 Found 930 hits with Last Name = 'ritter' and Initial = 'm'
Found 930 hits with Last Name = 'ritter' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50569654
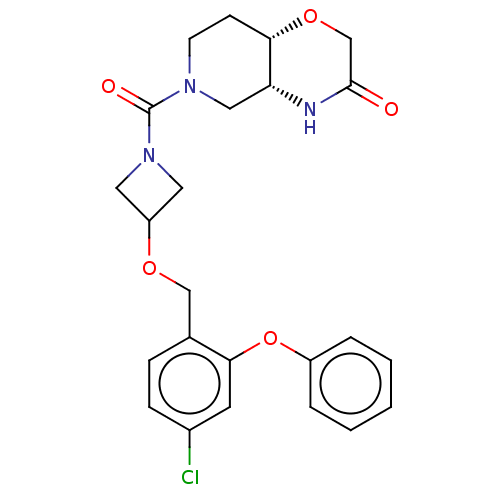
(CHEMBL4875383 | US11802133, Example 239)Show SMILES [H][C@@]12CN(CC[C@]1([H])OCC(=O)N2)C(=O)N1CC(C1)OCc1ccc(Cl)cc1Oc1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50569652

(CHEMBL4874643 | US11802133, Example 221)Show SMILES [H][C@@]12CN(CC[C@]1([H])OCC(=O)N2)C(=O)N1CC(CCc2ccc(cc2Br)C(F)(F)F)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50569653
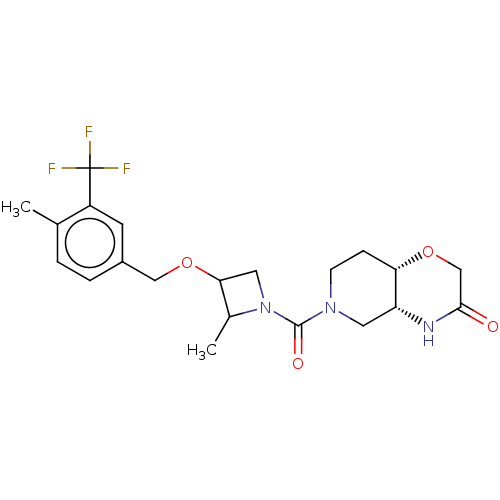
(CHEMBL4865707 | US11802133, Example 229)Show SMILES [H][C@@]12CN(CC[C@]1([H])OCC(=O)N2)C(=O)N1CC(OCc2ccc(C)c(c2)C(F)(F)F)C1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50569655

(CHEMBL4869836 | US11802133, Example 279)Show SMILES [H][C@@]12CN(CC[C@]1([H])OCC(=O)N2)C(=O)N1CC(CCc2ccc(cc2F)C(F)(F)F)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50569651

(CHEMBL4871897 | US11802133, Example 108)Show SMILES [H][C@@]12CN(CC[C@]1([H])OCC(=O)N2)C(=O)N1CC(C1)OCc1ccc(c(Cl)c1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631222
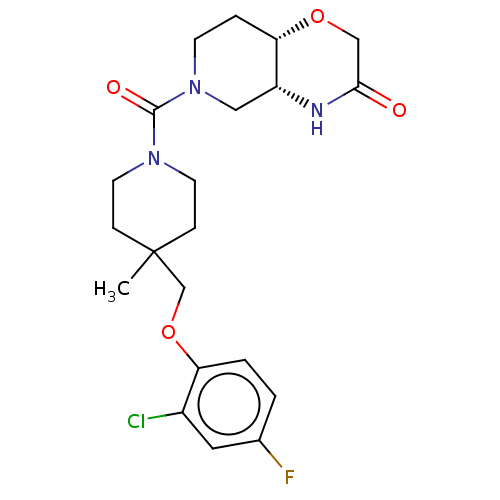
((4aR,8aS)-6-(4-((2-chloro-4-fluorophenoxy)methyl)-...)Show SMILES CC1(COc2ccc(F)cc2Cl)CCN(CC1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50569650
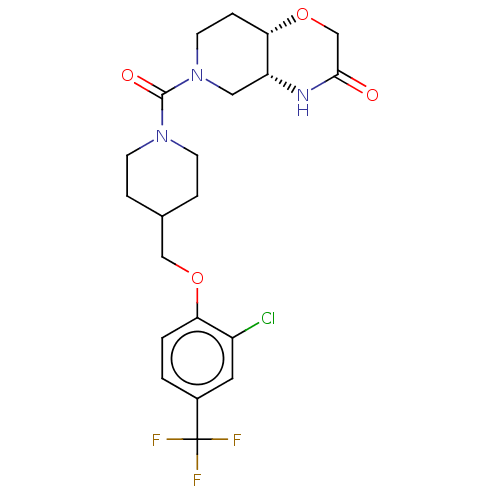
(CHEMBL4848022 | US11802133, Example 54)Show SMILES [H][C@@]12CN(CC[C@]1([H])OCC(=O)N2)C(=O)N1CCC(COc2ccc(cc2Cl)C(F)(F)F)CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631282

