 Found 90 hits with Last Name = 'rotondo' and Initial = 'a'
Found 90 hits with Last Name = 'rotondo' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069985
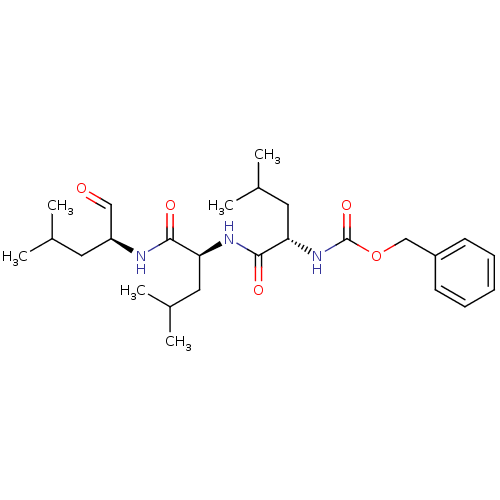
((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate measured for 10 mins by fluor... |
Eur J Med Chem 121: 578-591 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.049
BindingDB Entry DOI: 10.7270/Q28C9Z6K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50195937
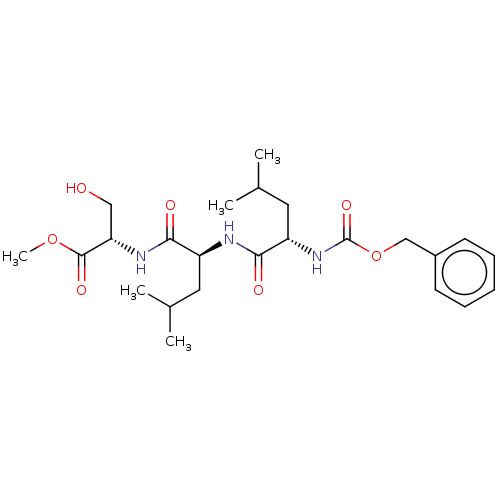
(CHEMBL3919583)Show SMILES COC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C24H37N3O7/c1-15(2)11-18(21(29)26-20(13-28)23(31)33-5)25-22(30)19(12-16(3)4)27-24(32)34-14-17-9-7-6-8-10-17/h6-10,15-16,18-20,28H,11-14H2,1-5H3,(H,25,30)(H,26,29)(H,27,32)/t18-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate measured for 10 mins by fluor... |
Eur J Med Chem 121: 578-591 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.049
BindingDB Entry DOI: 10.7270/Q28C9Z6K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444695
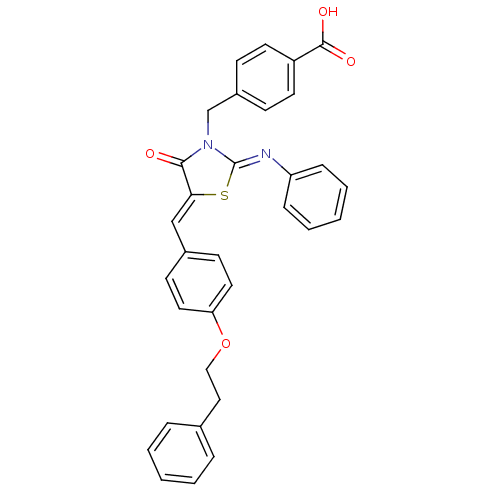
(CHEMBL3098852)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-24-13-17-28(18-14-24)38-20-19-23-7-3-1-4-8-23)39-32(33-27-9-5-2-6-10-27)34(30)22-25-11-15-26(16-12-25)31(36)37/h1-18,21H,19-20,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232100
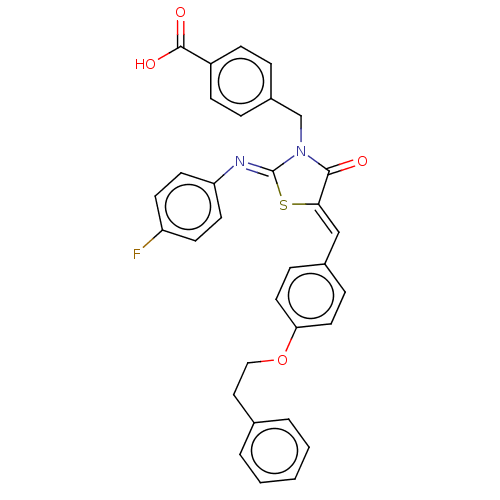
(CHEMBL4061225)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(OCCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C32H25FN2O4S/c33-26-12-14-27(15-13-26)34-32-35(21-24-6-10-25(11-7-24)31(37)38)30(36)29(40-32)20-23-8-16-28(17-9-23)39-19-18-22-4-2-1-3-5-22/h1-17,20H,18-19,21H2,(H,37,38)/b29-20-,34-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed non-competitive inhibition of full length recombinant human PTP1B assessed as enzyme-substrate-inhibitor complex using pNPP as substrate by dou... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444693
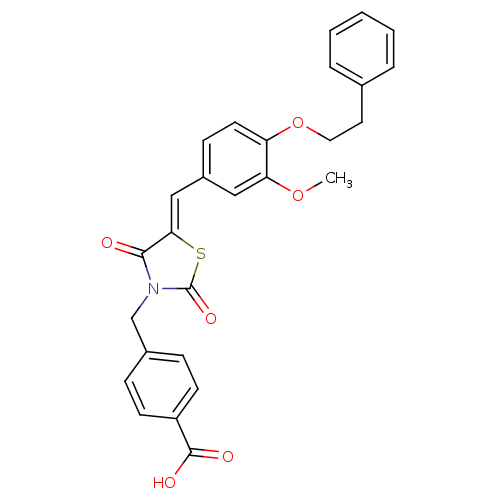
(CHEMBL3098944)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C27H23NO6S/c1-33-23-15-20(9-12-22(23)34-14-13-18-5-3-2-4-6-18)16-24-25(29)28(27(32)35-24)17-19-7-10-21(11-8-19)26(30)31/h2-12,15-16H,13-14,17H2,1H3,(H,30,31)/b24-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232102
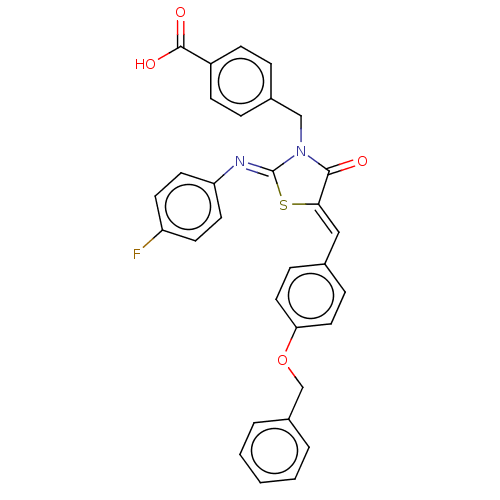
(CHEMBL4080177)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(OCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-25-12-14-26(15-13-25)33-31-34(19-22-6-10-24(11-7-22)30(36)37)29(35)28(39-31)18-21-8-16-27(17-9-21)38-20-23-4-2-1-3-5-23/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length recombinant human PTP1B assessed as enzyme-inhibitor complex using pNPP as substrate by double reciprocal plot ... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444692

