

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260415 (4-(2-Chloro-benzenesulfonyl)-2-(1-methyl-1,2,5,6-t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260414 (4-(4-tert-Butyl-benzenesulfonyl)-2-(1-methyl-1,2,5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260413 (4-(4-Methyl-benzenesulfonyl)-2-(1-methyl-1,2,5,6-t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300564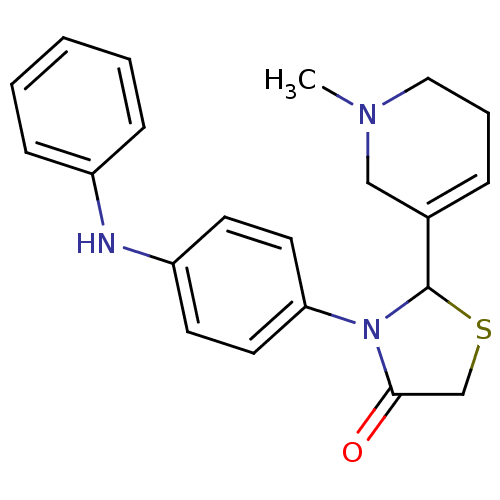 (2-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl)-3-(4-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260418 (4-Benzenesulfonyl-2-(1-methyl-1,2,5,6-tetrahydropy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300560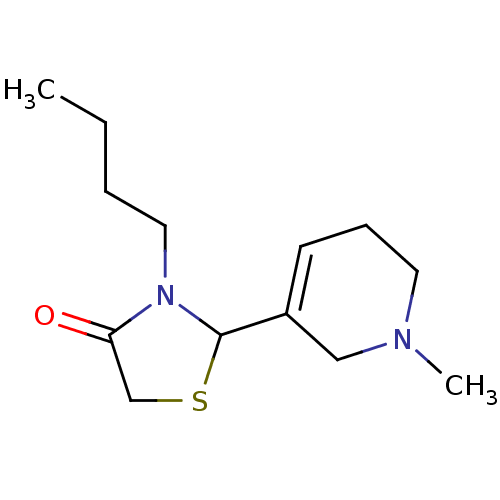 (3-butyl-2-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260417 (4-(2,5-Dichloro-benzenesulfonyl)-2-(1-methyl-1,2,5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300563 (4-[2-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl)-4-o...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300565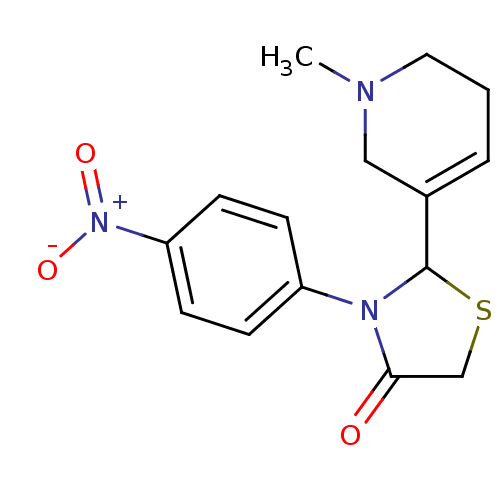 (2-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl)-3-(4-n...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260420 (4-(3-Nitrobenzenesulfonyl)-2-(1-methyl-1,2,5,6-tet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260421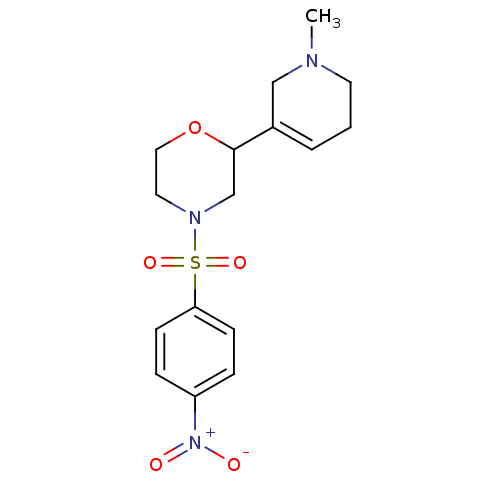 (4-(4-Nitro-benzenesulfonyl)-2-(1-methyl-1,2,5,6-te...