

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apelin receptor (Homo sapiens (Human)) | BDBM50014619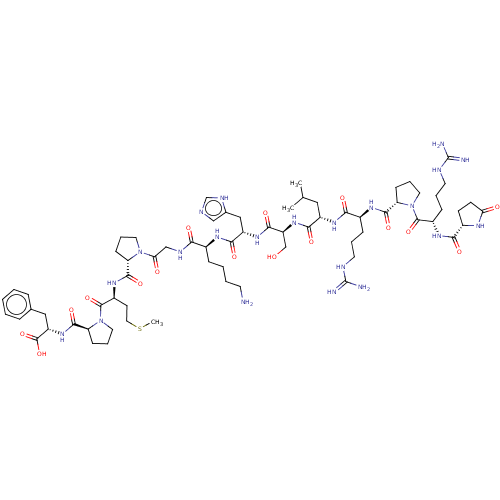 (CHEMBL3184840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544744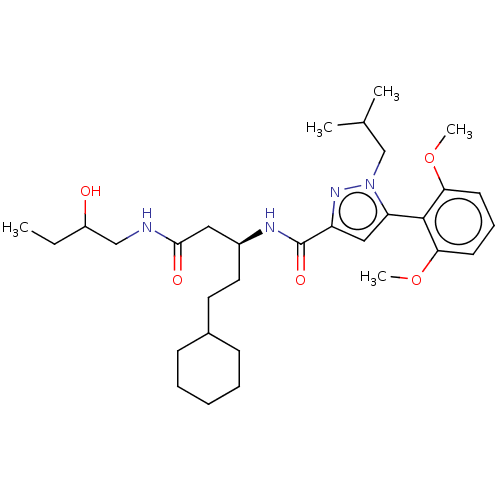 (CHEMBL4647996) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544735 (CHEMBL4640815) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544720 (CHEMBL4632564) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544742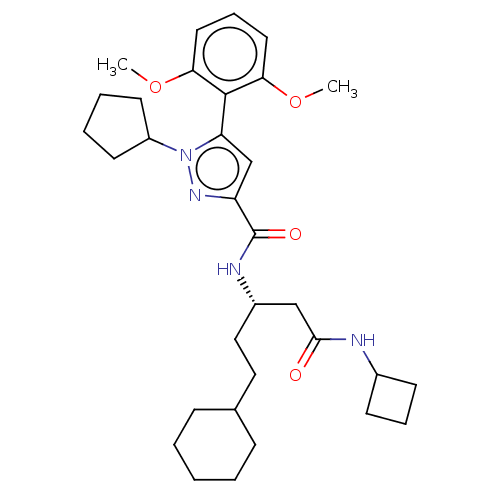 (CHEMBL4647910) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544721 (CHEMBL4644803) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544753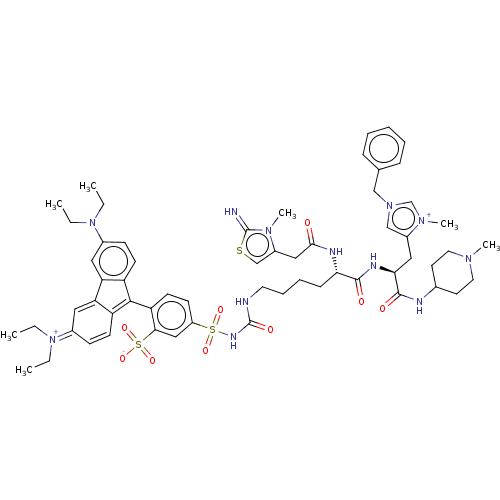 (CHEMBL4642754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from EGFP-fused human APJ receptor delta16 mutant stably expressed in HEK293 cells by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544749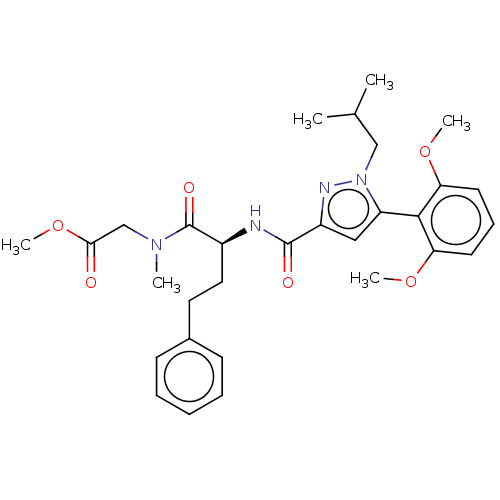 (CHEMBL4640653) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544729 (CHEMBL4647391) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544736 (CHEMBL4647853) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544747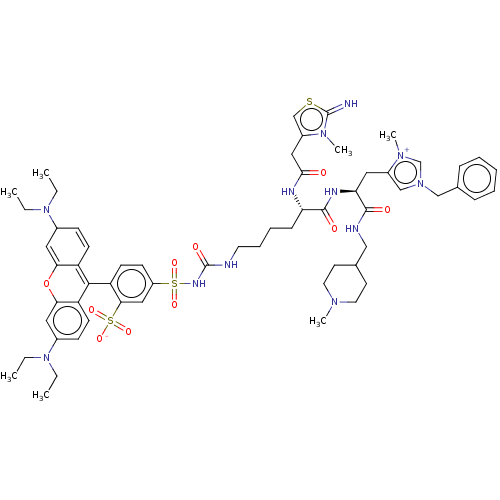 (CHEMBL4641593) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from EGFP-fused human APJ receptor delta16 mutant stably expressed in HEK293 cells by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544722 (CHEMBL4647332) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Rattus norvegicus) | BDBM50544745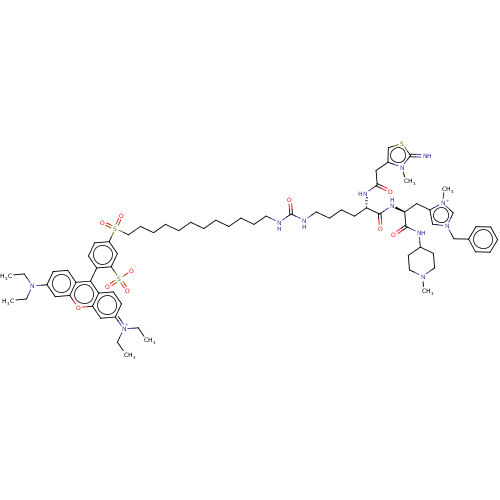 (CHEMBL4632773) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-pE13F