

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collagenase 3 (Homo sapiens (Human)) | BDBM50234334 (BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 by steady state kinetic assay | J Biol Chem 282: 27781-91 (2007) Article DOI: 10.1074/jbc.M703286200 BindingDB Entry DOI: 10.7270/Q2Z89C58 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009873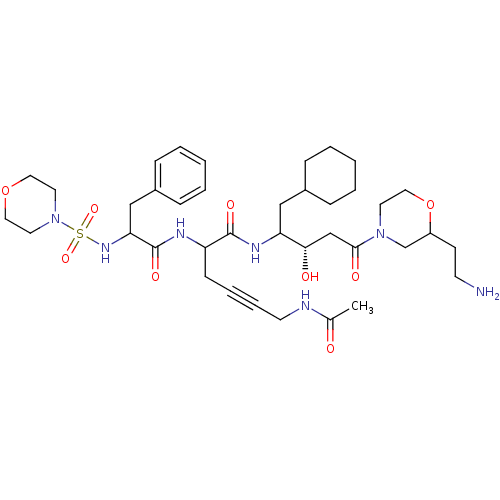 (6-Acetylamino-2-[2-(morpholine-4-sulfonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50265079 ((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP13 catalytic domain | J Biol Chem 282: 27781-91 (2007) Article DOI: 10.1074/jbc.M703286200 BindingDB Entry DOI: 10.7270/Q2Z89C58 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009879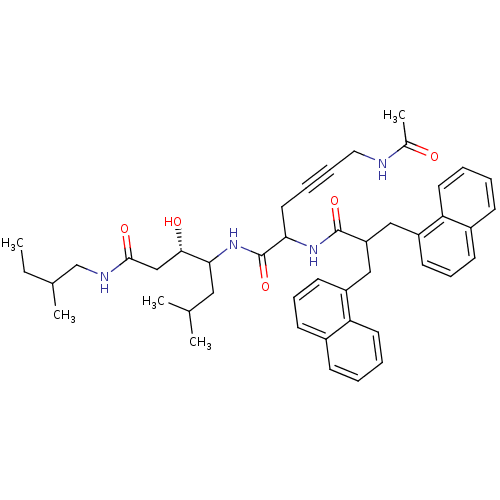 (4-[6-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009911 (6-(3-Methyl-thioureido)-2-[2-(morpholine-4-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009918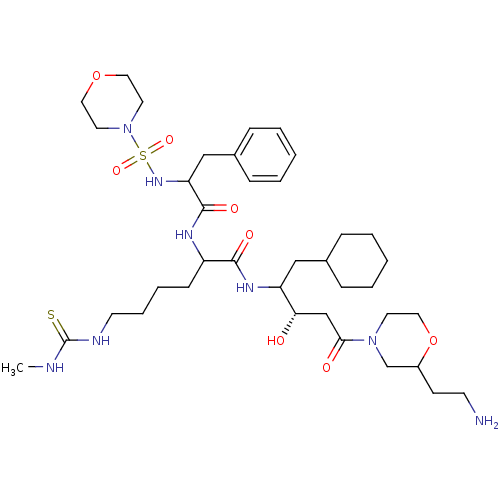 (6-(3-Methyl-thioureido)-2-[2-(morpholine-4-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP1 | J Biol Chem 282: 27781-91 (2007) Article DOI: 10.1074/jbc.M703286200 BindingDB Entry DOI: 10.7270/Q2Z89C58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009885 (6-(3-Methyl-thioureido)-2-[2-(morpholine-4-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009900 (CHEMBL23944 | {5-{4-[2-(2-Amino-ethyl)-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-17 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP17 catalytic domain | J Biol Chem 282: 27781-91 (2007) Article DOI: 10.1074/jbc.M703286200 BindingDB Entry DOI: 10.7270/Q2Z89C58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50289025 (CHEMBL164479 | N-(2,6-Diisopropyl-phenyl)-malonami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in intestinal microsomes isolated from cholesterol fed rabbits (IAI)... | Bioorg Med Chem Lett 6: 713-718 (1996) Article DOI: 10.1016/0960-894X(96)00098-4 BindingDB Entry DOI: 