 Found 346 hits with Last Name = 'porter' and Initial = 'wj'
Found 346 hits with Last Name = 'porter' and Initial = 'wj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-secretase 1
(Mus musculus (Mouse)) | BDBM400979
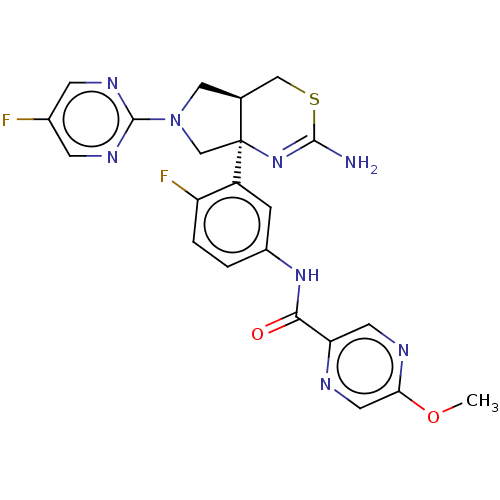
(US9999624, Compound 4)Show SMILES COc1cnc(cn1)C(=O)Nc1ccc(F)c(c1)[C@]12CN(C[C@H]1CSC(N)=N2)c1ncc(F)cn1 |r,c:29| Show InChI InChI=1S/C22H20F2N8O2S/c1-34-18-8-26-17(7-27-18)19(33)30-14-2-3-16(24)15(4-14)22-11-32(21-28-5-13(23)6-29-21)9-12(22)10-35-20(25)31-22/h2-8,12H,9-11H2,1H3,(H2,25,31)(H,30,33)/t12-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50474994
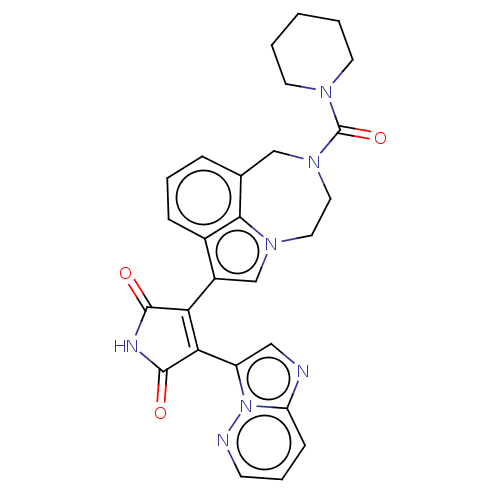
(Bisarylmaleimide 1)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cccnn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-14-28-21-8-5-9-29-34(20)21)19-16-32-12-13-33(27(37)31-10-2-1-3-11-31)15-17-6-4-7-18(19)24(17)32/h4-9,14,16H,1-3,10-13,15H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Mus musculus (Mouse)) | BDBM150693

(US8987254, 8 | US9999624, 9)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ncc(F)cc2F)ccc1F |r,t:1| Show InChI InChI=1S/C22H17F4N7OS/c23-12-3-17(26)18(28-5-12)19(34)31-14-1-2-16(25)15(4-14)22-10-33(21-29-6-13(24)7-30-21)8-11(22)9-35-20(27)32-22/h1-7,11H,8-10H2,(H2,27,32)(H,31,34)/t11-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM150688
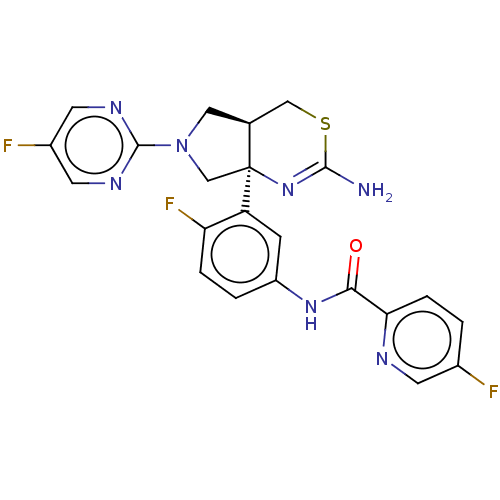
(US8987254, 3 | US9999624, 3)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C22H18F3N7OS/c23-13-1-4-18(27-6-13)19(33)30-15-2-3-17(25)16(5-15)22-11-32(21-28-7-14(24)8-29-21)9-12(22)10-34-20(26)31-22/h1-8,12H,9-11H2,(H2,26,31)(H,30,33)/t12-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.388 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Mus musculus (Mouse)) | BDBM150688