from rat EGFP-tagged APJ receptor stably expressed in CHO cell membrane measured after 1 hr | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544746 (CHEMBL4638070) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from EGFP-fused human APJ receptor delta16 mutant stably expressed in HEK293 cells by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544745 (CHEMBL4632773) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-pE13F from human APJ receptor stably expressed in CHO cell membrane after 1 hr | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544733 (CHEMBL4640390) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 449 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544728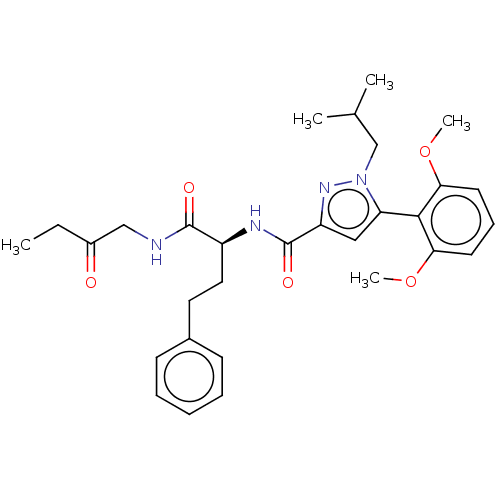 (CHEMBL4641474) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 571 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50544745 (CHEMBL4632773) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from EGFP-fused human APJ receptor delta16 mutant stably expressed in HEK293 cells by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50183341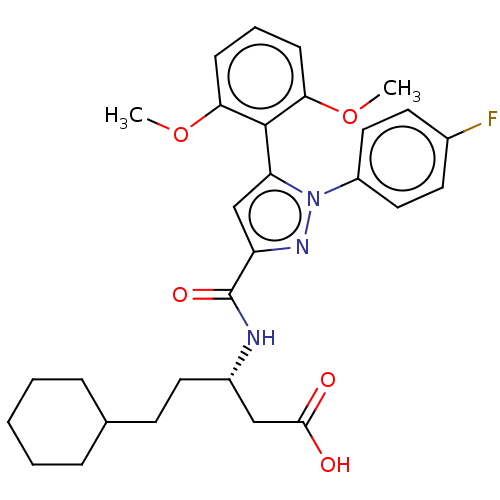 (CHEMBL3817879) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1/11/4/6/8 (Homo sapiens (Human)) | BDBM65470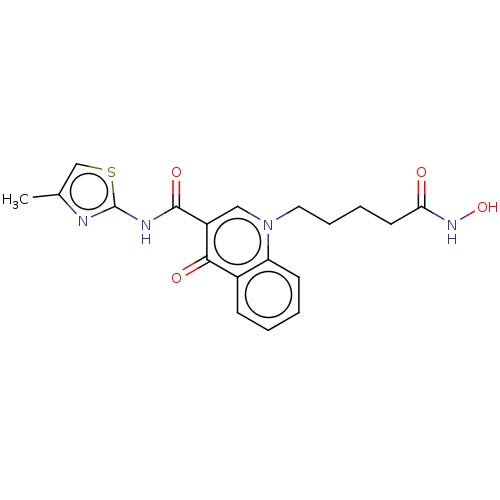 (Quinolone-based HDAC inhibitor 4i) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals & Pharmaceuticals Limited | Assay Description Histone Deacetylase (HDAC) Inhibition Assay using Boc-Lys(Ac)-AMC Substrate: Inhibition of HDAC has been implicated to modulate transcription and to ... | J Enzyme Inhib Med Chem 29: 555-62 (2014) Article