(US11802133, Example 219)Show SMILES Cc1ccc(CCC2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(c1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631297

(US11802133, Example 234)Show SMILES FC(F)(F)c1cnc(COC2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)cc1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631260

(US11802133, Example 139)Show SMILES Fc1cccc(c1CCC1CN(C1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631219
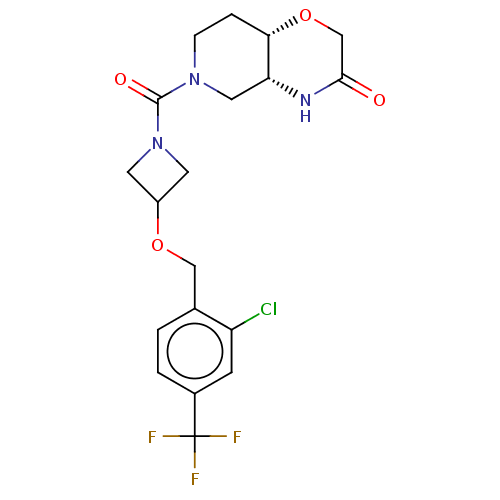
(US11802133, Example 75)Show SMILES FC(F)(F)c1ccc(COC2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631296
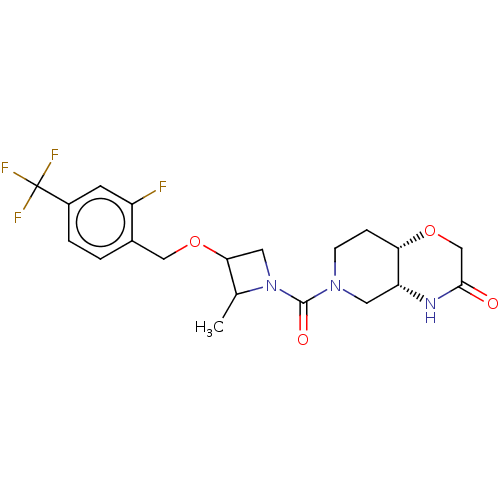
(US11802133, Example 233 | US11802133, Example 236 ...)Show SMILES CC1C(CN1C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)OCc1ccc(cc1F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM561384
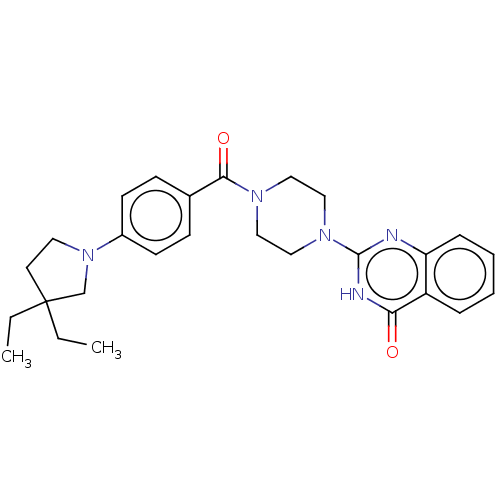
(US11390610, Example 213)Show SMILES CCC1(CC)CCN(C1)c1ccc(cc1)C(=O)N1CCN(CC1)c1nc2ccccc2c(=O)[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W0994M |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631352