(US8987254, 3 | US9999624, 3)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C22H18F3N7OS/c23-13-1-4-18(27-6-13)19(33)30-15-2-3-17(25)16(5-15)22-11-32(21-28-7-14(24)8-29-21)9-12(22)10-34-20(26)31-22/h1-8,12H,9-11H2,(H2,26,31)(H,30,33)/t12-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.481 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM150693

(US8987254, 8 | US9999624, 9)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ncc(F)cc2F)ccc1F |r,t:1| Show InChI InChI=1S/C22H17F4N7OS/c23-12-3-17(26)18(28-5-12)19(34)31-14-1-2-16(25)15(4-14)22-10-33(21-29-6-13(24)7-30-21)8-11(22)9-35-20(27)32-22/h1-7,11H,8-10H2,(H2,27,32)(H,31,34)/t11-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.555 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150688

(US8987254, 3 | US9999624, 3)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C22H18F3N7OS/c23-13-1-4-18(27-6-13)19(33)30-15-2-3-17(25)16(5-15)22-11-32(21-28-7-14(24)8-29-21)9-12(22)10-34-20(26)31-22/h1-8,12H,9-11H2,(H2,26,31)(H,30,33)/t12-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM400979

(US9999624, Compound 4)Show SMILES COc1cnc(cn1)C(=O)Nc1ccc(F)c(c1)[C@]12CN(C[C@H]1CSC(N)=N2)c1ncc(F)cn1 |r,c:29| Show InChI InChI=1S/C22H20F2N8O2S/c1-34-18-8-26-17(7-27-18)19(33)30-14-2-3-16(24)15(4-14)22-11-32(21-28-5-13(23)6-29-21)9-12(22)10-35-20(25)31-22/h2-8,12H,9-11H2,1H3,(H2,25,31)(H,30,33)/t12-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.615 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699
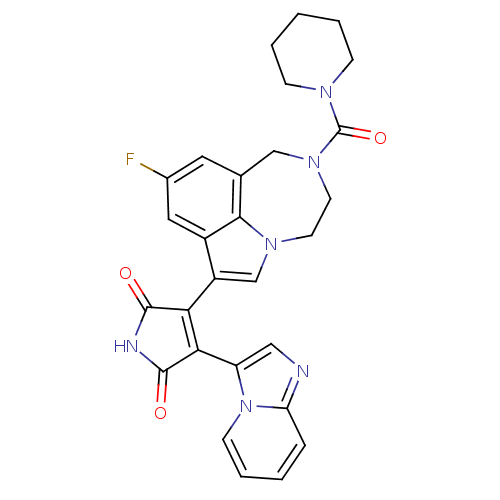
(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150693

(US8987254, 8 | US9999624, 9)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ncc(F)cc2F)ccc1F |r,t:1| Show InChI InChI=1S/C22H17F4N7OS/c23-12-3-17(26)18(28-5-12)19(34)31-14-1-2-16(25)15(4-14)22-10-33(21-29-6-13(24)7-30-21)8-11(22)9-35-20(27)32-22/h1-7,11H,8-10H2,(H2,27,32)(H,31,34)/t11-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475025

