

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558092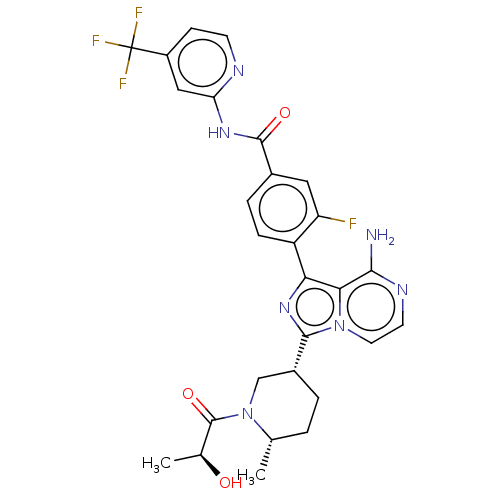 (CHEMBL4784814) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267789 (4-{8-amino-3-[(3R,6S)-1-(cyclopropylcarbonyl)-6-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558095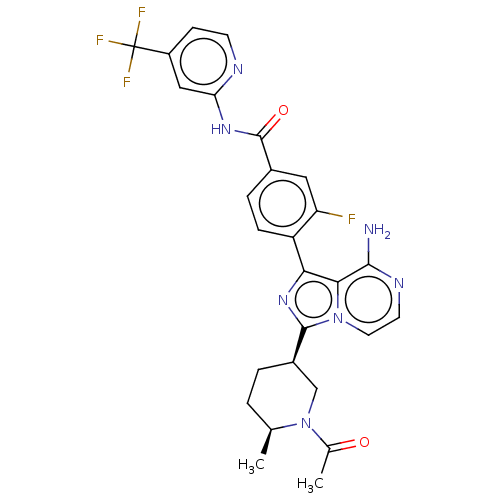 (CHEMBL4752314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558093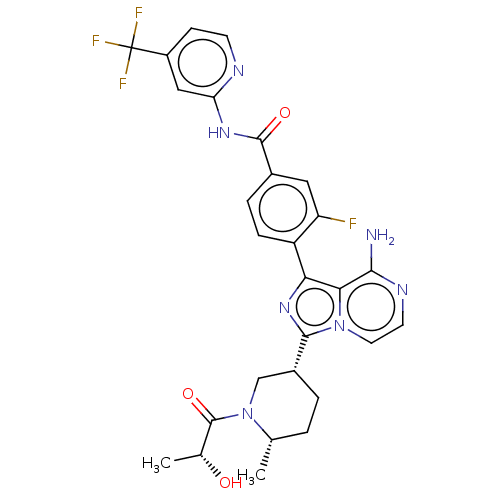 (CHEMBL4798236) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558088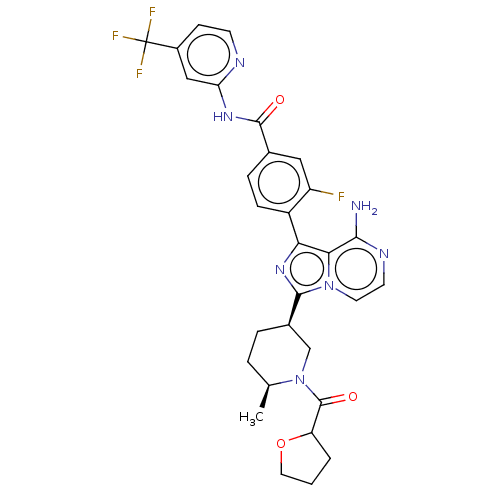 (CHEMBL4751655) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267703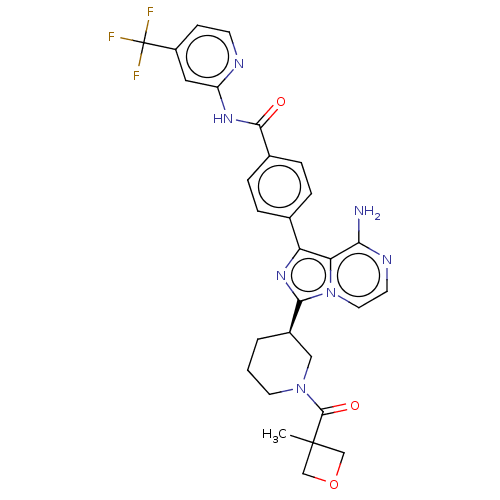 ((R)-4-(8-amino-5-deutero-3-(1-(3-methoxypropanoyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267791 (4-{8-amino-3-[(3R,6S)-1-(cyclopropylcarbonyl)-6-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267819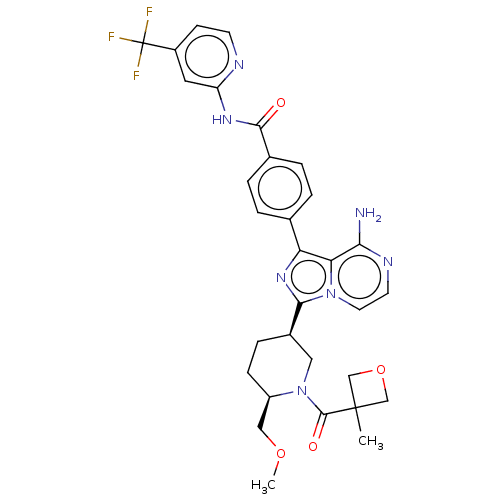 (4-(8-amino-3-{(3S,6S)-6-(methoxymethyl)-1-[(3-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267790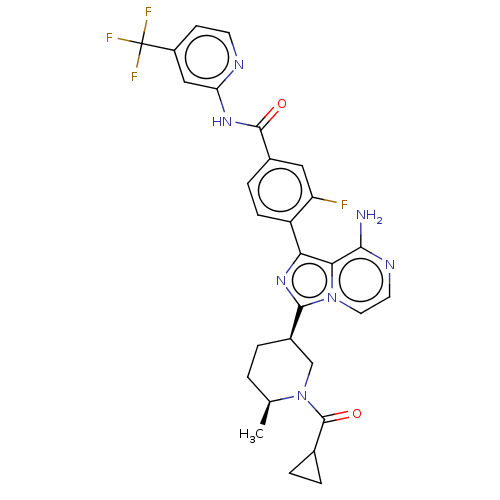 (4-(8-amino-3-{(3R,6S)-6-methyl-1-[(3-methyloxetan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558090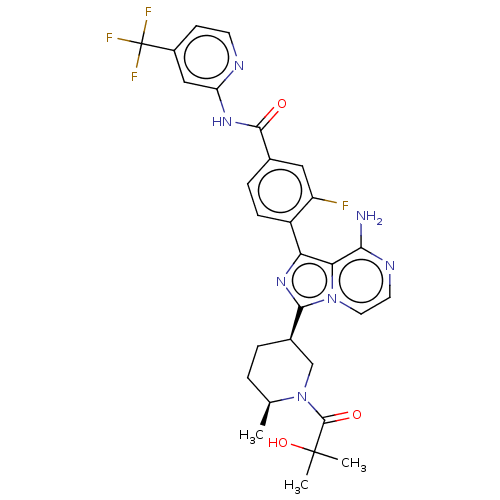 (CHEMBL4756290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558112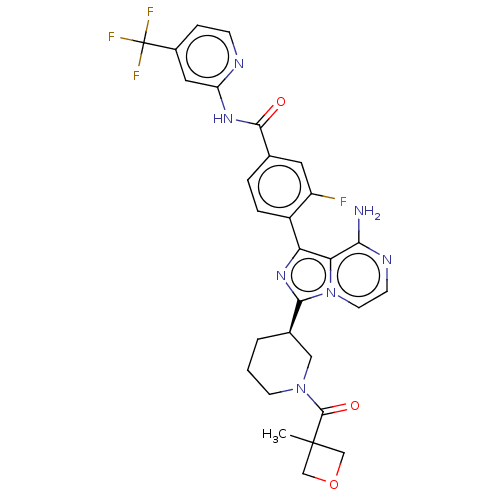 (CHEMBL4799627) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267792 (4-{8-amino-3-[(3R,6S)-6-methyl-1-propanoylpiperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267869 (4-(8-amino-3-{(3R)-1-[(3-methyloxetan-3-yl)carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558109 (CHEMBL4764215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558097 (CHEMBL4787427) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558107 (CHEMBL4743911) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558094 (CHEMBL4764139) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267870 (4-(8-amino-3-{(3R)-1-[(3-methyloxetan-3-yl)carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558089 (CHEMBL4788780) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558108 (CHEMBL4783242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558091 (CHEMBL4781173) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267867 (4-(8-amino-3-{(3R)-1-[(3-methyloxetan-3-yl)carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558110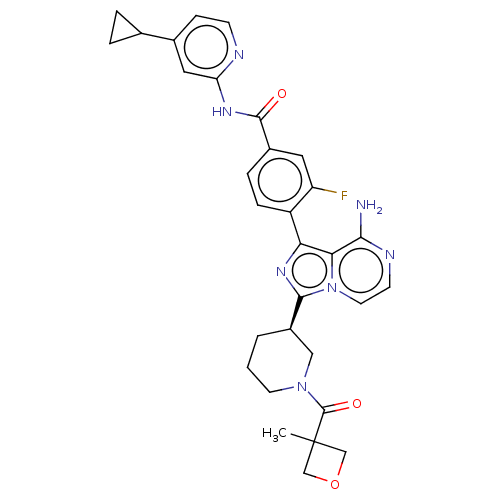 (CHEMBL4794031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267810 (4-{8-amino-3-[(3S,6R)-1-propanoyl-6-(trifluorometh...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558098 (CHEMBL4751902) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267864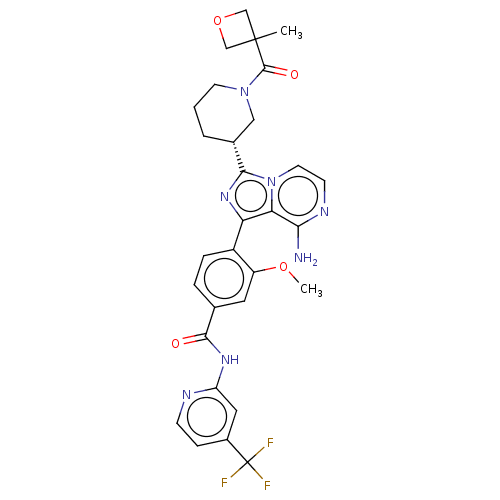 (4-(8-amino-3-{(3R,6S)-6-methyl-1-[(3-methyloxetan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558111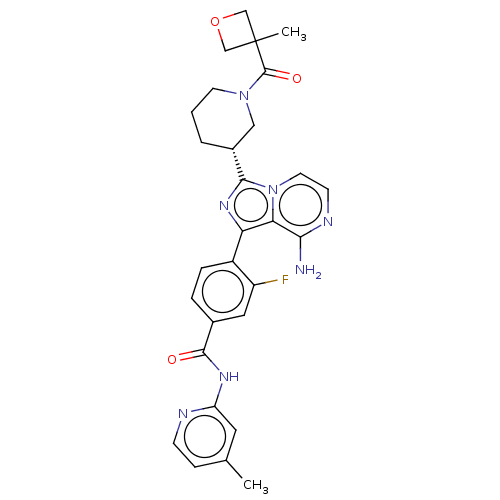 (CHEMBL4796764) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267791 (4-{8-amino-3-[(3R,6S)-1-(cyclopropylcarbonyl)-6-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267792 (4-{8-amino-3-[(3R,6S)-6-methyl-1-propanoylpiperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558092 (CHEMBL4784814) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267859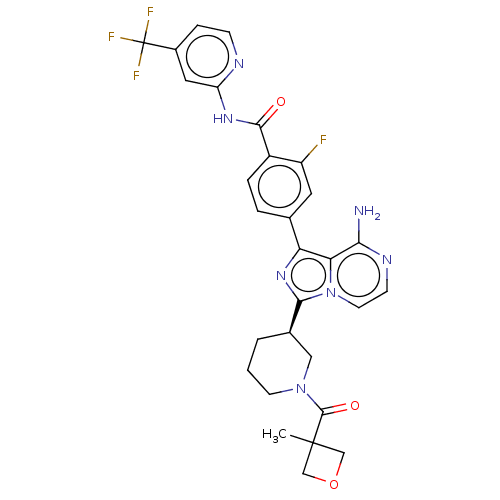 (4-[8-amino-5-(hydroxymethyl)-3-{(3R)-1-[(3-methylo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558088 (CHEMBL4751655) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267789 (4-{8-amino-3-[(3R,6S)-1-(cyclopropylcarbonyl)-6-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558101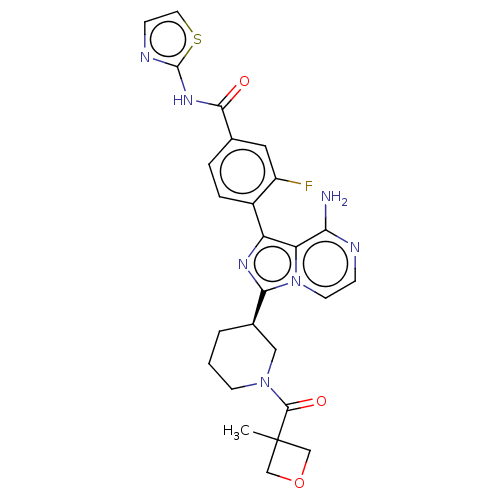 (CHEMBL4784093) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558096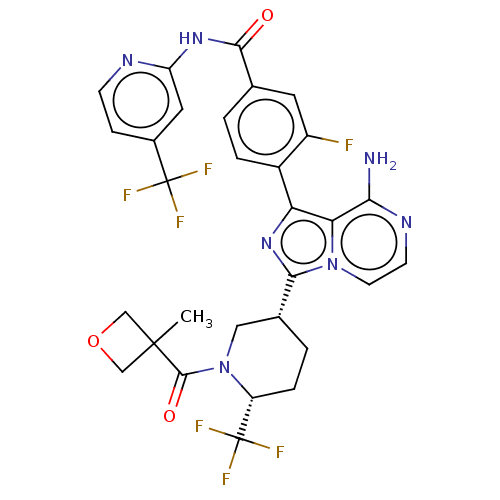 (CHEMBL4753001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558095 (CHEMBL4752314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267819 (4-(8-amino-3-{(3S,6S)-6-(methoxymethyl)-1-[(3-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267790 (4-(8-amino-3-{(3R,6S)-6-methyl-1-[(3-methyloxetan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558094 (CHEMBL4764139) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558093 (CHEMBL4798236) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558107 (CHEMBL4743911) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558090 (CHEMBL4756290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267860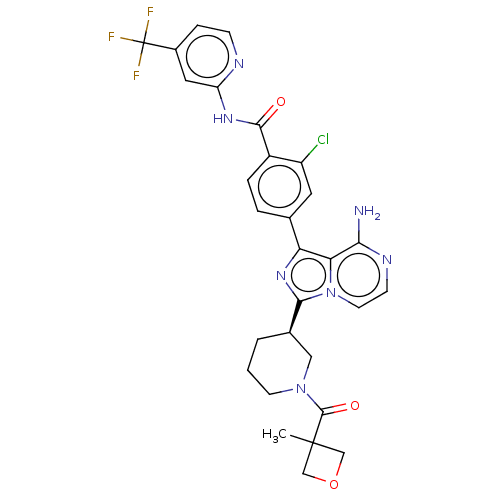 (4-(8-amino-3-{(3R)-1-[(3-methyloxetan-3-yl)carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of recombinant full length BTK (unknown origin) baculovirus infected Sf9 cells using biotinylated EQEDEPEGDYFEWLE-NH2 peptide