

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50537515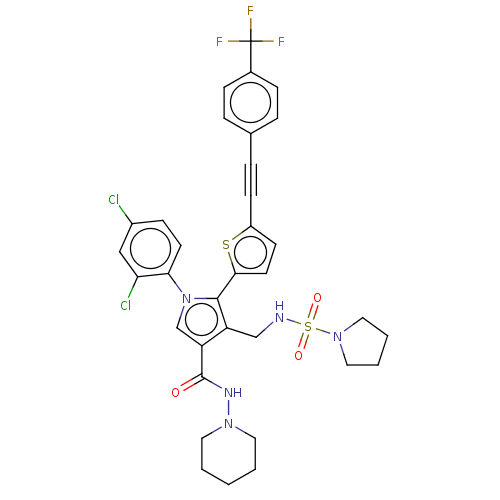 (CHEMBL4644088) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50537520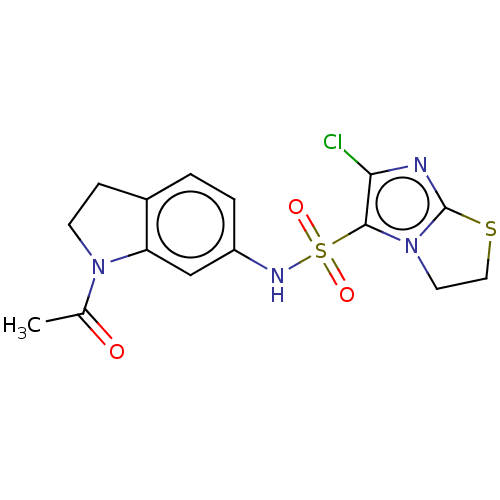 (CHEMBL4649783) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50537518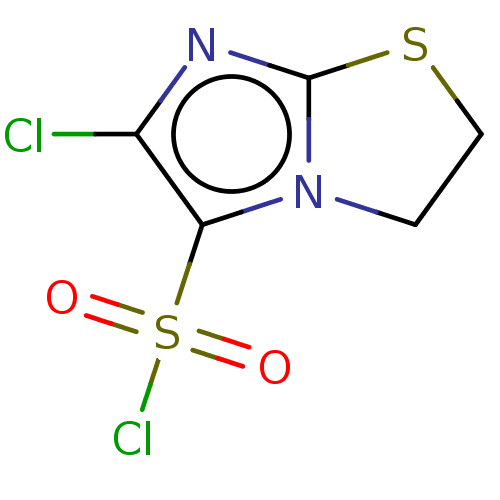 (CHEMBL4635924) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50537519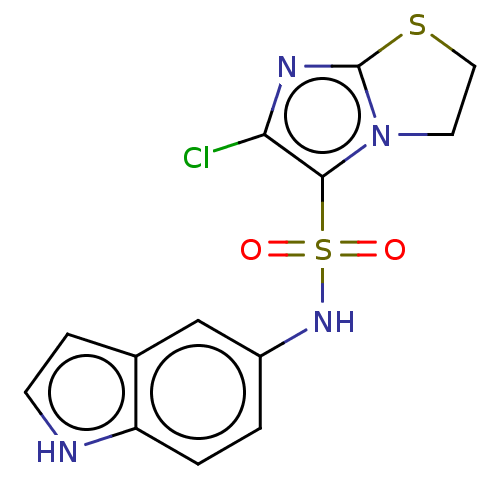 (CHEMBL4638578) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50537515 (CHEMBL4644088) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C eta expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528693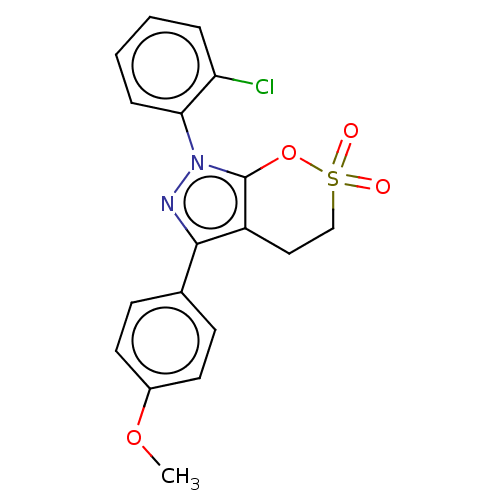 (CHEMBL4513389) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using varying levels of butyrylthiocholine iodide as substrate by Line weaver Burk reciprocal plot analysis | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50537512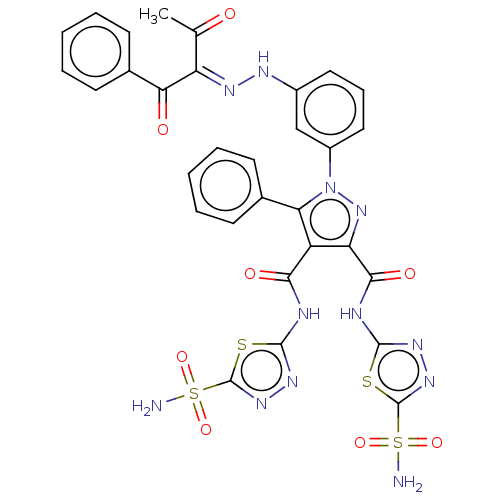 (CHEMBL4637934) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C eta expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528690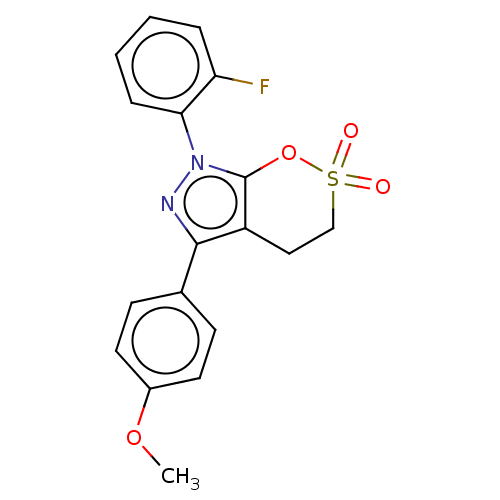 (CHEMBL4545271) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using varying levels of butyrylthiocholine iodide as substrate by Line weaver Burk reciprocal plot analysis | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34152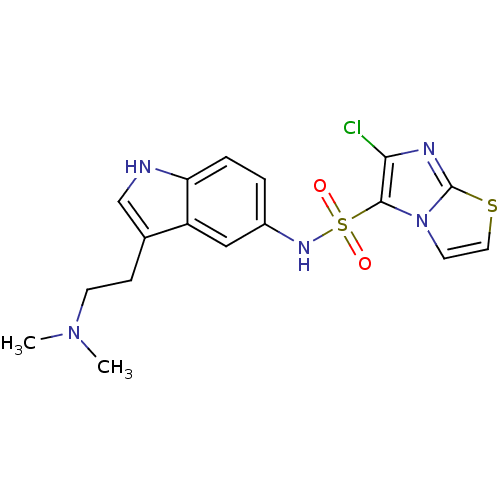 (CHEMBL362628 | E-6801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50537521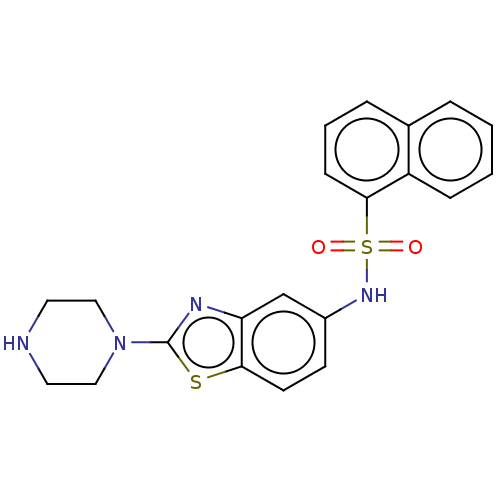 (CHEMBL4632617) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C delta expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM193743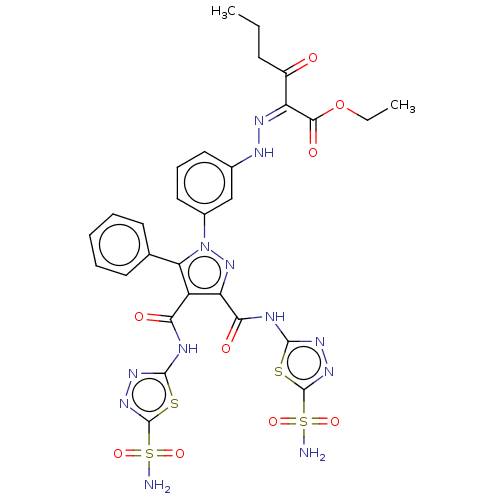 (Ethyl 3-oxo-2-(2-(3-(5-phenyl-3,4-bis((5-sulfamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 884 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50515806 (CHEMBL4533252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50537510 (CHEMBL4644942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C eta expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50537511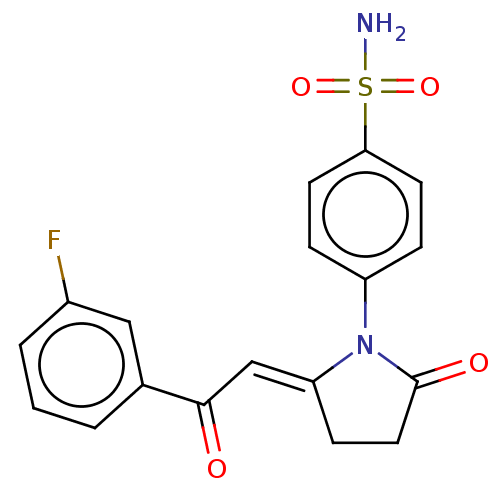 (CHEMBL4633178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C eta expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase after 1 hr by ELISA | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111566 BindingDB Entry DOI: 10.7270/Q2SN0DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252033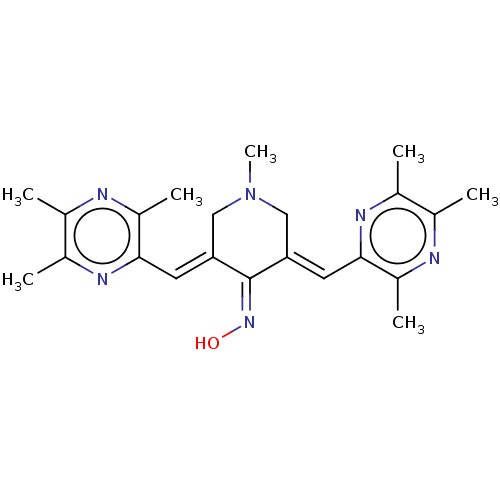 (CHEMBL4078023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252035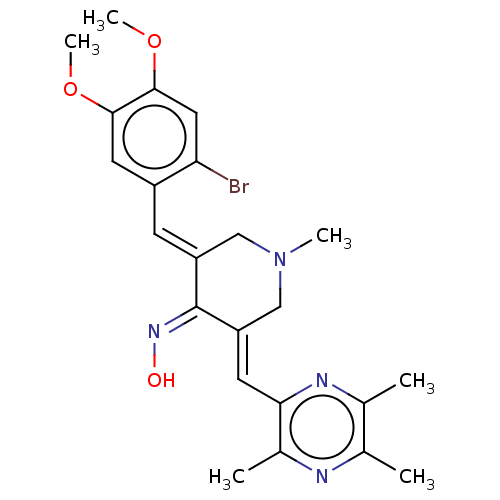 (CHEMBL4064361) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50515806 (CHEMBL4533252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C eta expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by El... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161803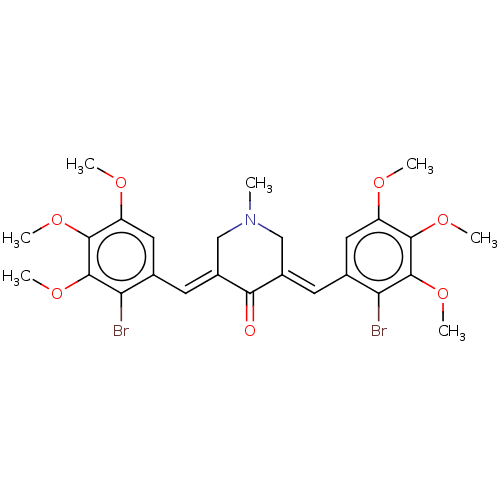 (CHEMBL3785375) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252089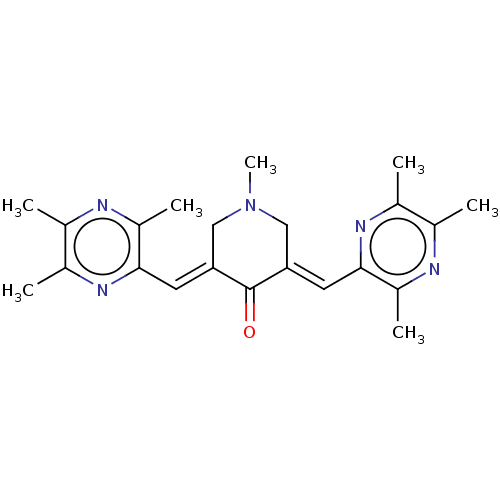 (CHEMBL4083642) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM203833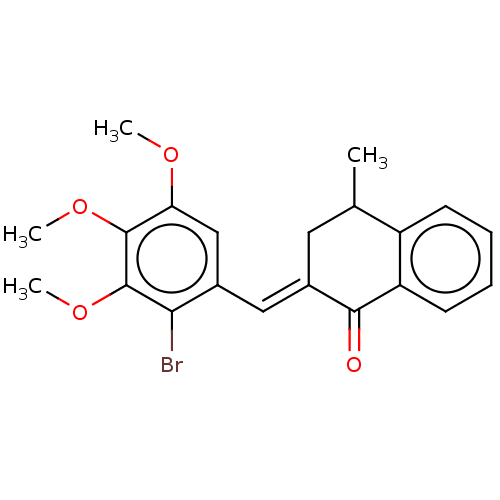 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-4-methyl-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50614390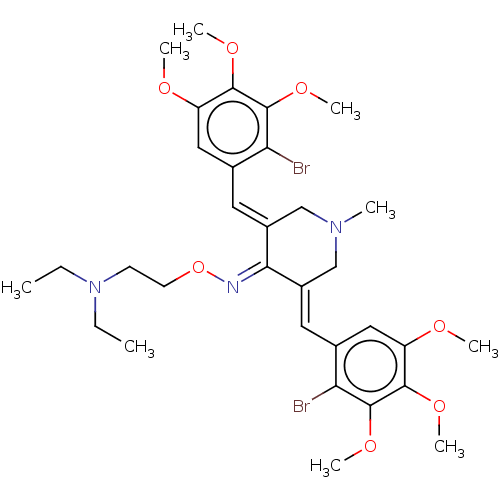 (CHEMBL5271106) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252090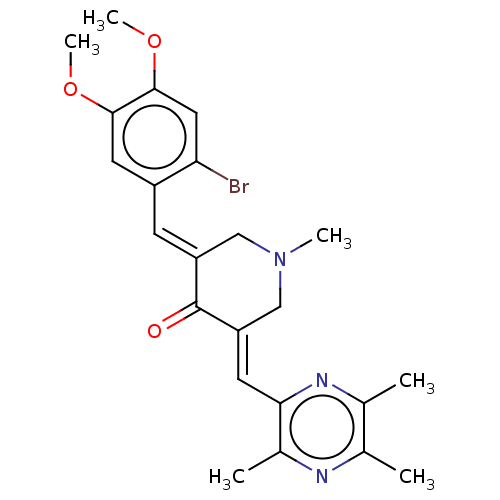 (CHEMBL4063890) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50614382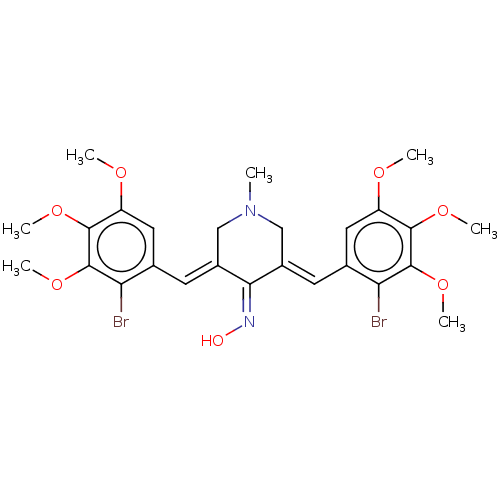 (CHEMBL5279636) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM203832 ((2E)-2-[(2-chloro-3,4-dimethoxyphenyl)methylidene]...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528685 (CHEMBL4457213) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM203851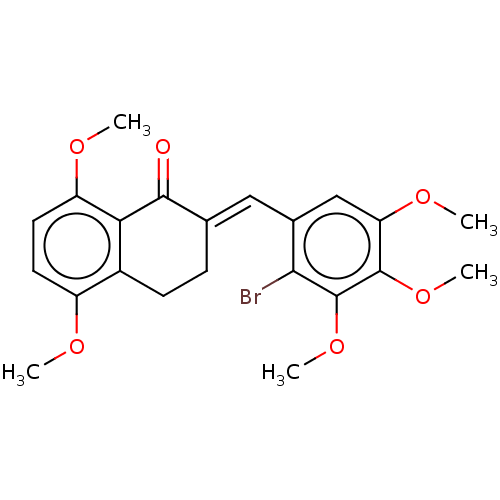 