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM46858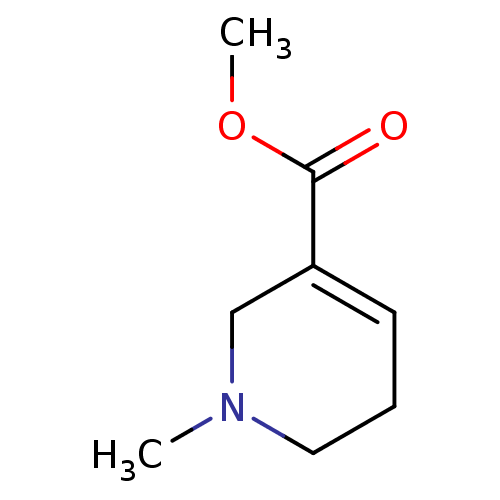 (1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM46858 (1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50260419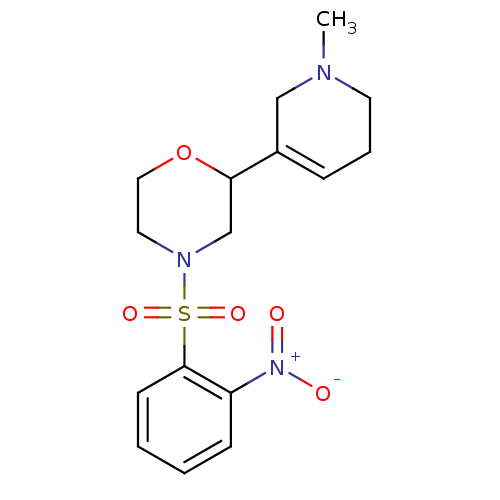 (4-(2-Nitrobenzenesulfonyl)-2-(1-methyl-1,2,5,6-tet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]Quinuclidinyl benzillate from muscarinic M1 receptor in Wistar rat cortex synaptosomal membrane | Bioorg Med Chem 16: 5157-63 (2008) Article DOI: 10.1016/j.bmc.2008.03.019 BindingDB Entry DOI: 10.7270/Q26973CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300561 (3-isopropyl-2-(1-methyl-1,2,5,6-tetrahydropyridin-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300567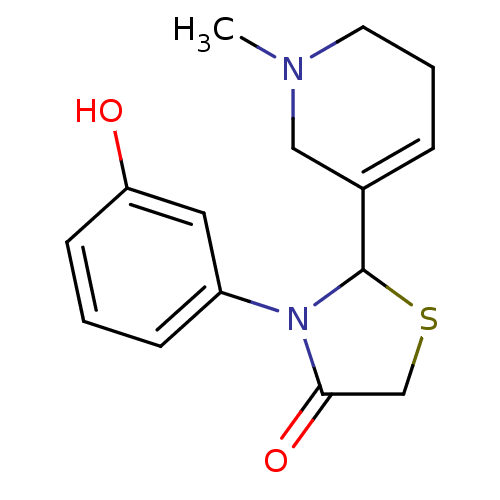 (3-(3-hydroxy-phenyl)-2-(1-methyl-1,2,5,6-tetrahydr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300558 (2-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl)-3-phen...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300559 (3-hexyl-2-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300562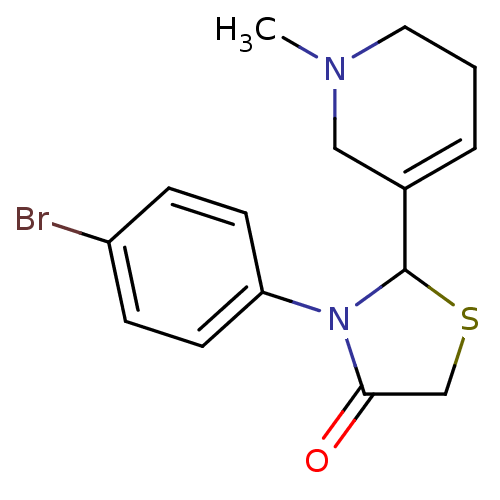 (3-(4-bromophenyl)-2-(1-methyl-1,2,5,6-tetrahydropy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300566 (3-(4-methyl-2-nitro-phenyl)-2-(1-methyl-1,2,5,6-te...