DOI: 10.3109/14756366.2013.827675 BindingDB Entry DOI: 10.7270/Q22J6916 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.011 BindingDB Entry DOI: 10.7270/Q2N58R28 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448084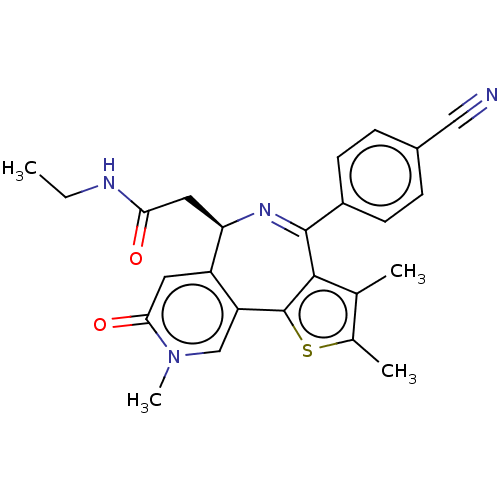 (US10689395, Compound k | US11267820, Compound k) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED US Patent | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | US Patent US10689395 (2020) BindingDB Entry DOI: 10.7270/Q21J9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [44-168] (Homo sapiens (Human)) | BDBM50260101 (CHEMBL4068487 | US10689390, Compound 38 | US113193...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448084 (US10689395, Compound k | US11267820, Compound k) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260101 (CHEMBL4068487 | US10689390, Compound 38 | US113193...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED US Patent | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | US Patent US10689390 (2020) BindingDB Entry DOI: 10.7270/Q25B05JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50057651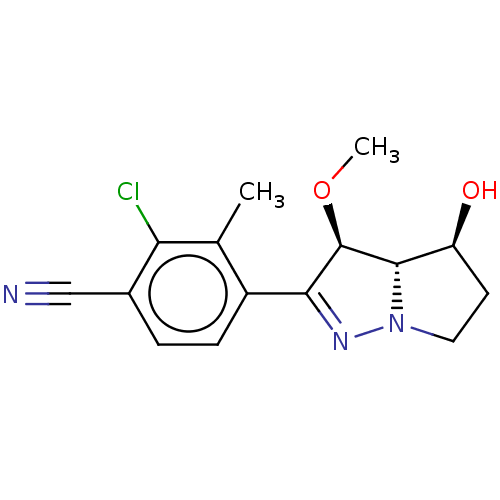 (CHEMBL3326454) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-435 cells after 30 mins | J Med Chem 57: 7396-411 (2014) Article DOI: 10.1021/jm5009049 BindingDB Entry DOI: 10.7270/Q2RB768M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448091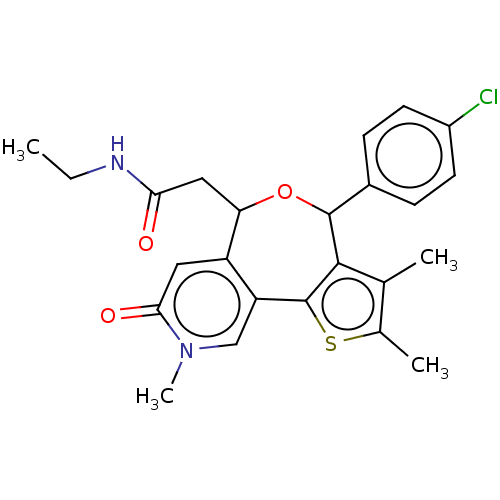 (US10689395, Compound bb | US11267820, Compound bb) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED US Patent | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | US Patent