10.7270/Q2DV1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50289051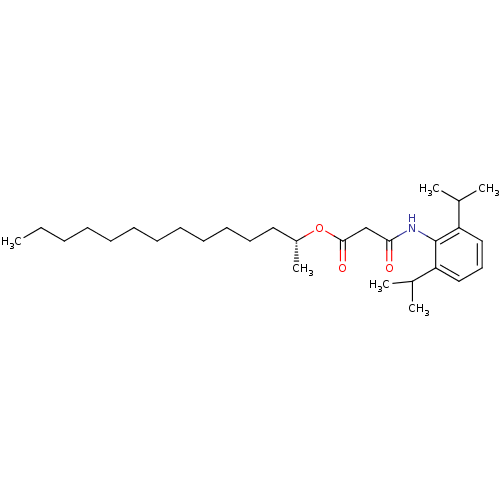 (CHEMBL160103 | N-(2,6-Diisopropyl-phenyl)-malonami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in intestinal microsomes isolated from cholesterol fed rabbits (IAI)... | Bioorg Med Chem Lett 6: 713-718 (1996) Article DOI: 10.1016/0960-894X(96)00098-4 BindingDB Entry DOI: 10.7270/Q2DV1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009879 (4-[6-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009879 (4-[6-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP14 catalytic domain | J Biol Chem 282: 27781-91 (2007) Article DOI: 10.1074/jbc.M703286200 BindingDB Entry DOI: 10.7270/Q2Z89C58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009884 (CHEMBL279921 | N-[1-{4-[2-(2-Amino-ethyl)-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP12 catalytic domain | J Biol Chem 282: 27781-91 (2007) Article DOI: 10.1074/jbc.M703286200 BindingDB Entry DOI: 10.7270/Q2Z89C58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP13 catalytic domain | J Biol Chem 282: 27781-91 (2007) Article DOI: 10.1074/jbc.M703286200 BindingDB Entry DOI: 10.7270/Q2Z89C58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009884 (CHEMBL279921 | N-[1-{4-[2-(2-Amino-ethyl)-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50016033 (CHEMBL3085574 | {1-[1-{1-[2-(1-Benzylcarbamoyl-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against human renin | J Med Chem 33: 838-45 (1990) BindingDB Entry DOI: 10.7270/Q2T152M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50289024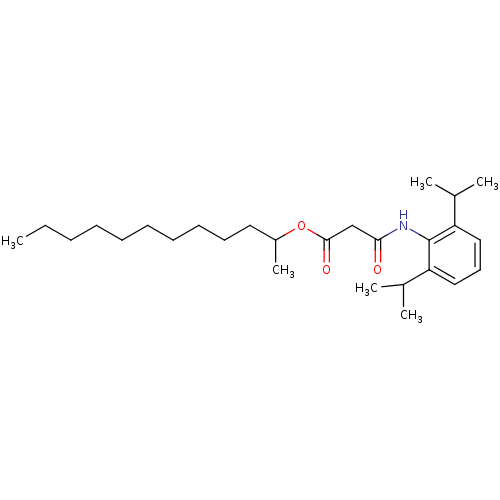 (CHEMBL422709 | N-(2,6-Diisopropyl-phenyl)-malonami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in intestinal microsomes isolated from cholesterol fed rabbits (IAI)... | Bioorg Med Chem Lett 6: 713-718 (1996) Article DOI: 10.1016/0960-894X(96)00098-4 BindingDB Entry DOI: 10.7270/Q2DV1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009872 (4-[6-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP9 | J Biol Chem 282: 27781-91 (2007) Article DOI: 10.1074/jbc.M703286200 BindingDB Entry DOI: 10.7270/Q2Z89C58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50069886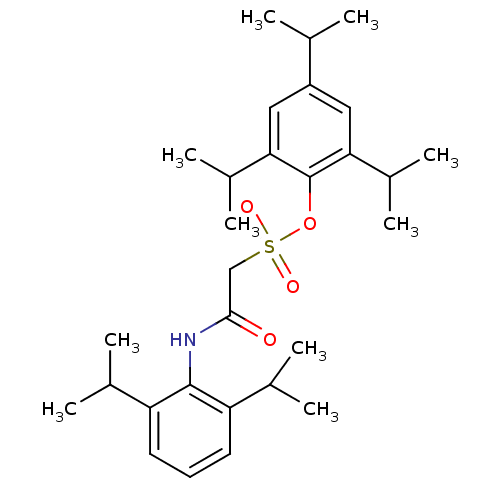 ((2,6-Diisopropyl-phenylcarbamoyl)-methanesulfonic ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of ACAT by incubation with [1-14C]-oleolyl-CoA and intestinal microsomes isolated from cholesterol-fed rabbits. | Bioorg Med Chem Lett 8: 289-94 (1999) BindingDB Entry DOI: 10.7270/Q2DN446R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50069883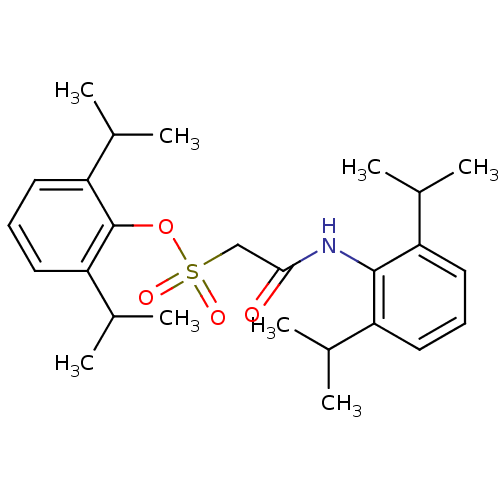 ((2,6-Diisopropyl-phenylcarbamoyl)-methanesulfonic ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of ACAT by incubation with [1-14C]-oleolyl-CoA and intestinal microsomes isolated from cholesterol-fed rabbits. | Bioorg Med Chem Lett 8: 289-94 (1999) BindingDB Entry DOI: 10.7270/Q2DN446R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50006409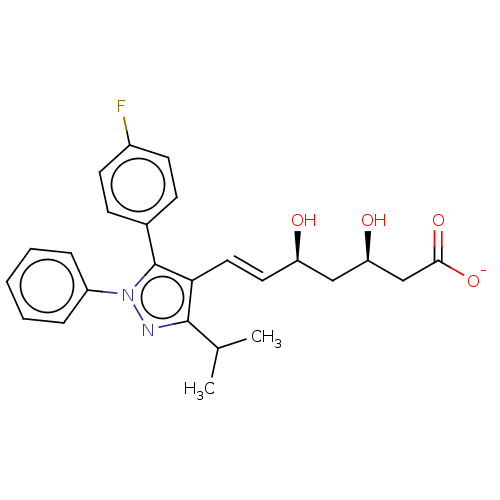 (CHEMBL2367478 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of HMG-CoA reductase from partially purified microsomal preparations. | J Med Chem 35: 2095-103 (1992) BindingDB Entry DOI: 10.7270/Q2HH6KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50006407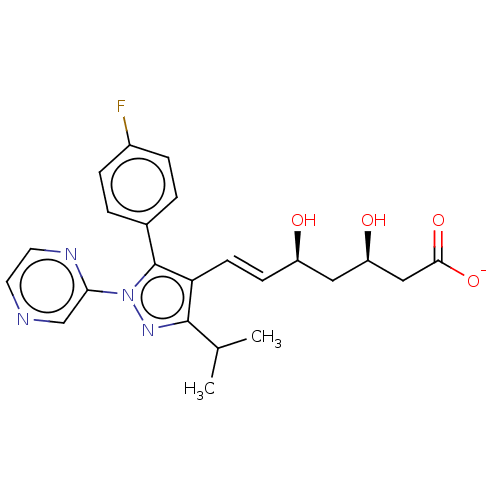 (CHEMBL2367472 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of HMG-CoA reductase from partially purified microsomal preparations. | J Med Chem 35: 2095-103 (1992) BindingDB Entry DOI: 10.7270/Q2HH6KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50005955 (CHEMBL40608 | Octadec-9-enoic acid (2,6-diisopropy...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of acyl coenzyme A:cholesterol acyltransferase, in intestinal microsomes isolated from cholesterol-fed rabbits | J Med Chem 35: 1609-17 (1992) BindingDB Entry DOI: 10.7270/Q2G44QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP3 catalytic domain | J Biol Chem 282: 27781-91 (2007) Article DOI: 