(CHEMBL181339)Show SMILES O=C(C1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H25N5O4/c34-26-23(24(27(35)30-26)21-14-29-22-6-1-2-9-33(21)22)20-16-31-10-11-32(28(36)17-7-12-37-13-8-17)15-18-4-3-5-19(20)25(18)31/h1-6,9,14,16-17H,7-8,10-13,15H2,(H,30,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475007

(Bisarylmaleimide 2)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H23N5O5/c33-25-21(19-13-28-12-16-4-9-37-24(16)19)22(26(34)29-25)20-15-31-5-6-32(27(35)30-7-10-36-11-8-30)14-17-2-1-3-18(20)23(17)31/h1-4,9,12-13,15H,5-8,10-11,14H2,(H,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM400979

(US9999624, Compound 4)Show SMILES COc1cnc(cn1)C(=O)Nc1ccc(F)c(c1)[C@]12CN(C[C@H]1CSC(N)=N2)c1ncc(F)cn1 |r,c:29| Show InChI InChI=1S/C22H20F2N8O2S/c1-34-18-8-26-17(7-27-18)19(33)30-14-2-3-16(24)15(4-14)22-11-32(21-28-5-13(23)6-29-21)9-12(22)10-35-20(25)31-22/h2-8,12H,9-11H2,1H3,(H2,25,31)(H,30,33)/t12-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475024

(CHEMBL181371)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H18N4O4/c1-13(29)27-6-7-28-12-18(16-4-2-3-15(11-27)21(16)28)20-19(23(30)26-24(20)31)17-10-25-9-14-5-8-32-22(14)17/h2-5,8-10,12H,6-7,11H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475018
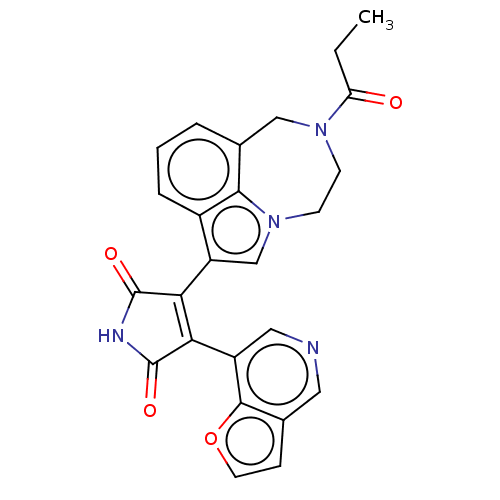
(CHEMBL181518)Show SMILES CCC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:10| Show InChI InChI=1S/C25H20N4O4/c1-2-19(30)28-7-8-29-13-18(16-5-3-4-15(12-28)22(16)29)21-20(24(31)27-25(21)32)17-11-26-10-14-6-9-33-23(14)17/h3-6,9-11,13H,2,7-8,12H2,1H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475031

(CHEMBL359871)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4CCOc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C25H21N3O4/c1-14(29)27-9-10-28-13-19(17-6-3-5-16(12-27)22(17)28)21-20(24(30)26-25(21)31)18-7-2-4-15-8-11-32-23(15)18/h2-7,13H,8-12H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475029

(CHEMBL180779)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H21N5O4/c1-28(2)25(33)30-8-7-29-13-18(16-5-3-4-15(12-30)21(16)29)20-19(23(31)27-24(20)32)17-11-26-10-14-6-9-34-22(14)17/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475008