(US11802133, Example 289)Show SMILES Fc1cccc(c1\C=C\C1CN(C1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631281
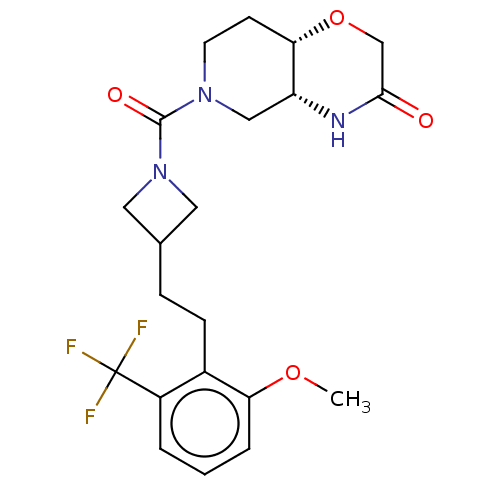
(US11802133, Example 218)Show SMILES COc1cccc(c1CCC1CN(C1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631283
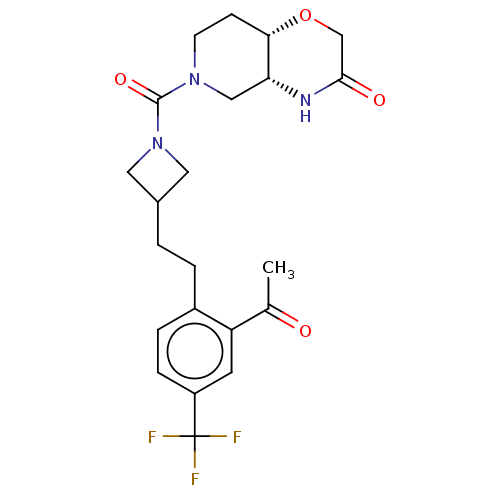
(US11802133, Example 220)Show SMILES CC(=O)c1cc(ccc1CCC1CN(C1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631275

(US11802133, Example 212)Show SMILES Fc1c(OC2CCN(CC2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)ccc(Cl)c1-c1ccc(Cl)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631208
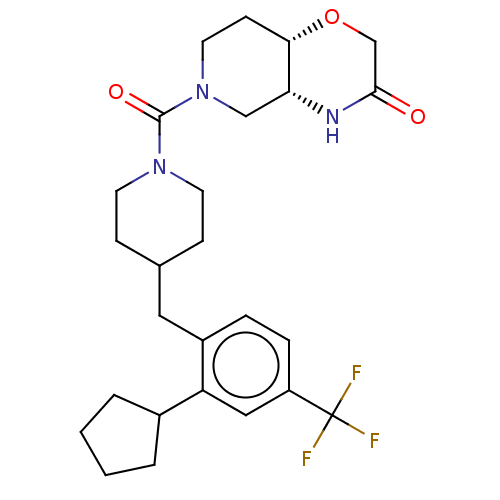
(US11802133, Example 58)Show SMILES FC(F)(F)c1ccc(CC2CCN(CC2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(c1)C1CCCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631218

(US11802133, Example 74)Show SMILES C[C@H]1CN(CC[C@H]1COc1ccc(F)cc1Cl)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631250

(US11802133, Example 115)Show SMILES COc1cc(ccc1COC1CN(C1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631271
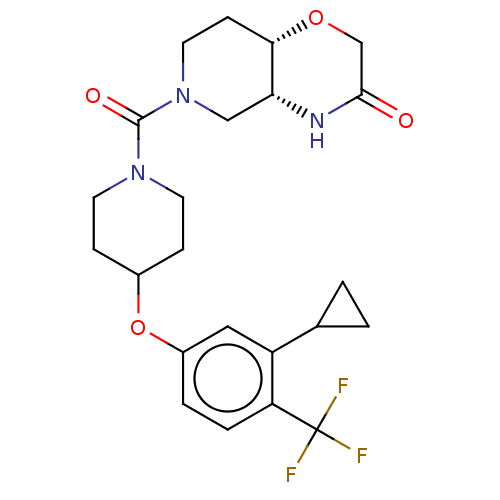
(US11802133, Example 208)Show SMILES FC(F)(F)c1ccc(OC2CCN(CC2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)cc1C1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631287
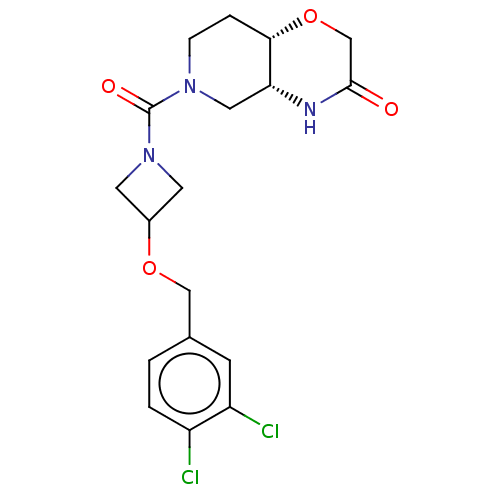
(US11802133, Example 224)Show SMILES Clc1ccc(COC2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)cc1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631158