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300556 (3-benzyl-2-(1-methyl-1,2,5,6-tetrahydropyridin-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300555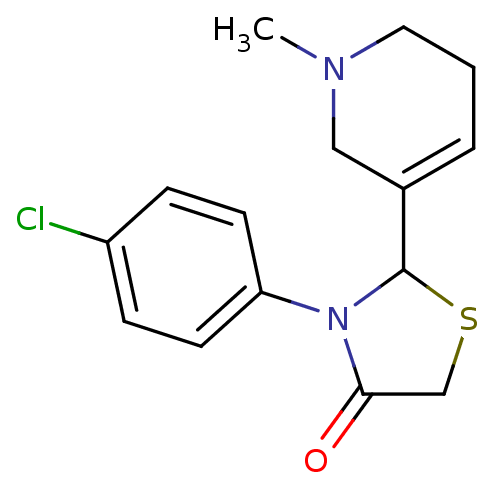 (3-(4-chlorophenyl)-2-(1,2,5,6-tetrahydro-1-methylp...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50300557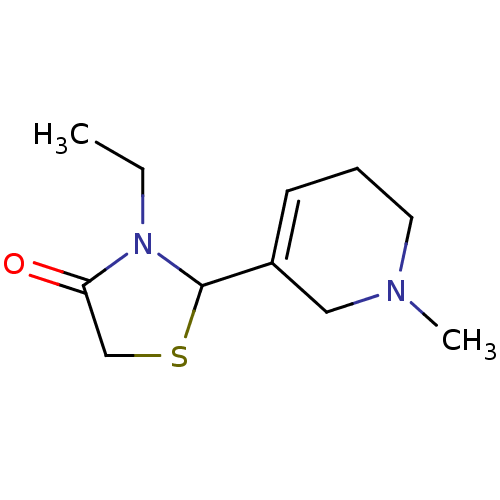 (3-ethyl-2-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic M1 receptor in Wistar rat brain cortex membrane after 2 hrs by liquid scintillation counting | Eur J Med Chem 44: 4848-54 (2009) Article DOI: 10.1016/j.ejmech.2009.07.026 BindingDB Entry DOI: 10.7270/Q23X86QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049128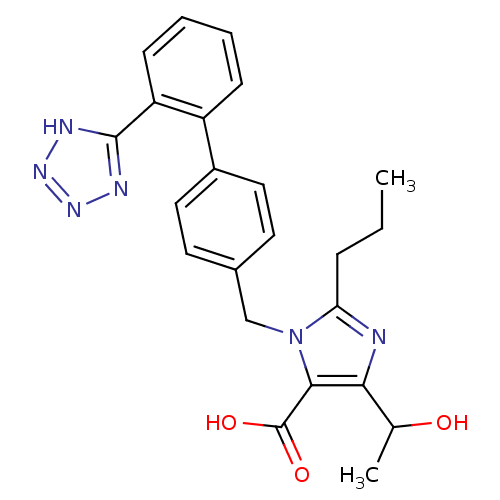 (5-(1-Hydroxy-ethyl)-2-propyl-3-[2'-(2H-tetrazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049118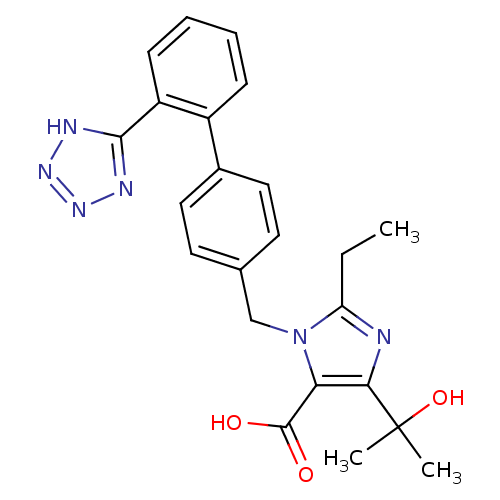 (2-Ethyl-5-(1-hydroxy-1-methyl-ethyl)-3-[2'-(2H-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049123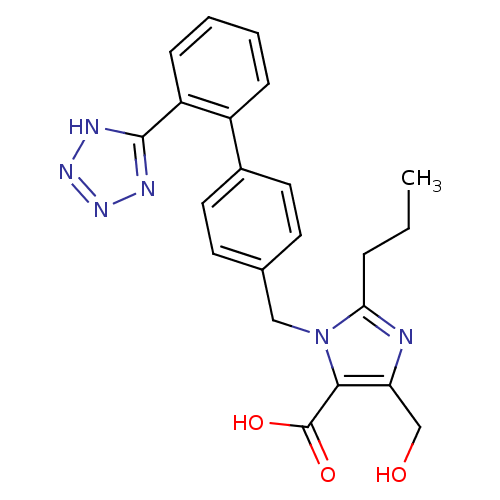 (5-Hydroxymethyl-2-propyl-3-[2'-(2H-tetrazol-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50241364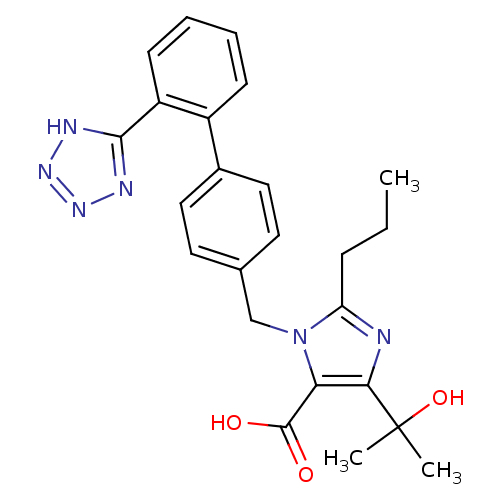 (4-(1-hydroxy-1-methylethyl)-2-propyl-1-{[2'-(1H-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049107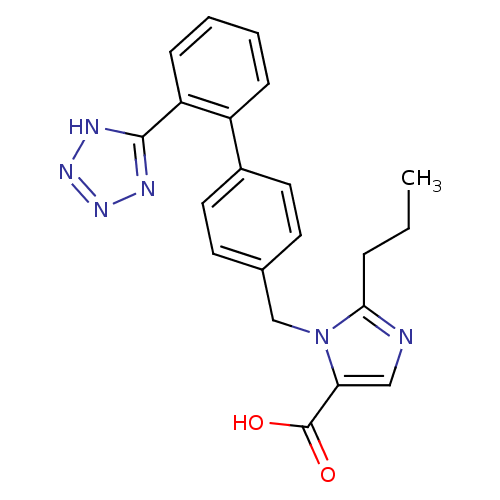 (2-Propyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049109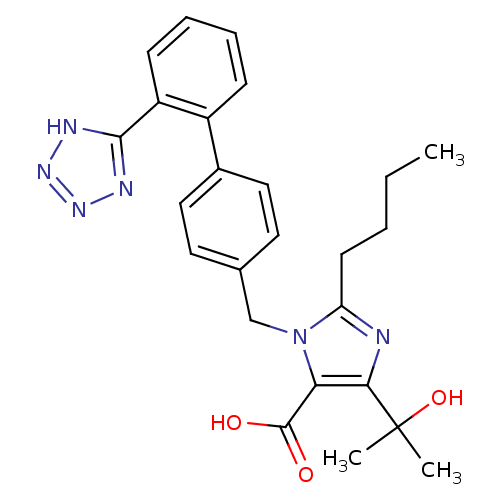 (2-Butyl-5-(1-hydroxy-1-methyl-ethyl)-3-[2'-(2H-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049124 (5-Methyl-2-propyl-3-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049116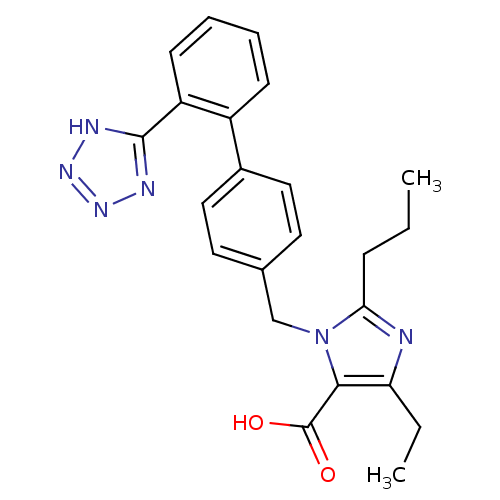 (5-Ethyl-2-propyl-3-(2'-tetrazol-1-yl-biphenyl-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50188016 (1-[bis(4-fluoro-phenyl)-methyl]-4-(2-pyrrolidin-1-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Inhibition of electric eel AchE | Bioorg Med Chem Lett 16: 3932-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.030 BindingDB Entry DOI: 10.7270/Q2RJ4J3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50188016 (1-[bis(4-fluoro-phenyl)-methyl]-4-(2-pyrrolidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Inhibition of rat brain homogenate AchE | Bioorg Med Chem Lett 16: 3932-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.030 BindingDB Entry DOI: 10.7270/Q2RJ4J3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50188016 (1-[bis(4-fluoro-phenyl)-methyl]-4-(2-pyrrolidin-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Inhibition of human serum AchE | Bioorg Med Chem Lett 16: 3932-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.030 BindingDB Entry DOI: 10.7270/Q2RJ4J3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50006909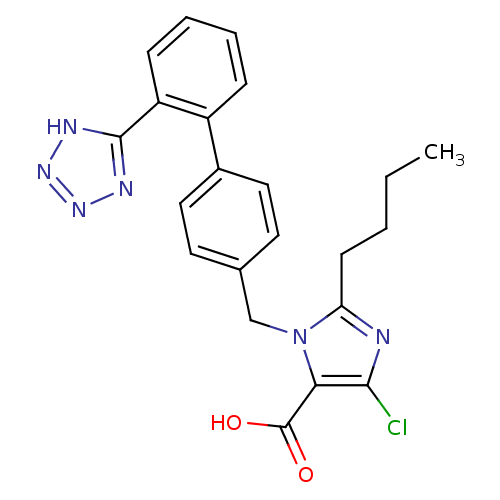 (2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50006909 (2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049132 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-(1-hydroxy-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049112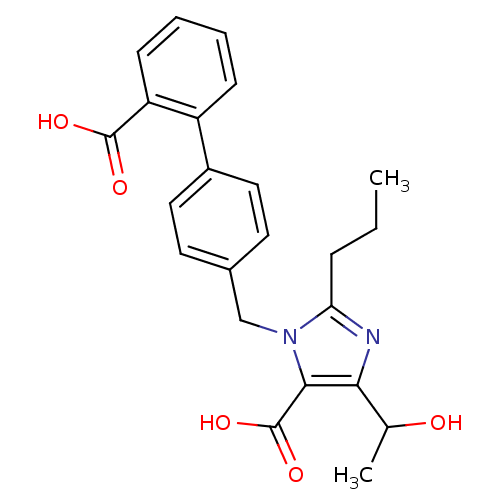 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-(1-hydroxy-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049122 (2-Butyl-3-(2'-carboxy-biphenyl-4-ylmethyl)-5-(1-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50022775 ((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Inhibition of Wistar rat brain AChE by Ellman colorimetric assay | Bioorg Med Chem 15: 7391-8 (2007) Article DOI: 10.1016/j.bmc.2007.07.014 BindingDB Entry DOI: 10.7270/Q2FB53R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049117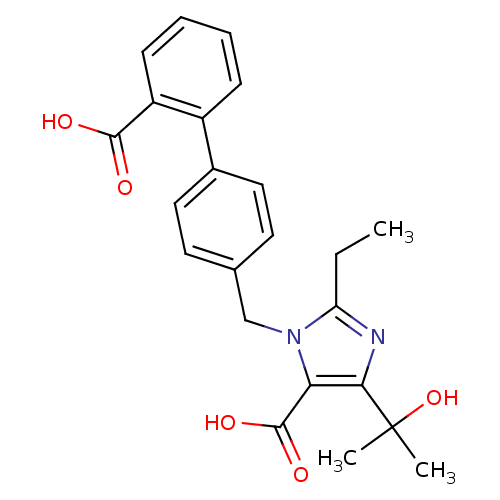 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-2-ethyl-5-(1-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50022775 ((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Inhibition of rat brain homogenate AchE | Bioorg Med Chem Lett 16: 3932-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.030 BindingDB Entry DOI: 10.7270/Q2RJ4J3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50188013 (1-[bis(4-fluoro-phenyl)-methyl]-4-1(benzyl)-4-(2-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Inhibition of rat brain homogenate AchE | Bioorg Med Chem Lett 16: 