(CHEMBL3098853)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-28-17-14-25(20-29(28)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal pl... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232101
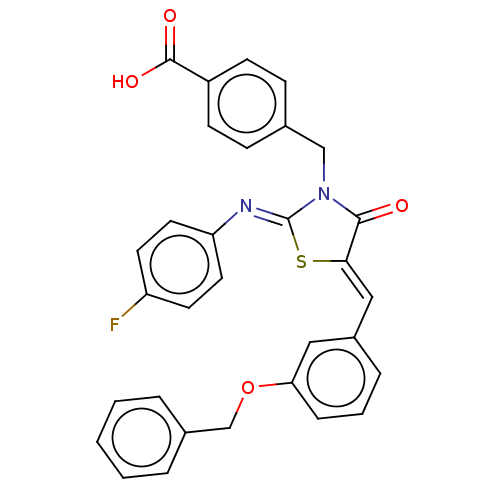
(CHEMBL4098207)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2cccc(OCc3ccccc3)c2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-25-13-15-26(16-14-25)33-31-34(19-21-9-11-24(12-10-21)30(36)37)29(35)28(39-31)18-23-7-4-8-27(17-23)38-20-22-5-2-1-3-6-22/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed non-competitive inhibition of full length recombinant human PTP1B assessed as enzyme-substrate-inhibitor complex using pNPP as substrate by dou... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444694

(CHEMBL3098854)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-29-20-25(14-17-28(29)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50195935

(CHEMBL3929092)Show SMILES CN\C(NS(=O)(=O)c1ccccc1)=N/CCSCc1ccc2ccccc2n1 Show InChI InChI=1S/C20H22N4O2S2/c1-21-20(24-28(25,26)18-8-3-2-4-9-18)22-13-14-27-15-17-12-11-16-7-5-6-10-19(16)23-17/h2-12H,13-15H2,1H3,(H2,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate measured for 10 mins by fluor... |
Eur J Med Chem 121: 578-591 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.049
BindingDB Entry DOI: 10.7270/Q28C9Z6K |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50195934