(US11802133, Example 24 | US11802133, Example 7)Show SMILES Fc1ccc(OCC2CCN(CC2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631251
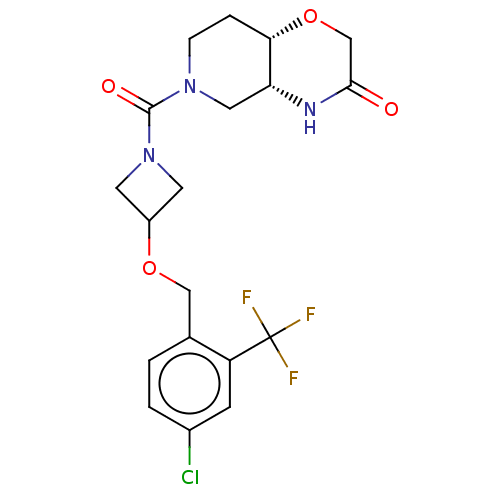
(US11802133, Example 116)Show SMILES FC(F)(F)c1cc(Cl)ccc1COC1CN(C1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631184

(US11802133, Example 33)Show SMILES Fc1cc(CC2CCN(CC2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)ccc1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM561025
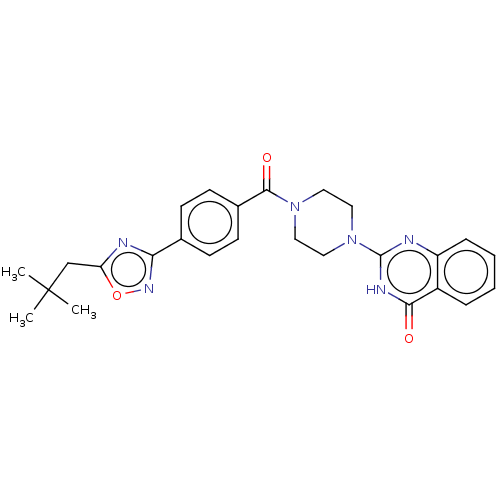
(US11390610, Example 67)Show SMILES CC(C)(C)Cc1nc(no1)-c1ccc(cc1)C(=O)N1CCN(CC1)c1nc2ccccc2c(=O)[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W0994M |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM561355

(US11390610, Example 184)Show SMILES Clc1cccc(OCc2ccc(cc2Cl)C(=O)N2CCN(CC2)c2nc3ccccc3c(=O)[nH]2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W0994M |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631249
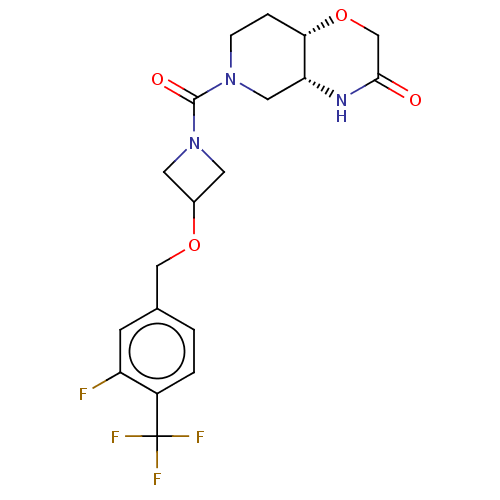
(US11802133, Example 114)Show SMILES Fc1cc(COC2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)ccc1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631314
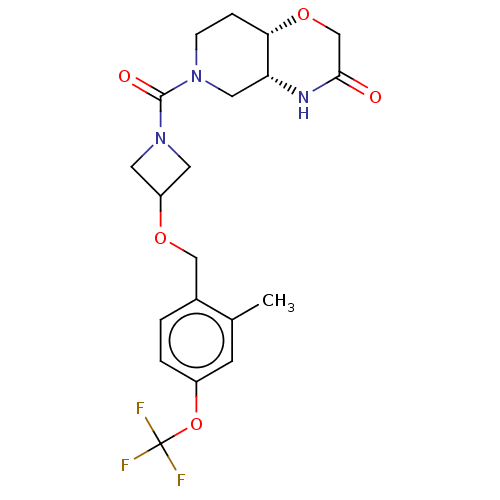
(US11802133, Example 251)Show SMILES Cc1cc(OC(F)(F)F)ccc1COC1CN(C1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631315