(CHEMBL369090)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3ccn4ncccc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(19-6-8-33-21(19)5-2-7-28-33)23(26(35)29-25)20-16-31-9-10-32(27(36)30-11-13-37-14-12-30)15-17-3-1-4-18(20)24(17)31/h1-8,16H,9-15H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150700
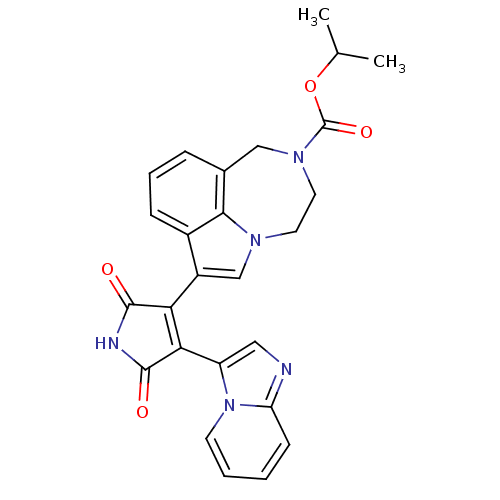
(7-(4-Imidazo[1,2-a]pyridin-3-yl-2,5-dioxo-2,5-dihy...)Show SMILES CC(C)OC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:12| Show InChI InChI=1S/C26H23N5O4/c1-15(2)35-26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698
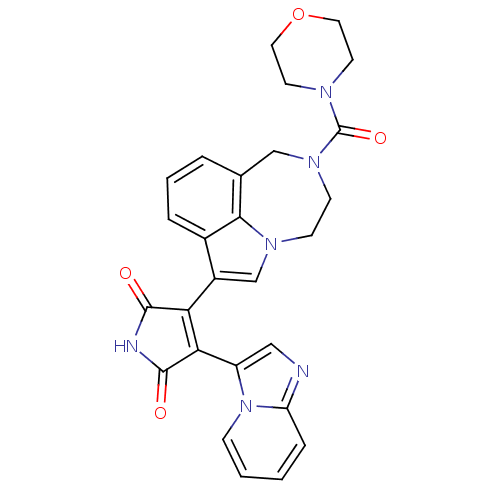
(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475022

(CHEMBL361765)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4ccoc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C25H19N3O4/c1-14(29)27-9-10-28-13-19(17-6-3-5-16(12-27)22(17)28)21-20(24(30)26-25(21)31)18-7-2-4-15-8-11-32-23(15)18/h2-8,11,13H,9-10,12H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150701
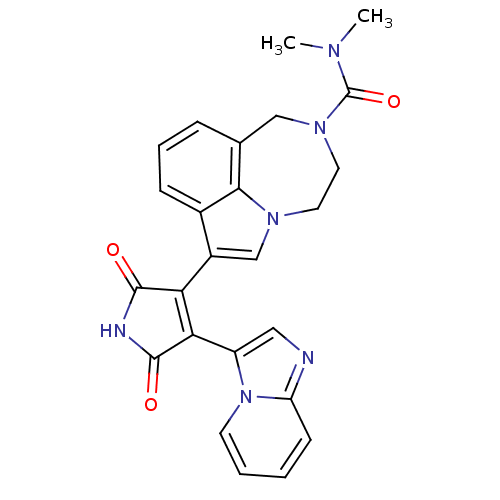
(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50474994

(Bisarylmaleimide 1)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cccnn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-14-28-21-8-5-9-29-34(20)21)19-16-32-12-13-33(27(37)31-10-2-1-3-11-31)15-17-6-4-7-18(19)24(17)32/h4-9,14,16H,1-3,10-13,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150701