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-5,8-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50526706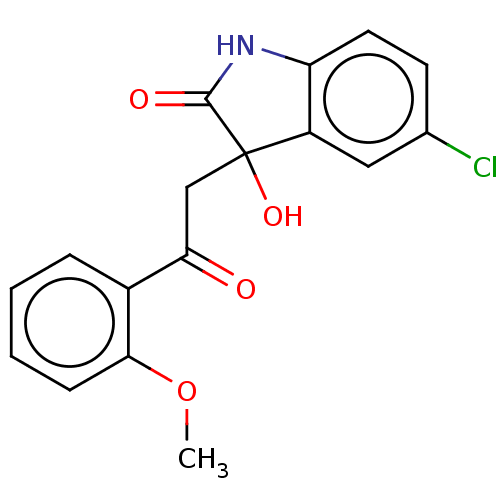 (CHEMBL4545676) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase after 1 hr by ELISA | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111566 BindingDB Entry DOI: 10.7270/Q2SN0DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528693 (CHEMBL4513389) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (RAT) | BDBM50537507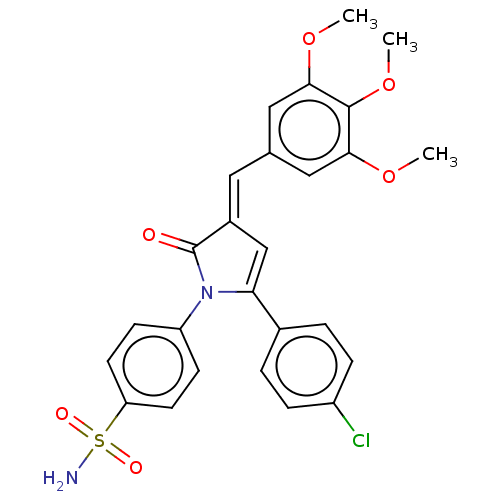 (CHEMBL4638485) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C eta expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528690 (CHEMBL4545271) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50614381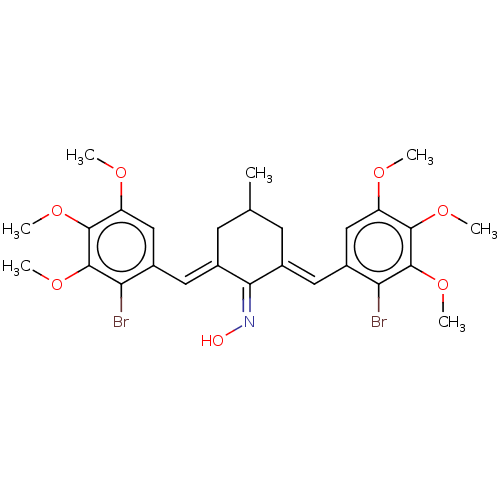 (CHEMBL5275872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161797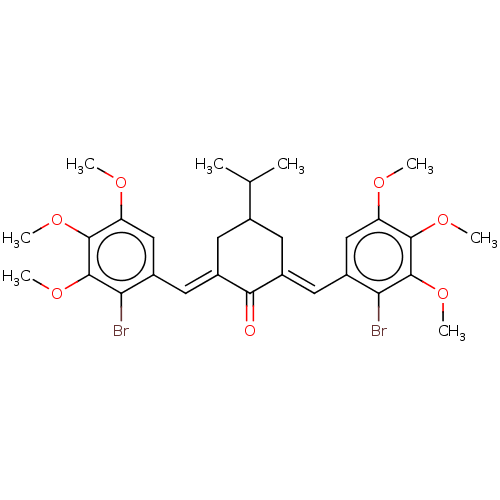 (CHEMBL3785402) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM203860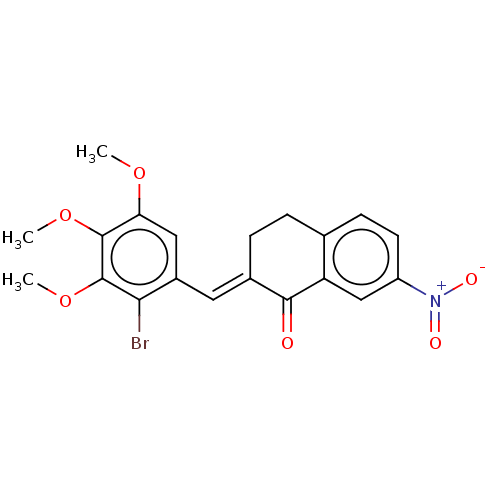 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-7-nitro-t...