10.1074/jbc.M703286200 BindingDB Entry DOI: 10.7270/Q2Z89C58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50006407 (CHEMBL2367472 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of HMG-CoA reductase from partially purified microsomal preparations. | J Med Chem 35: 2095-103 (1992) BindingDB Entry DOI: 10.7270/Q2HH6KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50063243 (CHEMBL161499 | N-(2,6-Diisopropyl-phenyl)-2-pyridi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibition in hepatic microsomes isolated from cholesterol-fed rats | J Med Chem 41: 682-90 (1998) Article DOI: 10.1021/jm970560h BindingDB Entry DOI: 10.7270/Q28051R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50368166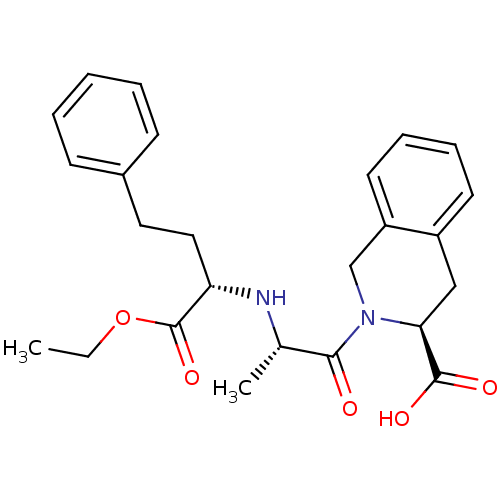 (Accupril | QUINAPRIL) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme from unpurified guinea pig serum | J Med Chem 28: 1291-5 (1985) BindingDB Entry DOI: 10.7270/Q2GF0V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009915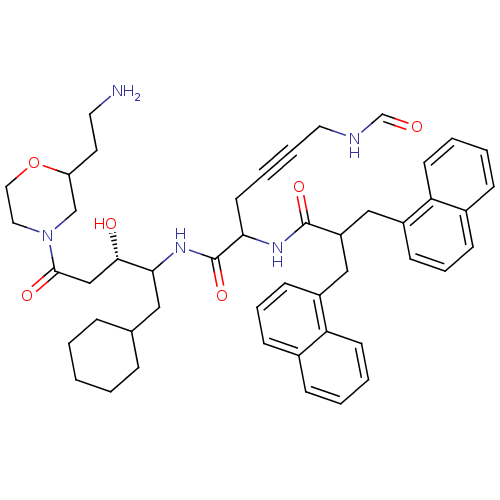 (6-Formylamino-2-(3-naphthalen-1-yl-2-naphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009916 (6-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP8 catalytic domain | J Biol Chem 282: 27781-91 (2007) Article DOI: 10.1074/jbc.M703286200 BindingDB Entry DOI: 10.7270/Q2Z89C58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009892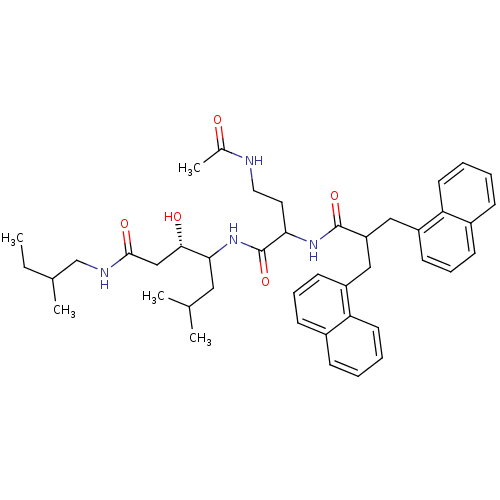 (4-[4-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM50063239 (CHEMBL161638 | N-(2,6-Diisopropyl-phenyl)-2-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Acyl coenzyme A:cholesterol acyltransferase by microsomal LAI assay | J Med Chem 41: 682-90 (1998) Article DOI: 10.1021/jm970560h BindingDB Entry DOI: 10.7270/Q28051R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50063239 (CHEMBL161638 | N-(2,6-Diisopropyl-phenyl)-2-phenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibition in hepatic microsomes isolated from cholesterol-fed rats | J Med Chem 41: 682-90 (1998) Article DOI: 