(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475010
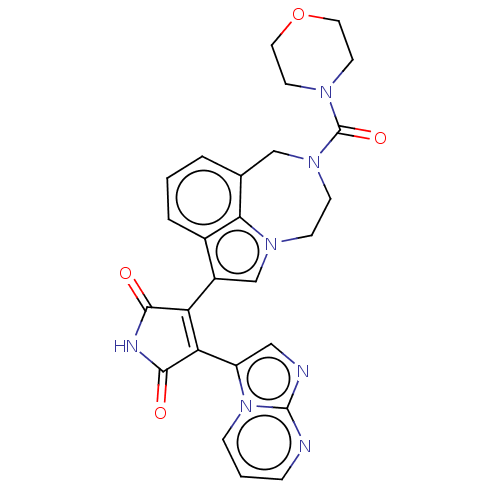
(CHEMBL369316)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ncccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C26H23N7O4/c34-23-20(21(24(35)29-23)19-13-28-25-27-5-2-6-33(19)25)18-15-31-7-8-32(26(36)30-9-11-37-12-10-30)14-16-3-1-4-17(18)22(16)31/h1-6,13,15H,7-12,14H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475001
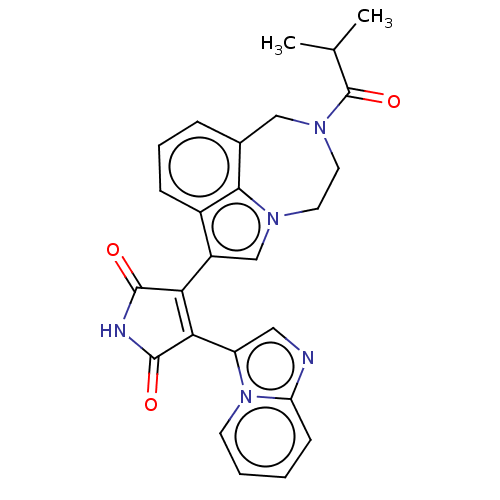
(CHEMBL368246)Show SMILES CC(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C26H23N5O3/c1-15(2)26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475004

(CHEMBL369572)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ncccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-24-21(22(25(36)30-24)20-14-29-26-28-8-5-11-34(20)26)19-16-32-12-13-33(27(37)31-9-2-1-3-10-31)15-17-6-4-7-18(19)23(17)32/h4-8,11,14,16H,1-3,9-10,12-13,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475014
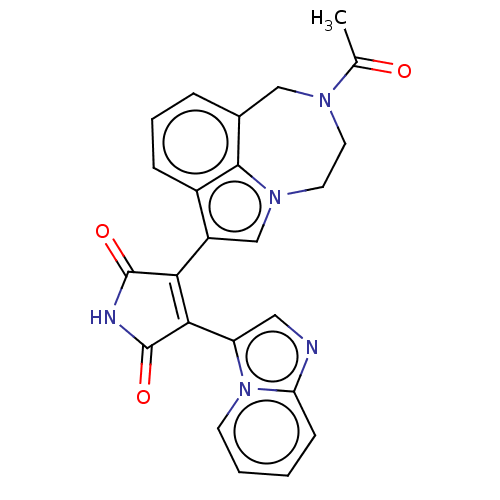
(CHEMBL361948)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H19N5O3/c1-14(30)27-9-10-28-13-17(16-6-4-5-15(12-27)22(16)28)20-21(24(32)26-23(20)31)18-11-25-19-7-2-3-8-29(18)19/h2-8,11,13H,9-10,12H2,1H3,(H,26,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50474996

(CHEMBL178646)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H25N5O4/c34-26-22(20-14-29-13-17-7-12-37-25(17)20)23(27(35)30-26)21-16-32-10-11-33(28(36)31-8-2-1-3-9-31)15-18-5-4-6-19(21)24(18)32/h4-7,12-14,16H,1-3,8-11,15H2,(H,30,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475006