US10689395 (2020) BindingDB Entry DOI: 10.7270/Q21J9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [44-168] (Homo sapiens (Human)) | BDBM448073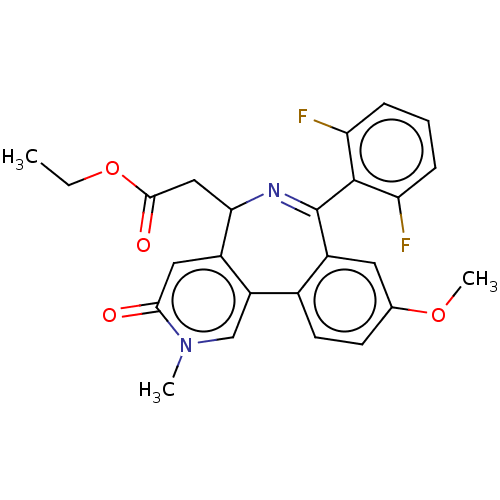 (US10689390, Compound 23 | US11319326, Compound 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448094 (US10689395, Compound ll | US11267820, Compound ll) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448091 (US10689395, Compound bb | US11267820, Compound bb) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448086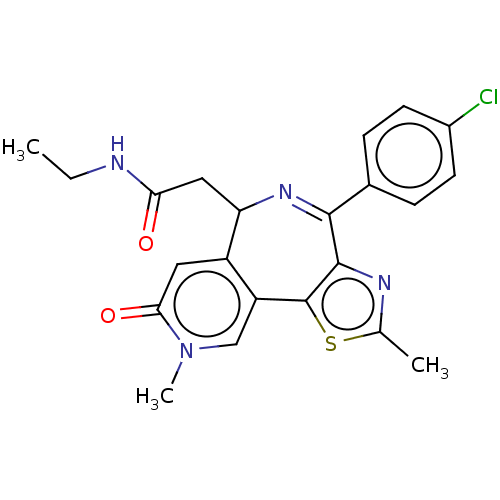 (US10689395, Compound m | US11267820, Compound m) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448073 (US10689390, Compound 23 | US11319326, Compound 23) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED US Patent | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | US Patent US10689390 (2020) BindingDB Entry DOI: 10.7270/Q25B05JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448086 (US10689395, Compound m | US11267820, Compound m) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED US Patent | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | US Patent US10689395 (2020) BindingDB Entry DOI: 10.7270/Q21J9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448094 (US10689395, Compound ll | US11267820, Compound ll) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED US Patent | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | US Patent US10689395 (2020) BindingDB Entry DOI: 10.7270/Q21J9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM247344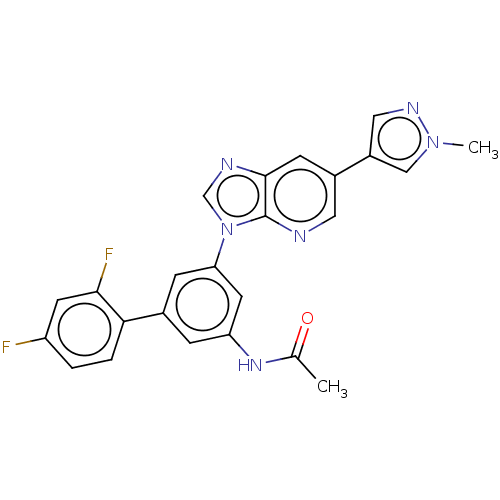 (US9447091, 131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Compounds were screened in the TR-FRET assay with FGFR1 kinase. 