(US11802133, Example 252)Show SMILES CC1C(CN1C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)OCc1ccc(OC(F)(F)F)cc1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631355
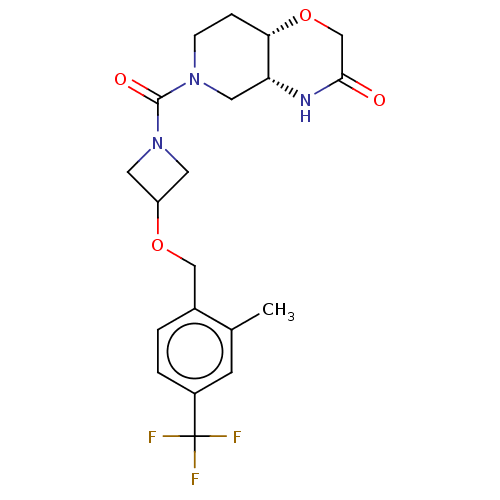
(US11802133, Example 292)Show SMILES Cc1cc(ccc1COC1CN(C1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM561217

(US11390610, Example 116)Show SMILES Clc1ccc(cc1Cl)-c1nc2ccc(cc2o1)C(=O)N1CCN(CC1)c1nc2ccccc2c(=O)[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W0994M |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631278

(US11802133, Example 215)Show SMILES Cc1ccc(CCC2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631294

(US11802133, Example 231)Show SMILES C[C@H]1CN(CC[C@H]1COc1cnc(c(C)c1)C(F)(F)F)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631178
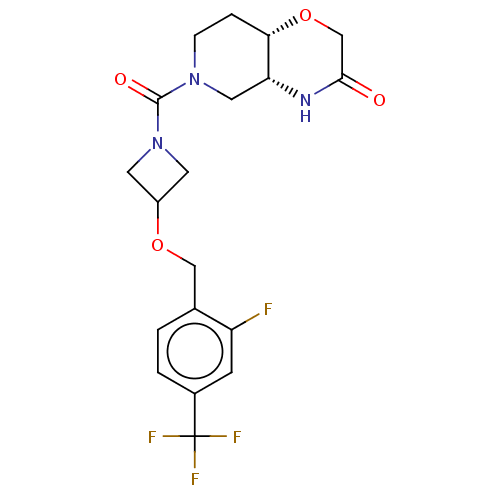
(US11802133, Example 27 | US11802133, Example 29)Show SMILES Fc1cc(ccc1COC1CN(C1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631196
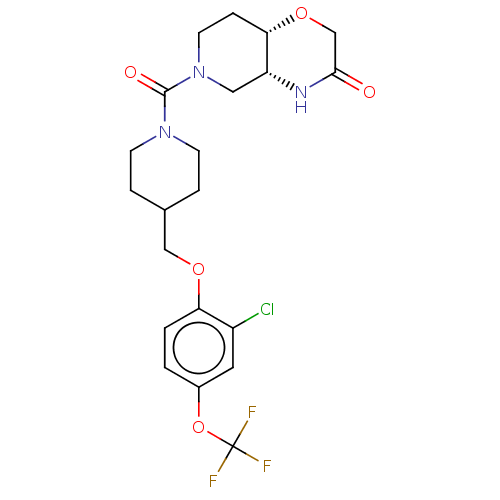
(US11802133, Example 46)Show SMILES FC(F)(F)Oc1ccc(OCC2CCN(CC2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631197
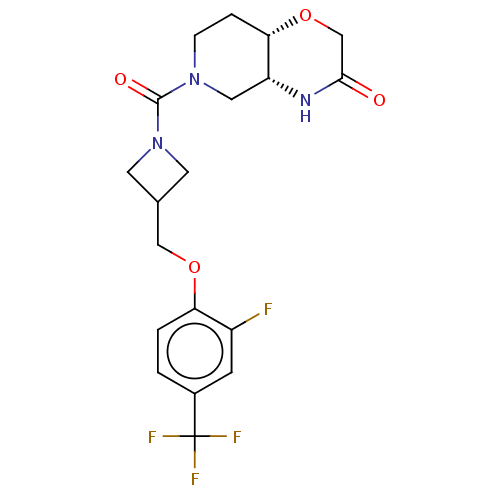
(US11802133, Example 47)Show SMILES Fc1cc(ccc1OCC1CN(C1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631225