(CHEMBL178851)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cnccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-13-29-21-14-28-7-10-34(20)21)19-16-32-11-12-33(27(37)31-8-2-1-3-9-31)15-17-5-4-6-18(19)24(17)32/h4-7,10,13-14,16H,1-3,8-9,11-12,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50012647
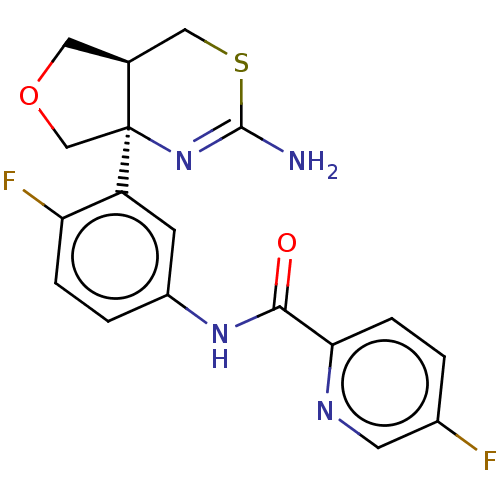
(CHEMBL2396989)Show SMILES [H][C@@]12COC[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:7| Show InChI InChI=1S/C18H16F2N4O2S/c19-11-1-4-15(22-6-11)16(25)23-12-2-3-14(20)13(5-12)18-9-26-7-10(18)8-27-17(21)24-18/h1-6,10H,7-9H2,(H2,21,24)(H,23,25)/t10-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475000

(CHEMBL181296)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4OCOc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H19N3O5/c1-13(28)26-8-9-27-11-17(15-5-2-4-14(10-26)21(15)27)20-19(23(29)25-24(20)30)16-6-3-7-18-22(16)32-12-31-18/h2-7,11H,8-10,12H2,1H3,(H,25,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475011