5 ng of FGFR1 [Upstate, USA] kinase was used for assay. The compound was incubated wi... | US Patent US9447091 (2016) BindingDB Entry DOI: 10.7270/Q2TM792J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1/11/4/6/8 (Homo sapiens (Human)) | BDBM65471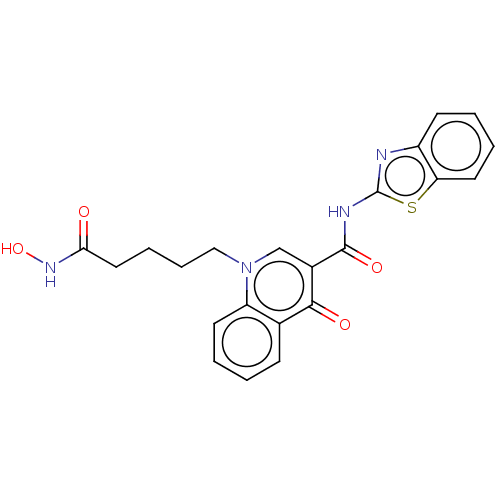 (Quinolone-based HDAC inhibitor 4j) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals & Pharmaceuticals Limited | Assay Description Histone Deacetylase (HDAC) Inhibition Assay using Boc-Lys(Ac)-AMC Substrate: Inhibition of HDAC has been implicated to modulate transcription and to ... | J Enzyme Inhib Med Chem 29: 555-62 (2014) Article DOI: 10.3109/14756366.2013.827675 BindingDB Entry DOI: 10.7270/Q22J6916 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [44-168] (Homo sapiens (Human)) | BDBM448074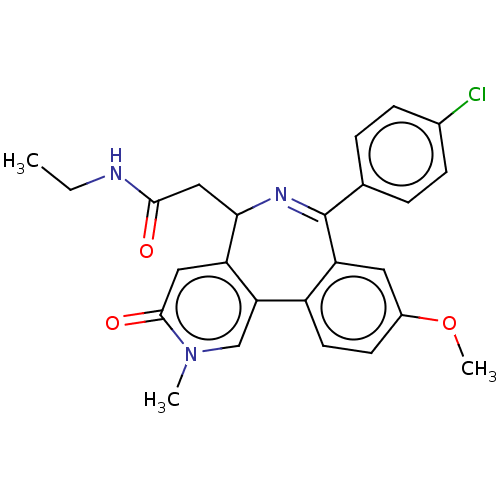 (US10689390, Compound 25 | US11319326, Compound 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448074 (US10689390, Compound 25 | US11319326, Compound 25) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED US Patent | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | US Patent US10689390 (2020) BindingDB Entry DOI: 10.7270/Q25B05JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM247340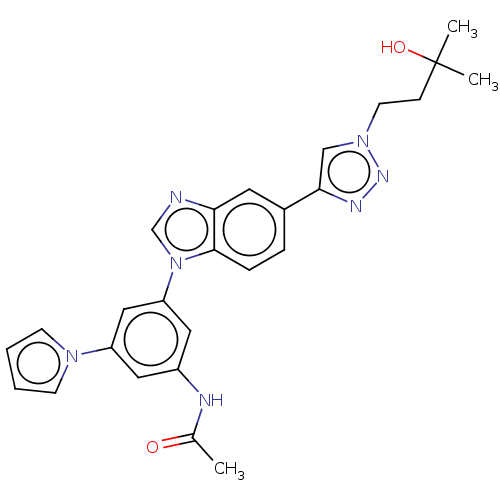 (US9447091, 112) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ORION CORPORATION US Patent | Assay Description Compounds were screened in the TR-FRET assay with FGFR1 kinase. 