(CHEMBL3977256)Show SMILES CC(C)CC(NC(=O)C(CO)NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C26H34N4O7/c1-16(2)12-21(24(34)28-20(23(27)33)13-17-8-10-19(32)11-9-17)29-25(35)22(14-31)30-26(36)37-15-18-6-4-3-5-7-18/h3-11,16,20-22,31-32H,12-15H2,1-2H3,(H2,27,33)(H,28,34)(H,29,35)(H,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate measured for 10 mins by fluor... |
Eur J Med Chem 121: 578-591 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.049
BindingDB Entry DOI: 10.7270/Q28C9Z6K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444696
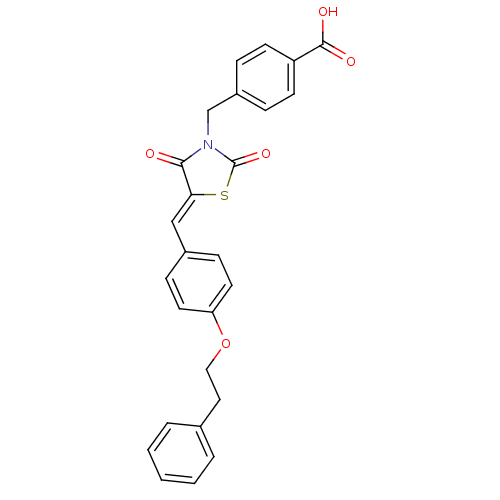
(CHEMBL3098942)Show SMILES OC(=O)c1ccc(CN2C(=O)S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C26H21NO5S/c28-24-23(33-26(31)27(24)17-20-6-10-21(11-7-20)25(29)30)16-19-8-12-22(13-9-19)32-15-14-18-4-2-1-3-5-18/h1-13,16H,14-15,17H2,(H,29,30)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232103
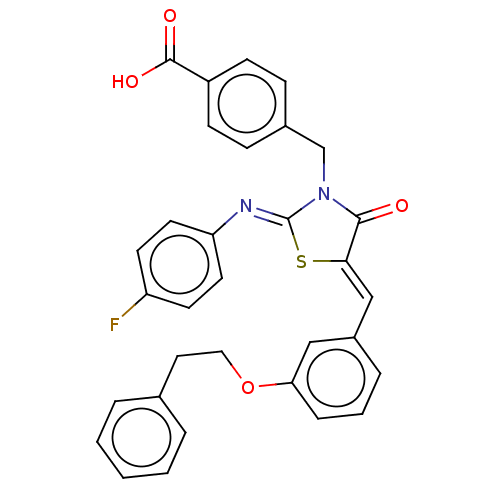
(CHEMBL4089378)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2cccc(OCCc3ccccc3)c2)cc1 Show InChI InChI=1S/C32H25FN2O4S/c33-26-13-15-27(16-14-26)34-32-35(21-23-9-11-25(12-10-23)31(37)38)30(36)29(40-32)20-24-7-4-8-28(19-24)39-18-17-22-5-2-1-3-6-22/h1-16,19-20H,17-18,21H2,(H,37,38)/b29-20-,34-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed non-competitive inhibition of full length recombinant human PTP1B assessed as enzyme-substrate-inhibitor complex using pNPP as substrate by dou... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232093
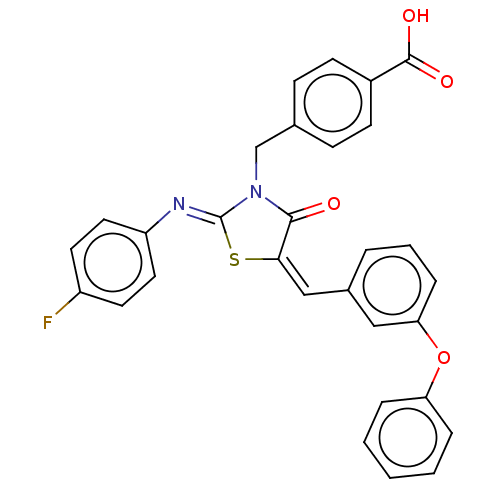
(CHEMBL4072175)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2cccc(Oc3ccccc3)c2)cc1 Show InChI InChI=1S/C30H21FN2O4S/c31-23-13-15-24(16-14-23)32-30-33(19-20-9-11-22(12-10-20)29(35)36)28(34)27(38-30)18-21-5-4-8-26(17-21)37-25-6-2-1-3-7-25/h1-18H,19H2,(H,35,36)/b27-18-,32-30- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length recombinant human PTP1B assessed as enzyme-inhibitor complex using pNPP as substrate by double reciprocal plot ... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50195936
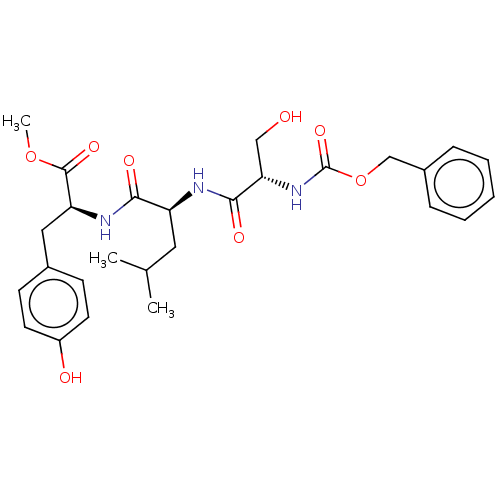
(CHEMBL3612420)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C27H35N3O8/c1-17(2)13-21(24(33)29-22(26(35)37-3)14-18-9-11-20(32)12-10-18)28-25(34)23(15-31)30-27(36)38-16-19-7-5-4-6-8-19/h4-12,17,21-23,31-32H,13-16H2,1-3H3,(H,28,34)(H,29,33)(H,30,36)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate measured for 10 mins by fluor... |
Eur J Med Chem 121: 578-591 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.049
BindingDB Entry DOI: 10.7270/Q28C9Z6K |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50069985