(CHEMBL178850)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4ccoc34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C26H22N4O4/c1-28(2)26(33)30-11-10-29-14-19(17-7-4-6-16(13-30)22(17)29)21-20(24(31)27-25(21)32)18-8-3-5-15-9-12-34-23(15)18/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475009
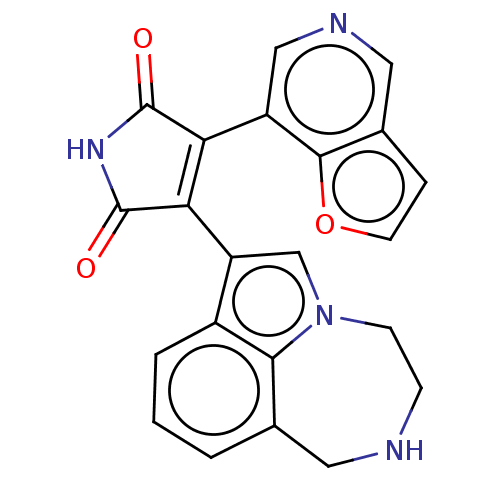
(CHEMBL361635)Show SMILES O=C1NC(=O)C(=C1c1cn2CCNCc3cccc1c23)c1cncc2ccoc12 |c:5| Show InChI InChI=1S/C22H16N4O3/c27-21-17(15-10-24-9-13-4-7-29-20(13)15)18(22(28)25-21)16-11-26-6-5-23-8-12-2-1-3-14(16)19(12)26/h1-4,7,9-11,23H,5-6,8H2,(H,25,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475021
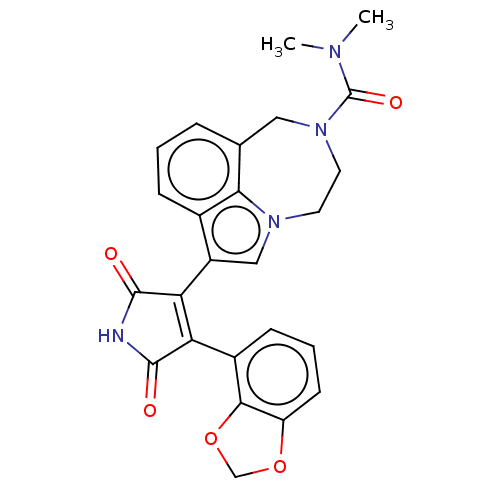
(CHEMBL445649)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4OCOc34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N4O5/c1-27(2)25(32)29-10-9-28-12-17(15-6-3-5-14(11-29)21(15)28)20-19(23(30)26-24(20)31)16-7-4-8-18-22(16)34-13-33-18/h3-8,12H,9-11,13H2,1-2H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475016
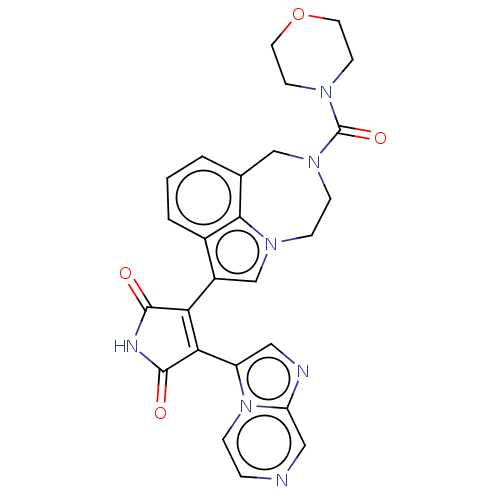
(CHEMBL361007)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cnccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C26H23N7O4/c34-24-21(22(25(35)29-24)19-12-28-20-13-27-4-5-33(19)20)18-15-31-6-7-32(26(36)30-8-10-37-11-9-30)14-16-2-1-3-17(18)23(16)31/h1-5,12-13,15H,6-11,14H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475005
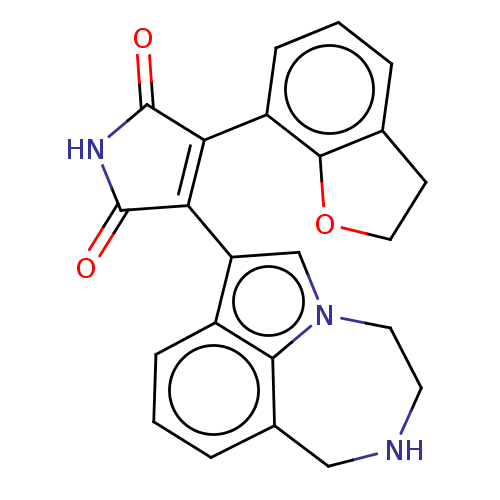
(CHEMBL178820)Show SMILES O=C1NC(=O)C(=C1c1cn2CCNCc3cccc1c23)c1cccc2CCOc12 |c:5| Show InChI InChI=1S/C23H19N3O3/c27-22-18(16-6-1-3-13-7-10-29-21(13)16)19(23(28)25-22)17-12-26-9-8-24-11-14-4-2-5-15(17)20(14)26/h1-6,12,24H,7-11H2,(H,25,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
