18-,20+,22+,24-,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.99 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome Tor Vergata
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 3B reverse transcriptase activity |
Bioorg Med Chem 19: 2084-9 (2011)
Article DOI: 10.1016/j.bmc.2011.01.024
BindingDB Entry DOI: 10.7270/Q2CN76Q4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50191163

(1-(2-(benzyloxy)-5-bromobenzyl)-5-methyl-1H-pyrazo...)Show InChI InChI=1S/C19H17BrN2O3/c1-13-9-17(19(23)24)21-22(13)11-15-10-16(20)7-8-18(15)25-12-14-5-3-2-4-6-14/h2-10H,11-12H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 1A2 |
Bioorg Med Chem Lett 16: 4767-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.086
BindingDB Entry DOI: 10.7270/Q2MC8ZN4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50191163

(1-(2-(benzyloxy)-5-bromobenzyl)-5-methyl-1H-pyrazo...)Show InChI InChI=1S/C19H17BrN2O3/c1-13-9-17(19(23)24)21-22(13)11-15-10-16(20)7-8-18(15)25-12-14-5-3-2-4-6-14/h2-10H,11-12H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 2D6 |
Bioorg Med Chem Lett 16: 4767-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.086
BindingDB Entry DOI: 10.7270/Q2MC8ZN4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50191163

(1-(2-(benzyloxy)-5-bromobenzyl)-5-methyl-1H-pyrazo...)Show InChI InChI=1S/C19H17BrN2O3/c1-13-9-17(19(23)24)21-22(13)11-15-10-16(20)7-8-18(15)25-12-14-5-3-2-4-6-14/h2-10H,11-12H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 3A4 |
Bioorg Med Chem Lett 16: 4767-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.086
BindingDB Entry DOI: 10.7270/Q2MC8ZN4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50191163

(1-(2-(benzyloxy)-5-bromobenzyl)-5-methyl-1H-pyrazo...)Show InChI InChI=1S/C19H17BrN2O3/c1-13-9-17(19(23)24)21-22(13)11-15-10-16(20)7-8-18(15)25-12-14-5-3-2-4-6-14/h2-10H,11-12H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 2C19 |
Bioorg Med Chem Lett 16: 4767-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.086
BindingDB Entry DOI: 10.7270/Q2MC8ZN4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50191163

(1-(2-(benzyloxy)-5-bromobenzyl)-5-methyl-1H-pyrazo...)Show InChI InChI=1S/C19H17BrN2O3/c1-13-9-17(19(23)24)21-22(13)11-15-10-16(20)7-8-18(15)25-12-14-5-3-2-4-6-14/h2-10H,11-12H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 2C9 |
Bioorg Med Chem Lett 16: 4767-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.086
BindingDB Entry DOI: 10.7270/Q2MC8ZN4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data