((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Competitive inhibition of post-glutamyl peptide hydrolyzing activity of human 20S proteasome using Cbz-Leu-Leu-Glu-AMC as substrate measured for 10 m... |
Eur J Med Chem 121: 578-591 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.049
BindingDB Entry DOI: 10.7270/Q28C9Z6K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50195934

(CHEMBL3977256)Show SMILES CC(C)CC(NC(=O)C(CO)NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C26H34N4O7/c1-16(2)12-21(24(34)28-20(23(27)33)13-17-8-10-19(32)11-9-17)29-25(35)22(14-31)30-26(36)37-15-18-6-4-3-5-7-18/h3-11,16,20-22,31-32H,12-15H2,1-2H3,(H2,27,33)(H,28,34)(H,29,35)(H,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Competitive inhibition of post-glutamyl peptide hydrolyzing activity of human 20S proteasome using Cbz-Leu-Leu-Glu-AMC as substrate measured for 10 m... |
Eur J Med Chem 121: 578-591 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.049
BindingDB Entry DOI: 10.7270/Q28C9Z6K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232094
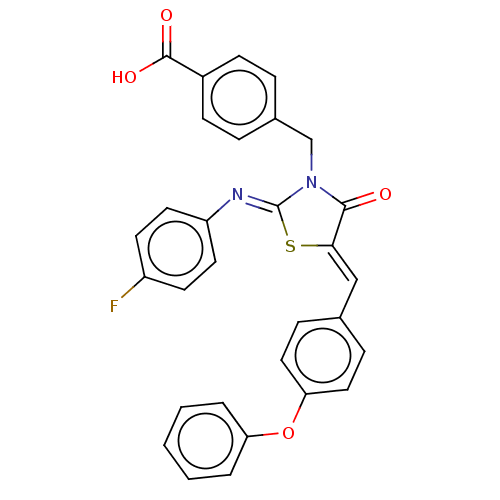
(CHEMBL4062661)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C30H21FN2O4S/c31-23-12-14-24(15-13-23)32-30-33(19-21-6-10-22(11-7-21)29(35)36)28(34)27(38-30)18-20-8-16-26(17-9-20)37-25-4-2-1-3-5-25/h1-18H,19H2,(H,35,36)/b27-18-,32-30- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length recombinant human PTP1B assessed as enzyme-inhibitor complex using pNPP as substrate by double reciprocal plot ... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50195933
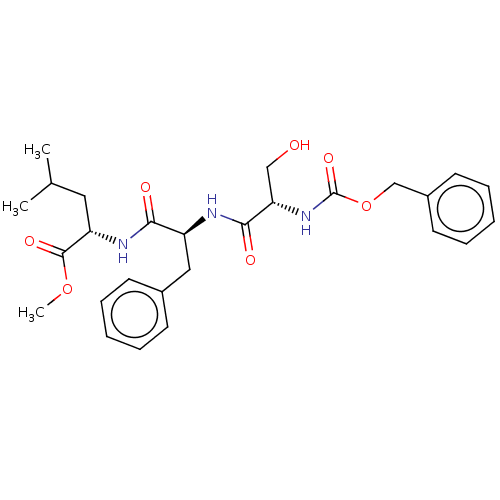
(CHEMBL3612421)Show SMILES COC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C27H35N3O7/c1-18(2)14-22(26(34)36-3)29-24(32)21(15-19-10-6-4-7-11-19)28-25(33)23(16-31)30-27(35)37-17-20-12-8-5-9-13-20/h4-13,18,21-23,31H,14-17H2,1-3H3,(H,28,33)(H,29,32)(H,30,35)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate measured for 10 mins by fluor... |
Eur J Med Chem 121: 578-591 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.049
BindingDB Entry DOI: 10.7270/Q28C9Z6K |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50195938
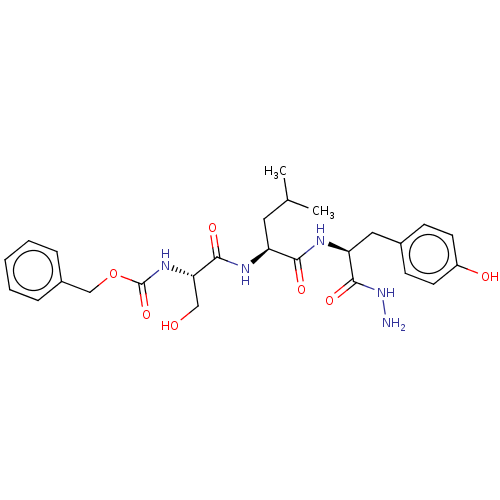
(CHEMBL3961750)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NN |r| Show InChI InChI=1S/C26H35N5O7/c1-16(2)12-20(23(34)29-21(25(36)31-27)13-17-8-10-19(33)11-9-17)28-24(35)22(14-32)30-26(37)38-15-18-6-4-3-5-7-18/h3-11,16,20-22,32-33H,12-15,27H2,1-2H3,(H,28,35)(H,29,34)(H,30,37)(H,31,36)/t20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate measured for 10 mins by fluor... |
Eur J Med Chem 121: 578-591 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.049
BindingDB Entry DOI: 10.7270/Q28C9Z6K |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50069985