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (RAT) | BDBM50487330 (CHEMBL4638767) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Tested for inhibition of cGMP-dependent protein kinase from bovine lung | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161802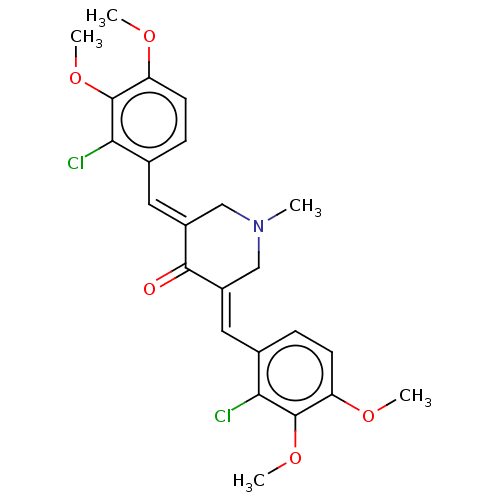 (CHEMBL3787299) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM222462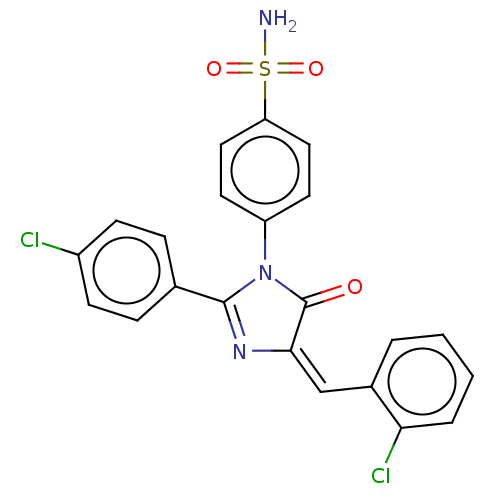 ((E)-4-[4-(2-Chlorobenzylidene)-2-(4-chlorophenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 by EIA | Eur J Med Chem 173: 117-153 (2019) Article DOI: 10.1016/j.ejmech.2019.03.063 BindingDB Entry DOI: 10.7270/Q2QJ7MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM203833 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-4-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 321 total ) | Next | Last >> |