

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260415 (4-(2-Chloro-benzenesulfonyl)-2-(1-methyl-1,2,5,6-t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260414 (4-(4-tert-Butyl-benzenesulfonyl)-2-(1-methyl-1,2,5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260413 (4-(4-Methyl-benzenesulfonyl)-2-(1-methyl-1,2,5,6-t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260418 (4-Benzenesulfonyl-2-(1-methyl-1,2,5,6-tetrahydropy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260417 (4-(2,5-Dichloro-benzenesulfonyl)-2-(1-methyl-1,2,5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260420 (4-(3-Nitrobenzenesulfonyl)-2-(1-methyl-1,2,5,6-tet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260421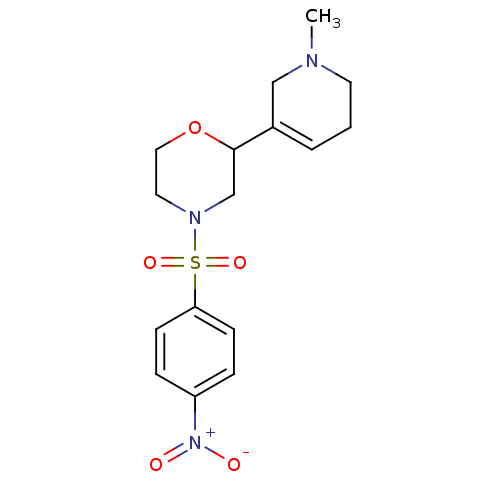 (4-(4-Nitro-benzenesulfonyl)-2-(1-methyl-1,2,5,6-te...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM46858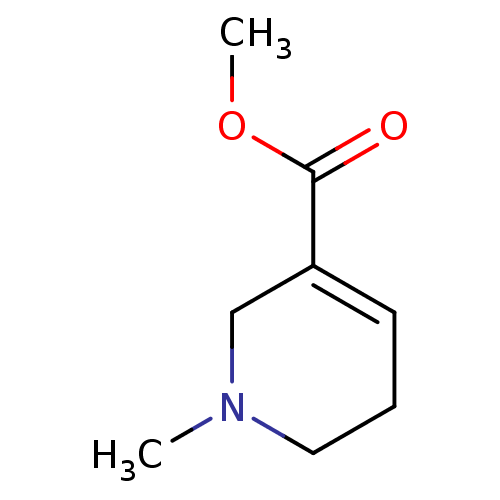 (1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260419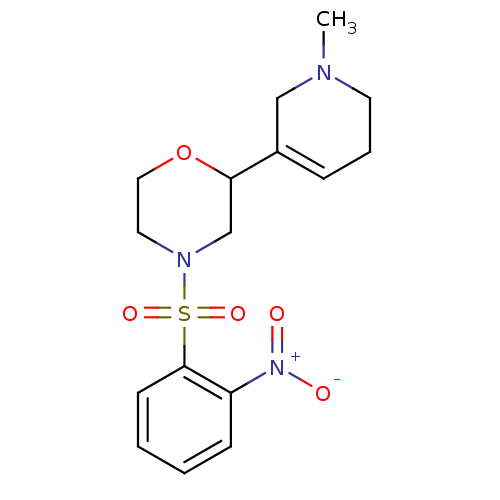 (4-(2-Nitrobenzenesulfonyl)-2-(1-methyl-1,2,5,6-tet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478381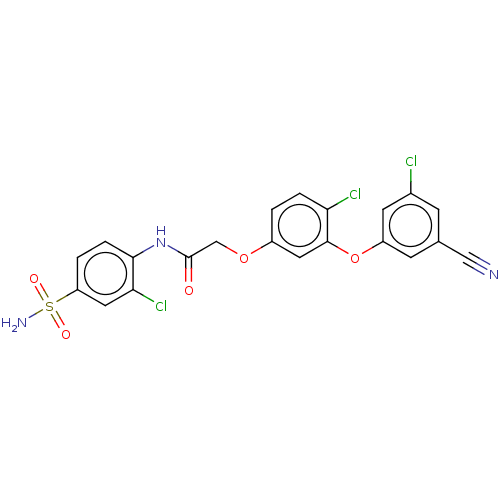 (CHEMBL256111) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479471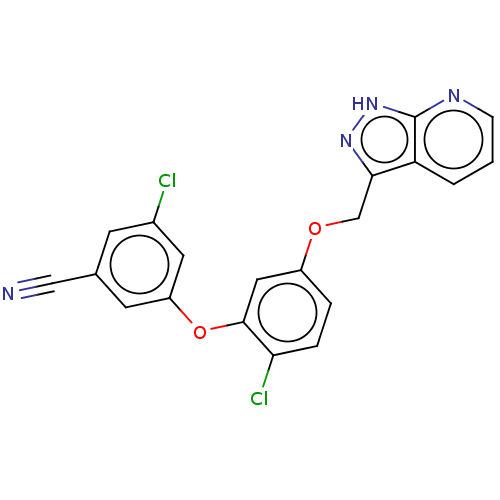 (CHEMBL491019 | MK-1107) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 RT polymerase Y181C mutant by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 RT polymerase by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50478381 (CHEMBL256111) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478381 (CHEMBL256111) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50478380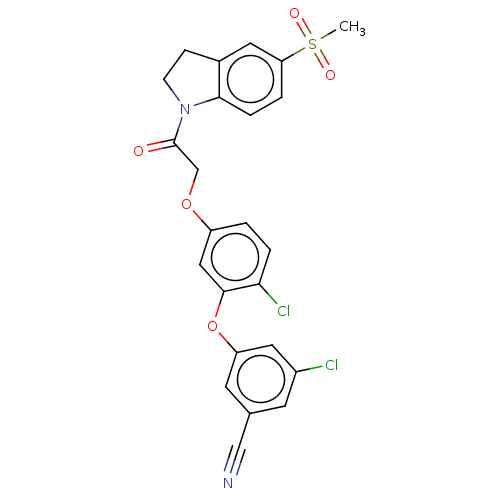 (CHEMBL257190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479471 (CHEMBL491019 | MK-1107) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 RT polymerase by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 RT polymerase Y181C mutant by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479471 (CHEMBL491019 | MK-1107) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 RT polymerase K103N mutant by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 RT polymerase K103N mutant by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478378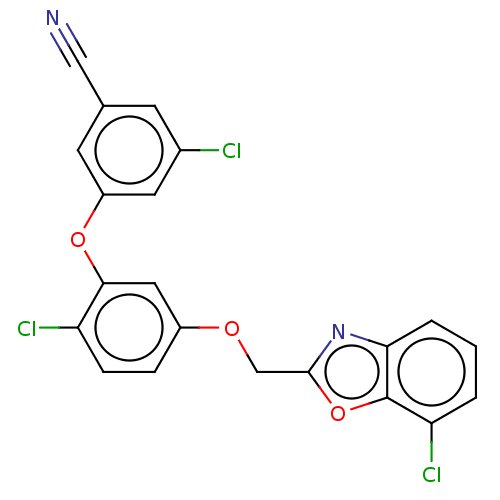 (CHEMBL403409) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479472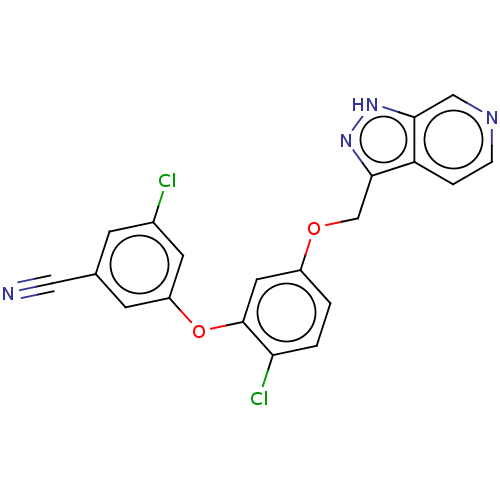 (CHEMBL523972) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 RT polymerase by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478380 (CHEMBL257190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478385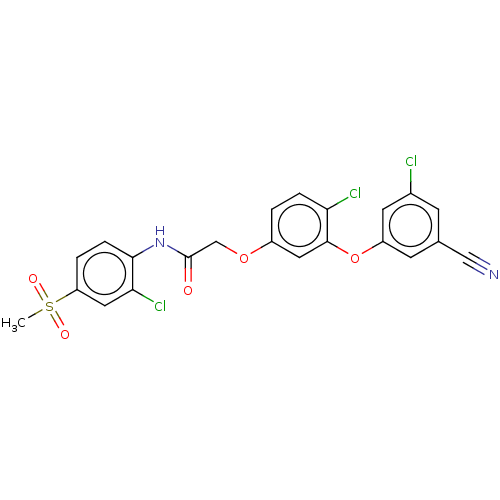 (CHEMBL257172) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478382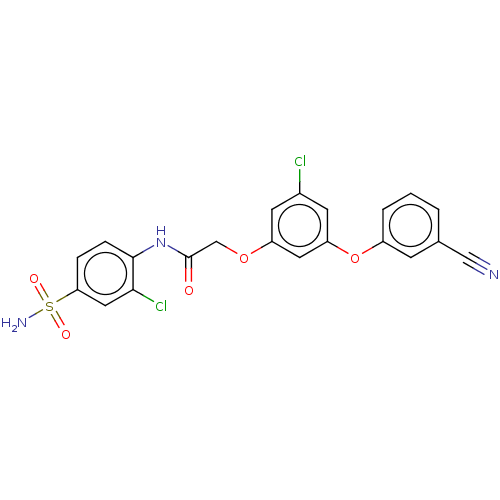 (CHEMBL254560) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478385 (CHEMBL257172) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50481850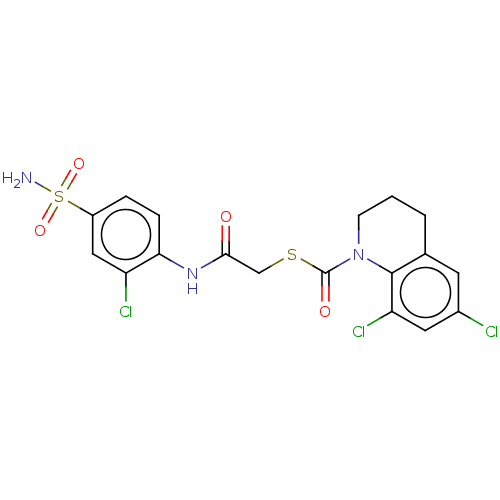 (CHEMBL1079267) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase expressed in human MT4 cells after 72 hrs by spread assay | Bioorg Med Chem Lett 19: 