((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Competitive inhibition of trypsin-like activity of human 20S proteasome using Boc-Leu-Arg-Arg-AMC as substrate measured for 10 mins by fluorescence a... |
Eur J Med Chem 121: 578-591 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.049
BindingDB Entry DOI: 10.7270/Q28C9Z6K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50195936

(CHEMBL3612420)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C27H35N3O8/c1-17(2)13-21(24(33)29-22(26(35)37-3)14-18-9-11-20(32)12-10-18)28-25(34)23(15-31)30-27(36)38-16-19-7-5-4-6-8-19/h4-12,17,21-23,31-32H,13-16H2,1-3H3,(H,28,34)(H,29,33)(H,30,36)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Competitive inhibition of post-glutamyl peptide hydrolyzing activity of human 20S proteasome using Cbz-Leu-Leu-Glu-AMC as substrate measured for 10 m... |
Eur J Med Chem 121: 578-591 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.049
BindingDB Entry DOI: 10.7270/Q28C9Z6K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444695

(CHEMBL3098852)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-24-13-17-28(18-14-24)38-20-19-23-7-3-1-4-8-23)39-32(33-27-9-5-2-6-10-27)34(30)22-25-11-15-26(16-12-25)31(36)37/h1-18,21H,19-20,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444697
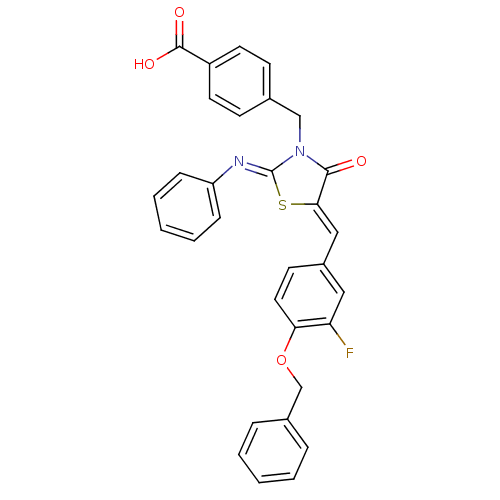
(CHEMBL3098857)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccccc2)=C\c2ccc(OCc3ccccc3)c(F)c2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-26-17-23(13-16-27(26)38-20-22-7-3-1-4-8-22)18-28-29(35)34(19-21-11-14-24(15-12-21)30(36)37)31(39-28)33-25-9-5-2-6-10-25/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444693

(CHEMBL3098944)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C27H23NO6S/c1-33-23-15-20(9-12-22(23)34-14-13-18-5-3-2-4-6-18)16-24-25(29)28(27(32)35-24)17-19-7-10-21(11-8-19)26(30)31/h2-12,15-16H,13-14,17H2,1H3,(H,30,31)/b24-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444695

(CHEMBL3098852)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-24-13-17-28(18-14-24)38-20-19-23-7-3-1-4-8-23)39-32(33-27-9-5-2-6-10-27)34(30)22-25-11-15-26(16-12-25)31(36)37/h1-18,21H,19-20,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232100