10.1021/jm970560h BindingDB Entry DOI: 10.7270/Q28051R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP2 | J Biol Chem 282: 27781-91 (2007) Article DOI: 10.1074/jbc.M703286200 BindingDB Entry DOI: 10.7270/Q2Z89C58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50289048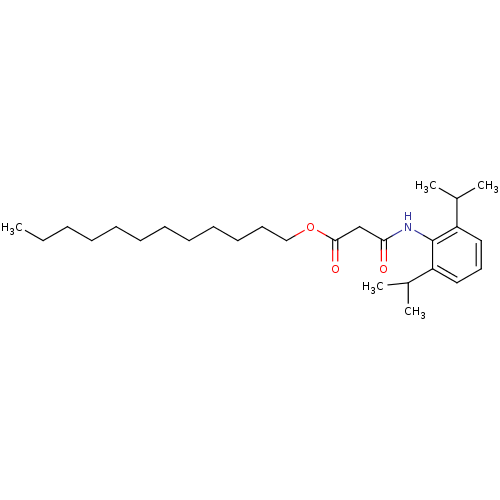 (CHEMBL164205 | N-(2,6-Diisopropyl-phenyl)-malonami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in intestinal microsomes isolated from cholesterol fed rabbits (IAI)... | Bioorg Med Chem Lett 6: 713-718 (1996) Article DOI: 10.1016/0960-894X(96)00098-4 BindingDB Entry DOI: 10.7270/Q2DV1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50063226 (CHEMBL160149 | N-(2,6-Diisopropyl-phenyl)-2-phenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibition in hepatic microsomes isolated from cholesterol-fed rats | J Med Chem 41: 682-90 (1998) Article DOI: 10.1021/jm970560h BindingDB Entry DOI: 10.7270/Q28051R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50289042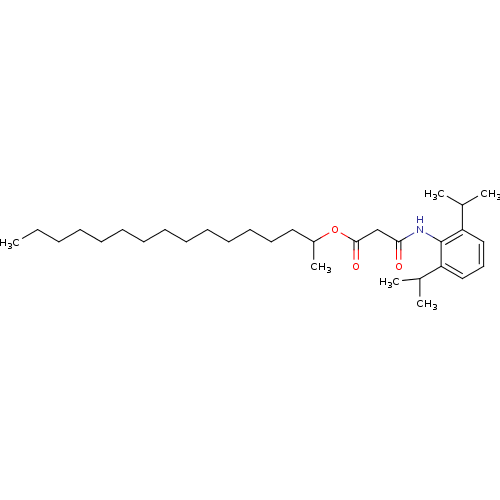 (CHEMBL162996 | N-(2,6-Diisopropyl-phenyl)-malonami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in intestinal microsomes isolated from cholesterol fed rabbits (IAI)... | Bioorg Med Chem Lett 6: 713-718 (1996) Article DOI: 10.1016/0960-894X(96)00098-4 BindingDB Entry DOI: 10.7270/Q2DV1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009894 (6-(N'-nitro-guanidino)-2-(3-naphthalen-1-yl-2-naph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey plasma renin. | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50289039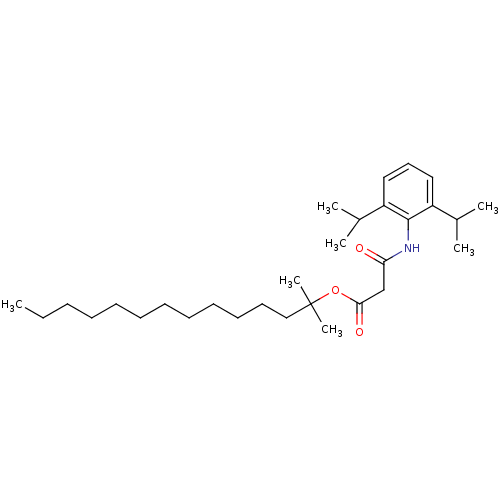 (CHEMBL163293 | N-(2,6-Diisopropyl-phenyl)-malonami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in intestinal microsomes isolated from cholesterol fed rabbits (IAI)... | Bioorg Med Chem Lett 6: 713-718 (1996) Article DOI: 10.1016/0960-894X(96)00098-4 BindingDB Entry DOI: 10.7270/Q2DV1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50042104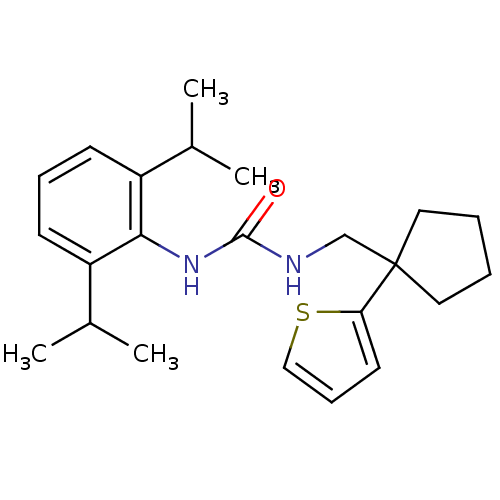 (1-(2,6-Diisopropyl-phenyl)-3-(1-thiophen-2-yl-cycl...