5119-23 (2009) Article DOI: 10.1016/j.bmcl.2009.07.031 BindingDB Entry DOI: 10.7270/Q2ZC85QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50498068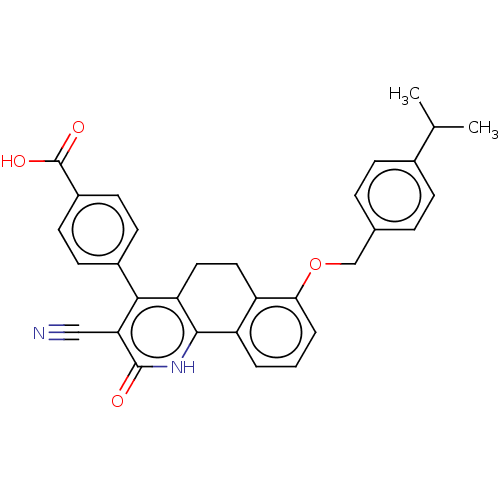 (CHEMBL3394234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis thymidylate kinase activity by pyruvate kinase-lactate dehydrogenase coupled assay | J Med Chem 58: 753-66 (2015) Article DOI: 10.1021/jm5012947 BindingDB Entry DOI: 10.7270/Q2JS9TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 RT polymerase by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50478383 (CHEMBL402823) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 RT polymerase K103N mutant by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50478383 (CHEMBL402823) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478382 (CHEMBL254560) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479473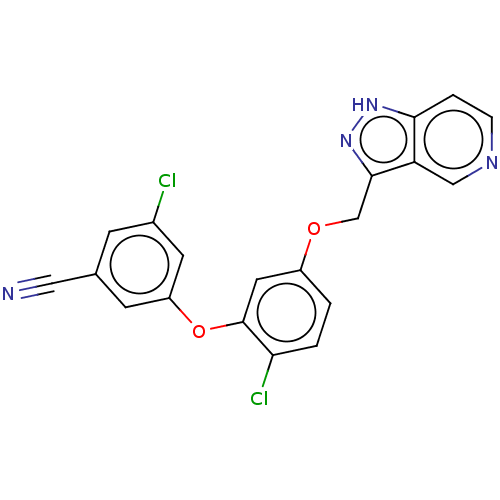 (CHEMBL491018) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 RT polymerase by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50478385 (CHEMBL257172) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479472 (CHEMBL523972) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 RT polymerase K103N mutant by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478383 (CHEMBL402823) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 RT polymerase by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50478378 (CHEMBL403409) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478383 (CHEMBL402823) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478380 (CHEMBL257190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50481845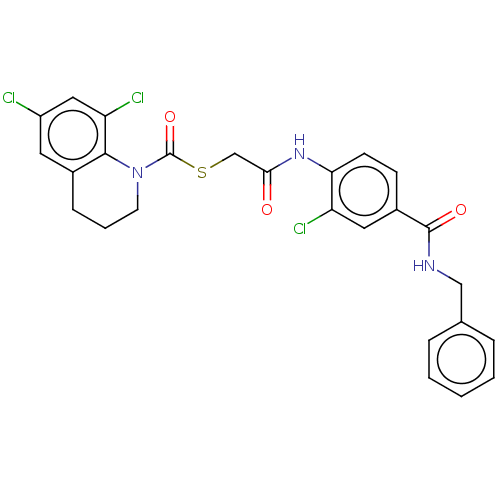 (CHEMBL1078846) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase expressed in human MT4 cells after 72 hrs by spread assay | Bioorg Med Chem Lett 19: 5119-23 (2009) Article DOI: 10.1016/j.bmcl.2009.07.031 BindingDB Entry DOI: 10.7270/Q2ZC85QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50481840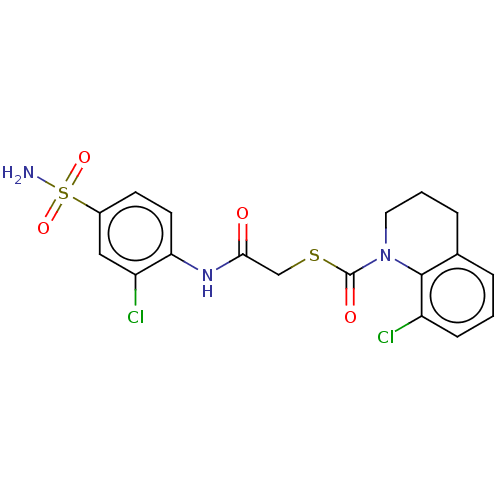 (CHEMBL1079266) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase expressed in human MT4 cells after 72 hrs by spread assay | Bioorg Med Chem Lett 19: 5119-23 (2009) Article DOI: 10.1016/j.bmcl.2009.07.031 BindingDB Entry DOI: 10.7270/Q2ZC85QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50498093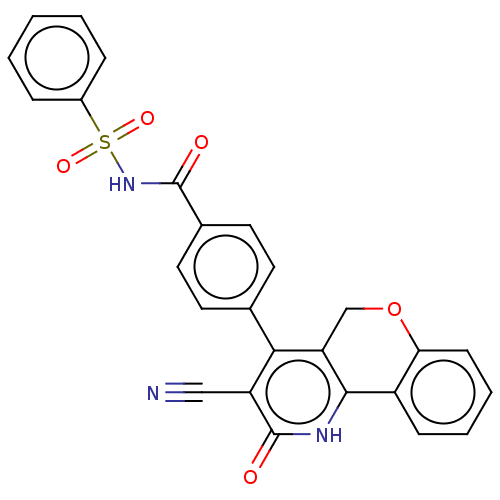 (CHEMBL3394236) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis thymidylate kinase activity by pyruvate kinase-lactate dehydrogenase coupled assay | J Med Chem 58: 753-66 (2015) Article DOI: 10.1021/jm5012947 BindingDB Entry DOI: 10.7270/Q2JS9TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50498069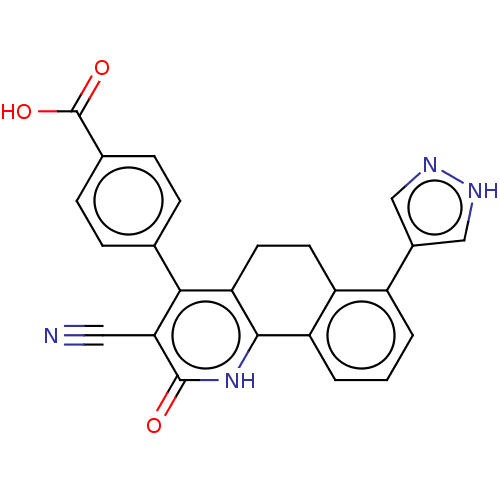 (CHEMBL3394233) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis thymidylate kinase activity by pyruvate kinase-lactate dehydrogenase coupled assay | J Med Chem 58: 753-66 (2015) Article DOI: 10.1021/jm5012947 BindingDB Entry DOI: 10.7270/Q2JS9TD7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50481843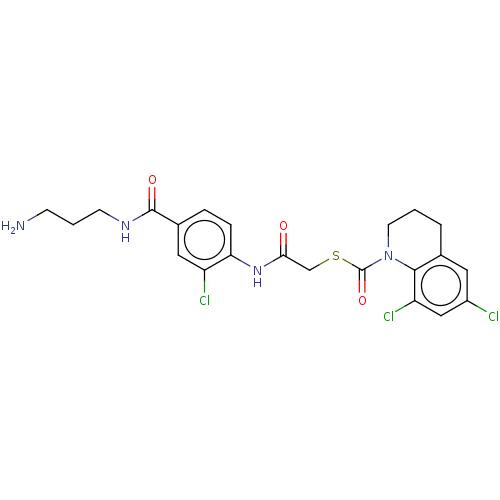 (CHEMBL1078945) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase expressed in human MT4 cells after 72 hrs by spread assay | Bioorg Med Chem Lett 19: 5119-23 (2009) Article DOI: 10.1016/j.bmcl.2009.07.031 BindingDB Entry DOI: 10.7270/Q2ZC85QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50193591 (CHEMBL3949218) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of His-tagged full length human recombinant GSK-3beta expressed in Baculovirus using Ser/Thr9 peptide as substrate by Z' LYTE assay | Eur J Med Chem 121: 727-736 (2016) Article DOI: 10.1016/j.ejmech.2016.04.075 BindingDB Entry DOI: 10.7270/Q2SJ1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478387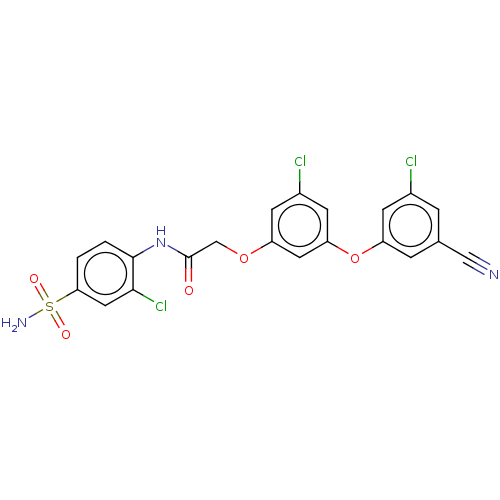 (CHEMBL255689) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478026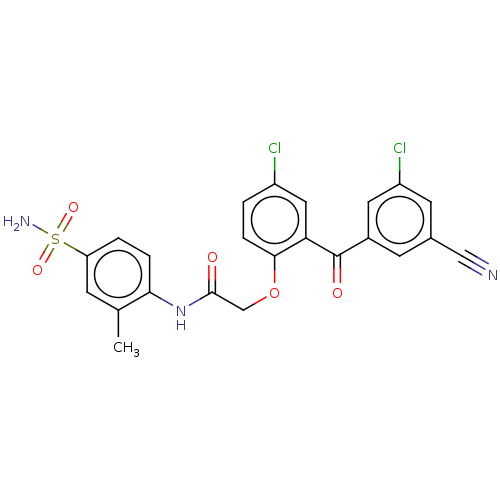 (CHEMBL203420 | GW678248 | GW8248) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478026 (CHEMBL203420 | GW678248 | GW8248) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478383 (CHEMBL402823) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 RT polymerase Y181C mutant by SPA | J Med Chem 51: 6503-11 (2008) Article DOI: 10.1021/jm800856c BindingDB Entry DOI: 10.7270/Q2H41V7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478383 (CHEMBL402823) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478387 (CHEMBL255689) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 18: 2959-66 (2008) Article DOI: 10.1016/j.bmcl.2008.03.064 BindingDB Entry DOI: 10.7270/Q2HT2S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 354 total ) | Next | Last >> |