(CHEMBL4061225)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(OCCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C32H25FN2O4S/c33-26-12-14-27(15-13-26)34-32-35(21-24-6-10-25(11-7-24)31(37)38)30(36)29(40-32)20-23-8-16-28(17-9-23)39-19-18-22-4-2-1-3-5-22/h1-17,20H,18-19,21H2,(H,37,38)/b29-20-,34-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444698
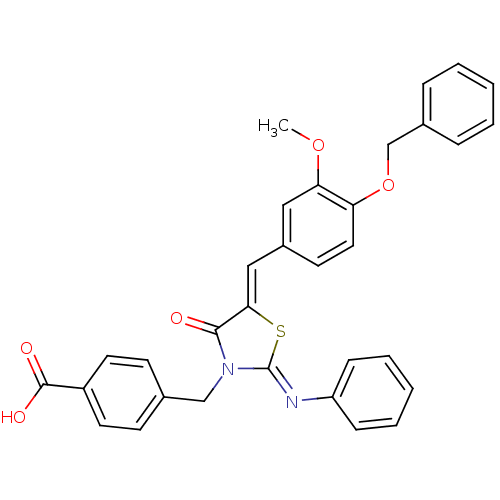
(CHEMBL3098856)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C32H26N2O5S/c1-38-28-18-24(14-17-27(28)39-21-23-8-4-2-5-9-23)19-29-30(35)34(20-22-12-15-25(16-13-22)31(36)37)32(40-29)33-26-10-6-3-7-11-26/h2-19H,20-21H2,1H3,(H,36,37)/b29-19-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444692

(CHEMBL3098853)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-28-17-14-25(20-29(28)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232102

(CHEMBL4080177)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(OCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-25-12-14-26(15-13-25)33-31-34(19-22-6-10-24(11-7-22)30(36)37)29(35)28(39-31)18-21-8-16-27(17-9-21)38-20-23-4-2-1-3-5-23/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444700
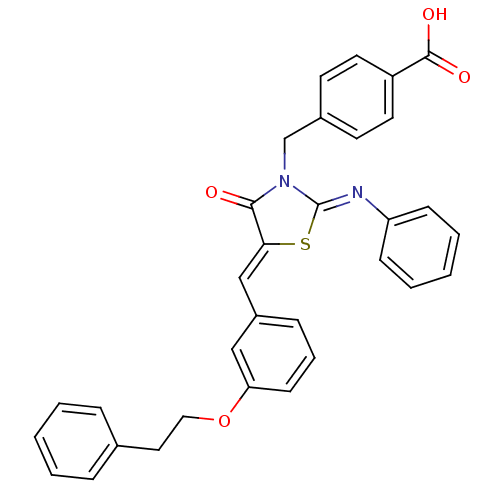
(CHEMBL3098851)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3cccc(OCCc4ccccc4)c3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-25-10-7-13-28(20-25)38-19-18-23-8-3-1-4-9-23)39-32(33-27-11-5-2-6-12-27)34(30)22-24-14-16-26(17-15-24)31(36)37/h1-17,20-21H,18-19,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232095
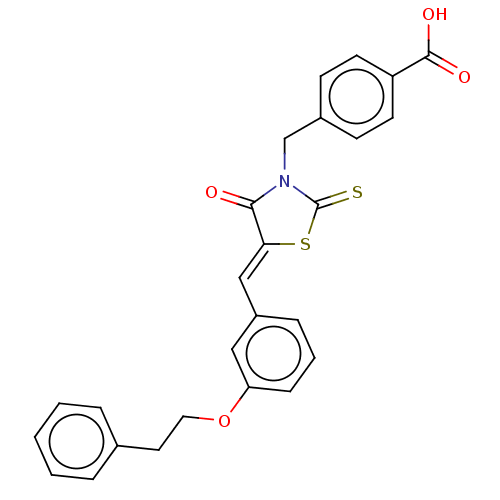
(CHEMBL4081501)Show SMILES OC(=O)c1ccc(CN2C(=S)S\C(=C/c3cccc(OCCc4ccccc4)c3)C2=O)cc1 Show InChI InChI=1S/C26H21NO4S2/c28-24-23(33-26(32)27(24)17-19-9-11-21(12-10-19)25(29)30)16-20-7-4-8-22(15-20)31-14-13-18-5-2-1-3-6-18/h1-12,15-16H,13-14,17H2,(H,29,30)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444697

(CHEMBL3098857)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccccc2)=C\c2ccc(OCc3ccccc3)c(F)c2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-26-17-23(13-16-27(26)38-20-22-7-3-1-4-8-22)18-28-29(35)34(19-21-11-14-24(15-12-21)30(36)37)31(39-28)33-25-9-5-2-6-10-25/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444696