5 ng of FGFR1 [Upstate, USA] kinase was used for assay. The compound was incubated wi... | US Patent US9447091 (2016) BindingDB Entry DOI: 10.7270/Q2TM792J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM556400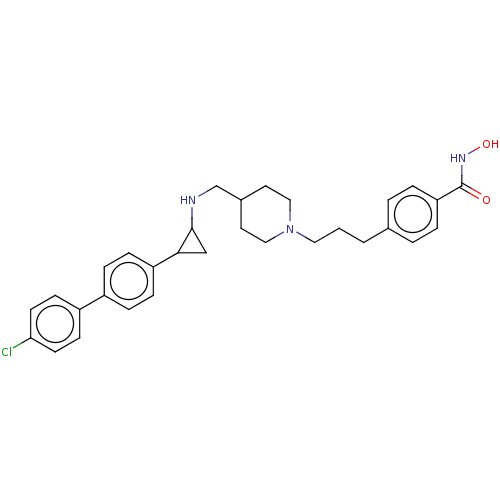 (4-(3-(4-(((2-(4′-chloro-[1,1′-biphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LSD1 enzyme was produced in house. Tranylcypromine (TCP), LSD1 inhibitor was procured from Selleckchem. LSD1 enzyme, TCP and Biotinylated peptide sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NS0Z4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM556509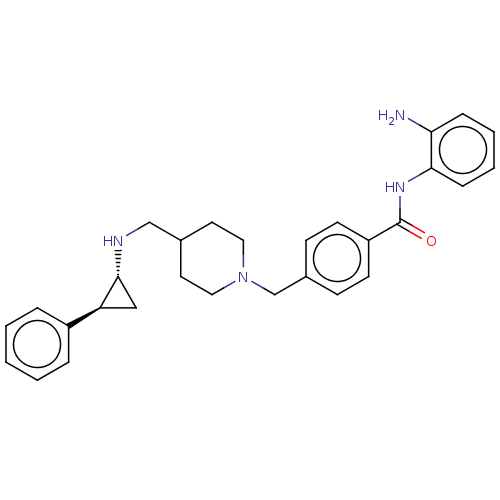 (N-(2-aminophenyl)-4-((4-((((1R,2S)-2-phenylcyclopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LSD1 enzyme was produced in house. Tranylcypromine (TCP), LSD1 inhibitor was procured from Selleckchem. LSD1 enzyme, TCP and Biotinylated peptide sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NS0Z4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448075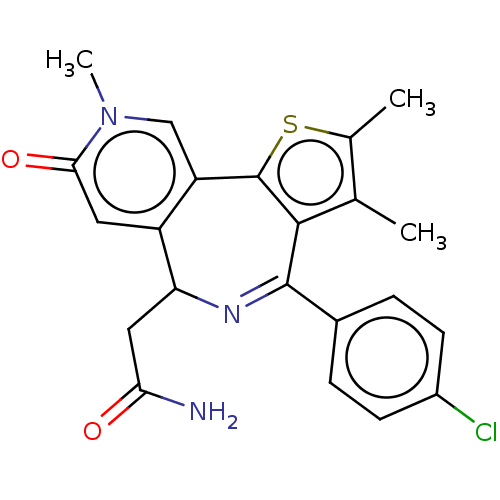 (US10689395, Compound e | US11267820, Compound e) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED US Patent | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | US Patent US10689395 (2020) BindingDB Entry DOI: 10.7270/Q21J9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM556509 (N-(2-aminophenyl)-4-((4-((((1R,2S)-2-phenylcyclopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LSD1 enzyme was produced in house. Tranylcypromine (TCP), LSD1 inhibitor was procured from Selleckchem. LSD1 enzyme, TCP and Biotinylated peptide sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NS0Z4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448088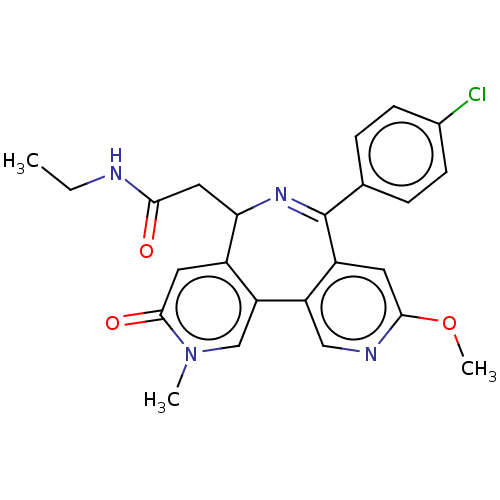 (US10689395, Compound t | US11267820, Compound t) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED US Patent | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | US Patent US10689395 (2020) BindingDB Entry DOI: 10.7270/Q21J9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448092 (US10689395, Compound ee | US11267820, Compound ee) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED US Patent | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | US Patent US10689395 (2020) BindingDB Entry DOI: 10.7270/Q21J9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM556400 (4-(3-(4-(((2-(4′-chloro-[1,1′-biphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LSD1 enzyme was produced in house. Tranylcypromine (TCP), LSD1 inhibitor was procured from Selleckchem. LSD1 enzyme, TCP and Biotinylated peptide sub... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NS0Z4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260078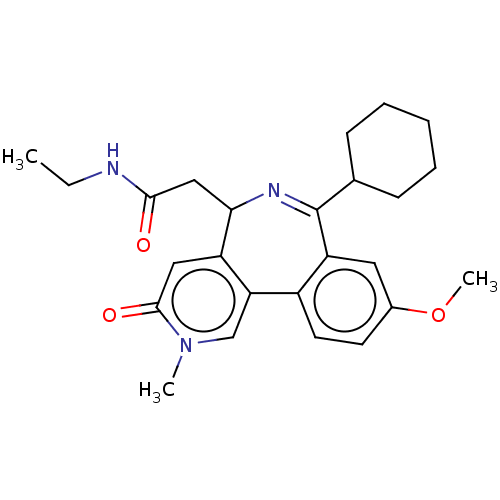 (CHEMBL4095826 | US10689390, Compound 26 | US113193...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED US Patent | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | US Patent US10689390 (2020) BindingDB Entry DOI: 10.7270/Q25B05JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448075 (US10689395, Compound e | US11267820, Compound e) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448088 (US10689395, Compound t | US11267820, Compound t) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM448092 (US10689395, Compound ee | US11267820, Compound ee) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1301 total ) | Next | Last >> |