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase using intestinal microsomes isolated from cholesterol fed rabbits | J Med Chem 36: 3300-7 (1993) BindingDB Entry DOI: 10.7270/Q2M044GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50063231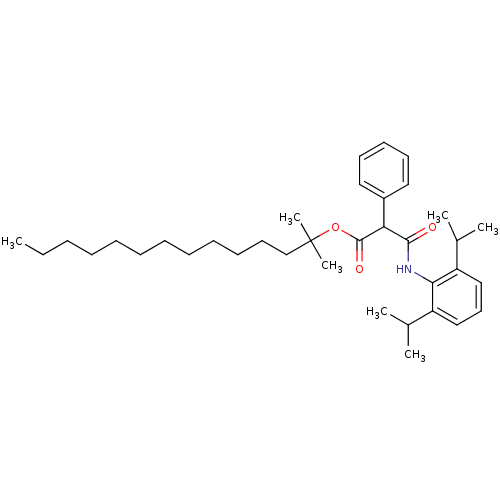 (CHEMBL159621 | N-(2,6-Diisopropyl-phenyl)-2-phenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibition in hepatic microsomes isolated from cholesterol-fed rats | J Med Chem 41: 682-90 (1998) Article DOI: 10.1021/jm970560h BindingDB Entry DOI: 10.7270/Q28051R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50063236 (CHEMBL159871 | N-(2,6-Diisopropyl-phenyl)-2-phenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibition in hepatic microsomes isolated from cholesterol-fed rats | J Med Chem 41: 682-90 (1998) Article DOI: 10.1021/jm970560h BindingDB Entry DOI: 10.7270/Q28051R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50289050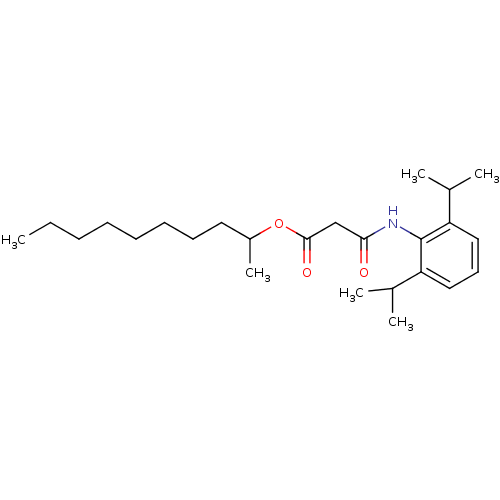 (CHEMBL159666 | N-(2,6-Diisopropyl-phenyl)-malonami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in intestinal microsomes isolated from cholesterol fed rabbits (IAI)... | Bioorg Med Chem Lett 6: 713-718 (1996) Article DOI: 10.1016/0960-894X(96)00098-4 BindingDB Entry DOI: 10.7270/Q2DV1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009905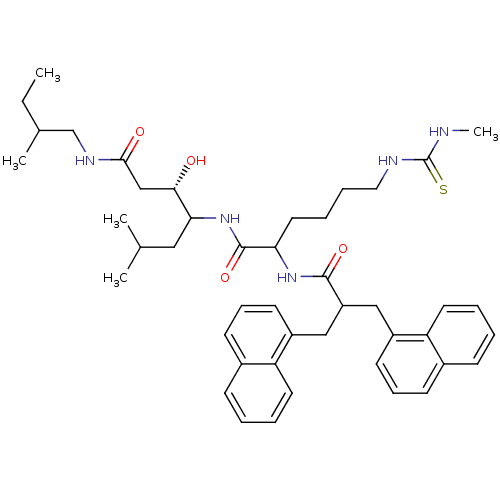 (3-Hydroxy-6-methyl-4-[6-(3-methyl-thioureido)-2-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | J Med Chem 34: 1258-71 (1991) BindingDB Entry DOI: 10.7270/Q21C1VT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50063220 (CHEMBL161270 | N-(2,6-Diisopropyl-phenyl)-2-phenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibition in hepatic microsomes isolated from cholesterol-fed rats | J Med Chem 41: 682-90 (1998) Article DOI: 10.1021/jm970560h BindingDB Entry DOI: 10.7270/Q28051R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 555 total ) | Next | Last >> |