(CHEMBL3098942)Show SMILES OC(=O)c1ccc(CN2C(=O)S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C26H21NO5S/c28-24-23(33-26(31)27(24)17-20-6-10-21(11-7-20)25(29)30)16-19-8-12-22(13-9-19)32-15-14-18-4-2-1-3-5-18/h1-13,16H,14-15,17H2,(H,29,30)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444694

(CHEMBL3098854)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-29-20-25(14-17-28(29)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50444697

(CHEMBL3098857)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccccc2)=C\c2ccc(OCc3ccccc3)c(F)c2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-26-17-23(13-16-27(26)38-20-22-7-3-1-4-8-22)18-28-29(35)34(19-21-11-14-24(15-12-21)30(36)37)31(39-28)33-25-9-5-2-6-10-25/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LMW-PTP IF1 expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444694

(CHEMBL3098854)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-29-20-25(14-17-28(29)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444693

(CHEMBL3098944)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C27H23NO6S/c1-33-23-15-20(9-12-22(23)34-14-13-18-5-3-2-4-6-18)16-24-25(29)28(27(32)35-24)17-19-7-10-21(11-8-19)26(30)31/h2-12,15-16H,13-14,17H2,1H3,(H,30,31)/b24-16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444702
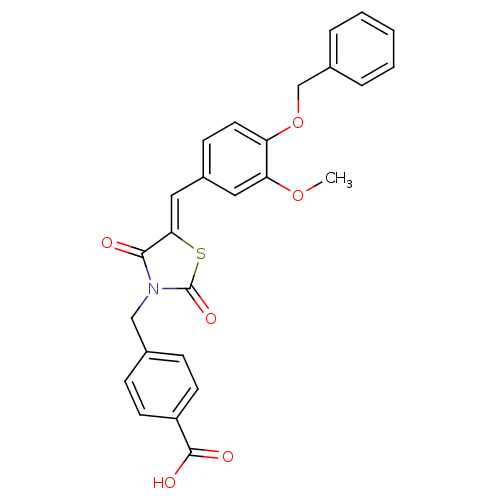
(CHEMBL3098946)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H21NO6S/c1-32-22-13-19(9-12-21(22)33-16-18-5-3-2-4-6-18)14-23-24(28)27(26(31)34-23)15-17-7-10-20(11-8-17)25(29)30/h2-14H,15-16H2,1H3,(H,29,30)/b23-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232096
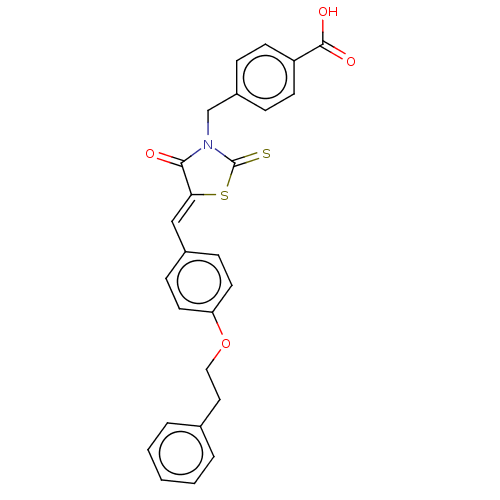
(CHEMBL4099520)Show SMILES OC(=O)c1ccc(CN2C(=S)S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C26H21NO4S2/c28-24-23(33-26(32)27(24)17-20-6-10-21(11-7-20)25(29)30)16-19-8-12-22(13-9-19)31-15-14-18-4-2-1-3-5-18/h1-13,16H,14-15,17H2,(H,29,30)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444699
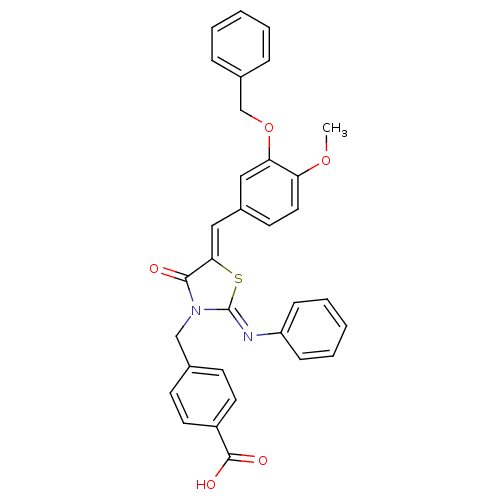
(CHEMBL3098855)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCc1ccccc1 Show InChI InChI=1S/C32H26N2O5S/c1-38-27-17-14-24(18-28(27)39-21-23-8-4-2-5-9-23)19-29-30(35)34(20-22-12-15-25(16-13-22)31(36)37)32(40-29)33-26-10-6-3-7-11-26/h2-19H,20-21H2,1H3,(H,36,37)/b29-19-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444703
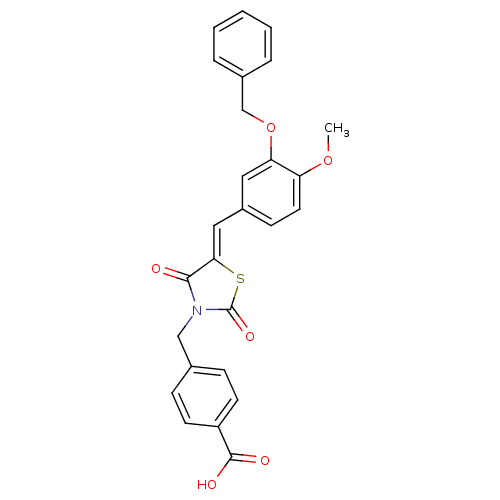
(CHEMBL3098945)Show SMILES COc1ccc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCc1ccccc1 Show InChI InChI=1S/C26H21NO6S/c1-32-21-12-9-19(13-22(21)33-16-18-5-3-2-4-6-18)14-23-24(28)27(26(31)34-23)15-17-7-10-20(11-8-17)25(29)30/h2-14H,15-16H2,1H3,(H,29,30)/b23-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50444695