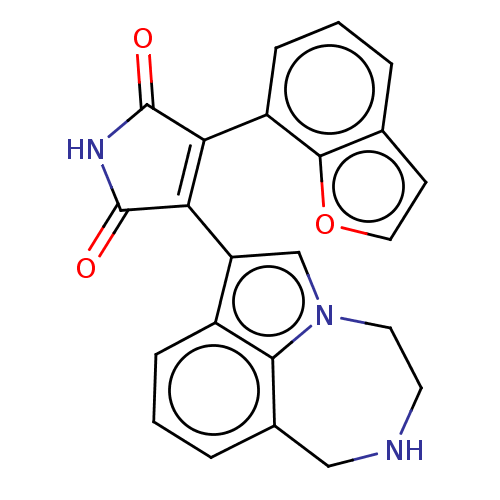
(Homo sapiens (Human)) | BDBM50474993
(CHEMBL361996)Show SMILES O=C1NC(=O)C(=C1c1cn2CCNCc3cccc1c23)c1cccc2ccoc12 |c:5| Show InChI InChI=1S/C23H17N3O3/c27-22-18(16-6-1-3-13-7-10-29-21(13)16)19(23(28)25-22)17-12-26-9-8-24-11-14-4-2-5-15(17)20(14)26/h1-7,10,12,24H,8-9,11H2,(H,25,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
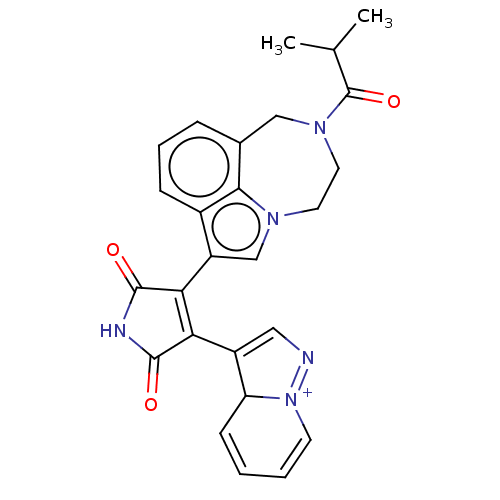
(Homo sapiens (Human)) | BDBM50475028
(CHEMBL179725)Show SMILES CC(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)C3=CN=[N+]4C=CC=CC34)c3cccc(C1)c23 |c:23,25,t:11,19,21| Show InChI InChI=1S/C26H23N5O3/c1-15(2)26(34)30-11-10-29-14-19(17-7-5-6-16(13-30)23(17)29)22-21(24(32)28-25(22)33)18-12-27-31-9-4-3-8-20(18)31/h3-9,12,14-15,20H,10-11,13H2,1-2H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
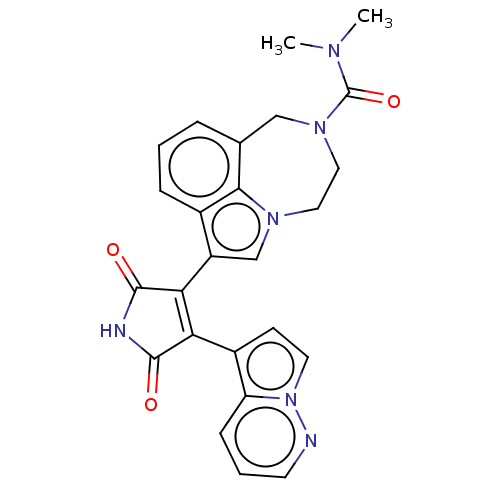
(Homo sapiens (Human)) | BDBM50475020
(CHEMBL181464)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3ccn4ncccc34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-12-11-29-14-18(16-6-3-5-15(13-30)22(16)29)21-20(23(32)27-24(21)33)17-8-10-31-19(17)7-4-9-26-31/h3-10,14H,11-13H2,1-2H3,(H,27,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
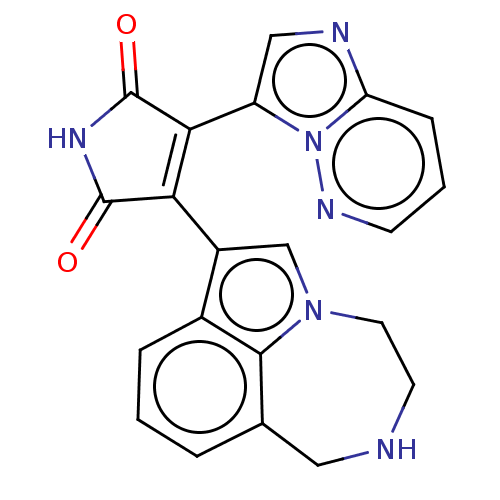
(Homo sapiens (Human)) | BDBM50475027
(CHEMBL359537)Show SMILES O=C1NC(=O)C(=C1c1cn2CCNCc3cccc1c23)c1cnc2cccnn12 |c:5| Show InChI InChI=1S/C21H16N6O2/c28-20-17(14-11-26-8-7-22-9-12-3-1-4-13(14)19(12)26)18(21(29)25-20)15-10-23-16-5-2-6-24-27(15)16/h1-6,10-11,22H,7-9H2,(H,25,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50475001

(CHEMBL368246)Show SMILES CC(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C26H23N5O3/c1-15(2)26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data