(US11802133, Example 88)Show SMILES COc1cc(COC2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)ccc1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631240

(US11802133, Example 103 | US11802133, Example 104 ...)Show SMILES CC1C(CN1C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)OCc1ccc(cc1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631248
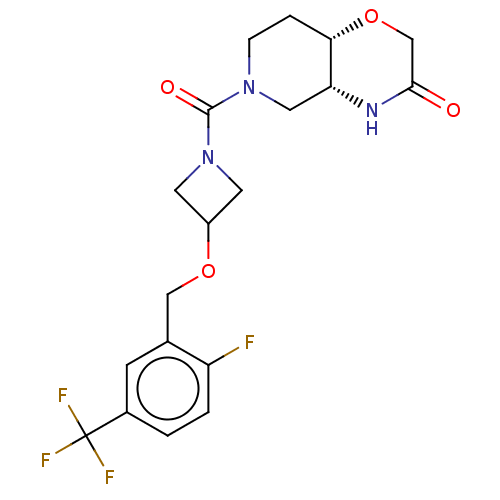
(US11802133, Example 113)Show SMILES Fc1ccc(cc1COC1CN(C1)C(=O)N1CC[C@@H]2OCC(=O)N[C@@H]2C1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631312

(US11802133, Example 249)Show SMILES FC(F)(F)c1ccc(COC2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(c1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM561381
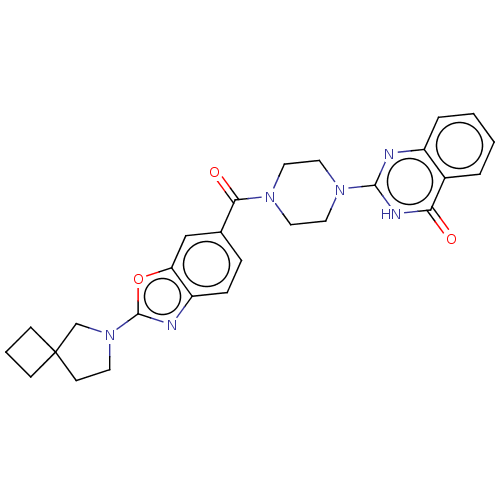
(US11390610, Example 210)Show SMILES O=C(N1CCN(CC1)c1nc2ccccc2c(=O)[nH]1)c1ccc2nc(oc2c1)N1CCC2(CCC2)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W0994M |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631328