(CHEMBL3098852)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-24-13-17-28(18-14-24)38-20-19-23-7-3-1-4-8-23)39-32(33-27-9-5-2-6-10-27)34(30)22-25-11-15-26(16-12-25)31(36)37/h1-18,21H,19-20,22H2,(H,36,37)/b29-21-,33-32- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LMW-PTP IF1 expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444699

(CHEMBL3098855)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCc1ccccc1 Show InChI InChI=1S/C32H26N2O5S/c1-38-27-17-14-24(18-28(27)39-21-23-8-4-2-5-9-23)19-29-30(35)34(20-22-12-15-25(16-13-22)31(36)37)32(40-29)33-26-10-6-3-7-11-26/h2-19H,20-21H2,1H3,(H,36,37)/b29-19-,33-32- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232101

(CHEMBL4098207)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2cccc(OCc3ccccc3)c2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-25-13-15-26(16-14-25)33-31-34(19-21-9-11-24(12-10-21)30(36)37)29(35)28(39-31)18-23-7-4-8-27(17-23)38-20-22-5-2-1-3-6-22/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444692

(CHEMBL3098853)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-28-17-14-25(20-29(28)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50444698

(CHEMBL3098856)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C32H26N2O5S/c1-38-28-18-24(14-17-27(28)39-21-23-8-4-2-5-9-23)19-29-30(35)34(20-22-12-15-25(16-13-22)31(36)37)32(40-29)33-26-10-6-3-7-11-26/h2-19H,20-21H2,1H3,(H,36,37)/b29-19-,33-32- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LMW-PTP IF1 expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444702

(CHEMBL3098946)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H21NO6S/c1-32-22-13-19(9-12-21(22)33-16-18-5-3-2-4-6-18)14-23-24(28)27(26(31)34-23)15-17-7-10-20(11-8-17)25(29)30/h2-14H,15-16H2,1H3,(H,29,30)/b23-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444700

(CHEMBL3098851)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3cccc(OCCc4ccccc4)c3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-25-10-7-13-28(20-25)38-19-18-23-8-3-1-4-9-23)39-32(33-27-11-5-2-6-12-27)34(30)22-24-14-16-26(17-15-24)31(36)37/h1-17,20-21H,18-19,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232097
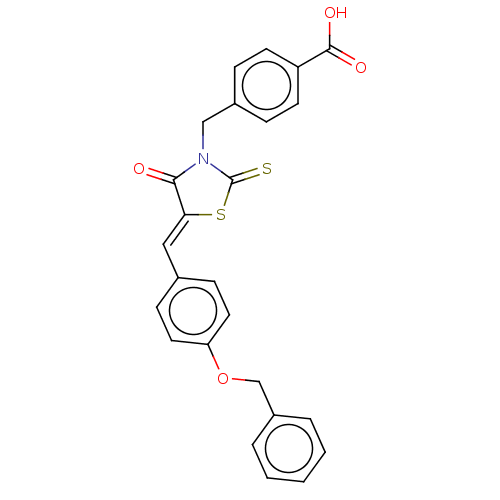
(CHEMBL4091784)Show SMILES OC(=O)c1ccc(CN2C(=S)S\C(=C/c3ccc(OCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C25H19NO4S2/c27-23-22(32-25(31)26(23)15-18-6-10-20(11-7-18)24(28)29)14-17-8-12-21(13-9-17)30-16-19-4-2-1-3-5-19/h1-14H,15-16H2,(H,28,29)/b22-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data