a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558112 (CHEMBL4799627) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267703 ((R)-4-(8-amino-5-deutero-3-(1-(3-methoxypropanoyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50558089 (CHEMBL4788780) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in human PBMC assessed as reduction in cell surface CD69 expression preincubated for 1 hr followed by stimulation with goat anti-hu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00463 BindingDB Entry DOI: 10.7270/Q25B065B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267904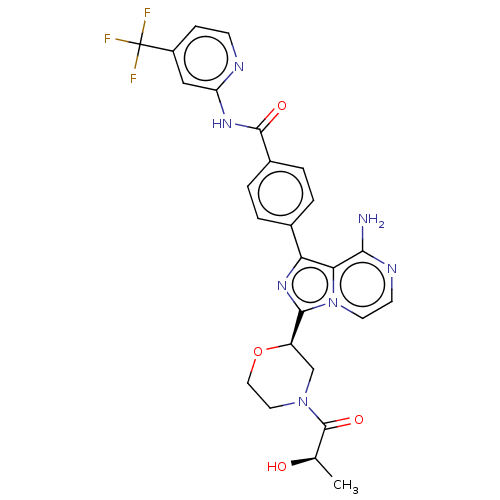 (4-(8-amino-3-{(2R)-4-[(2S)-2-hydroxypropanoyl]morp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267905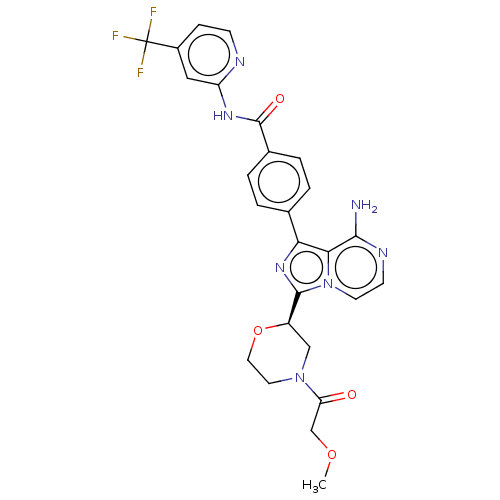 (4-(8-amino-3-{(2R)-4-[(2R)-2-hydroxypropanoyl]morp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267906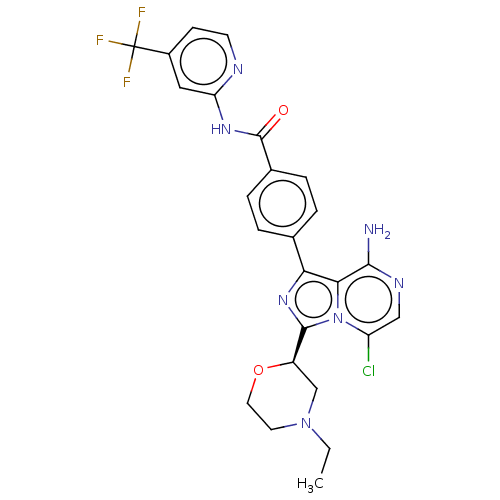 (4-{8-amino-3-[(2R)-4-(methoxyacetyl)morpholin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267907 (4-{8-amino-5-chloro-3-[(2R)-4-ethylmorpholin-2-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2598 total ) | Next | Last >> |