((+)-(4aR,8aS)-6-[3-[3-(2-Azaspiro[3.3]heptan-2-yl)...)Show SMILES FC(F)(F)c1ccc(OC2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)cc1N1CC2(CCC2)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631350
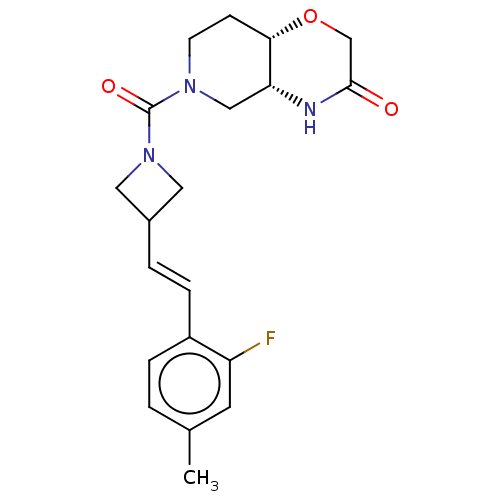
(US11802133, Example 287)Show SMILES Cc1ccc(\C=C\C2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631224
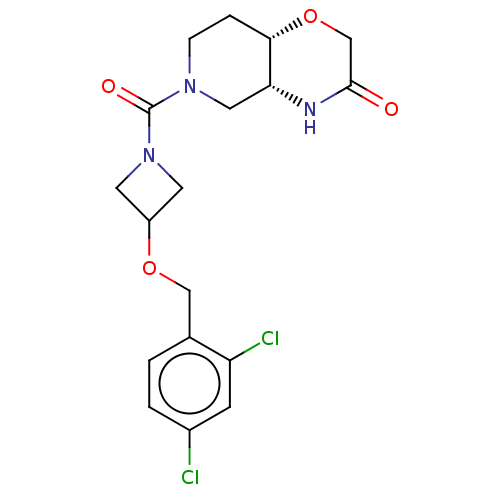
(US11802133, Example 87)Show SMILES Clc1ccc(COC2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631203
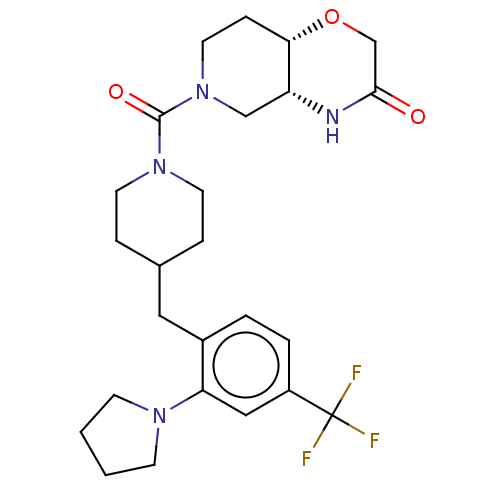
(US11802133, Example 53)Show SMILES FC(F)(F)c1ccc(CC2CCN(CC2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(c1)N1CCCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631223

(US11802133, Example 81)Show SMILES FC(F)(F)c1ccc(CC2CCN(CC2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(c1)-c1cn[nH]c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM631279

(US11802133, Example 216)Show SMILES COc1ccc(CCC2CN(C2)C(=O)N2CC[C@@H]3OCC(=O)N[C@@H]3C2)c(c1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM561341
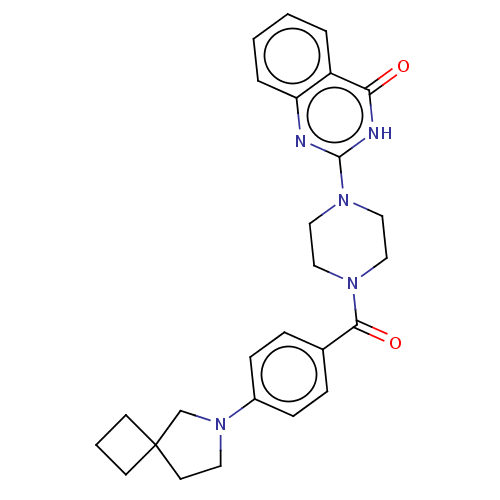
(US11390610, Example 170)Show SMILES O=C(N1CCN(CC1)c1nc2ccccc2c(=O)[nH]1)c1ccc(cc1)N1CCC2(CCC2)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W0994M |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM561367
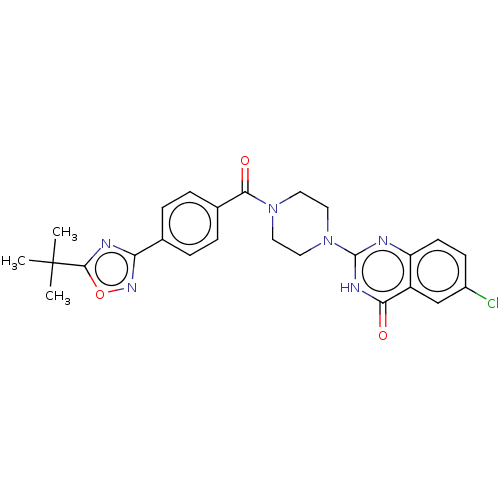
(US11390610, Example 196)Show SMILES CC(C)(C)c1nc(no1)-c1ccc(cc1)C(=O)N1CCN(CC1)c1nc2ccc(Cl)cc2c(=O)[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W0994M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data