3932-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.030 BindingDB Entry DOI: 10.7270/Q2RJ4J3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50022775 ((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Inhibition of Wistar rat brain homogenate AChE by Ellman's assay | Eur J Med Chem 44: 4057-62 (2009) Article DOI: 10.1016/j.ejmech.2009.04.042 BindingDB Entry DOI: 10.7270/Q2MK6CZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50022775 ((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Inhibition of human serum AChE by Ellman's assay | Eur J Med Chem 44: 4057-62 (2009) Article DOI: 10.1016/j.ejmech.2009.04.042 BindingDB Entry DOI: 10.7270/Q2MK6CZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50022775 ((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 42.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Inhibition of human serum AchE | Bioorg Med Chem Lett 16: 3932-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.030 BindingDB Entry DOI: 10.7270/Q2RJ4J3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50022775 ((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Inhibition of human serum AChE by Ellman colorimetric assay | Bioorg Med Chem 15: 7391-8 (2007) Article DOI: 10.1016/j.bmc.2007.07.014 BindingDB Entry DOI: 10.7270/Q2FB53R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049130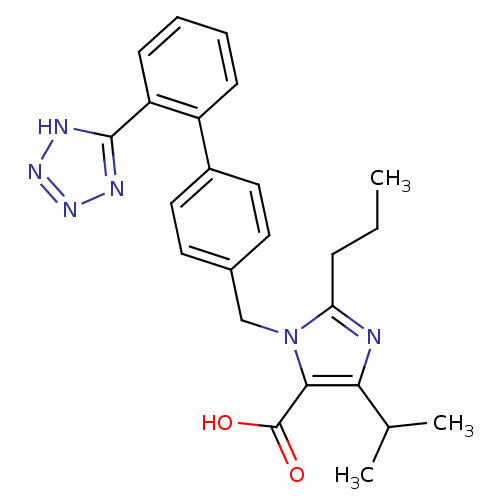 (5-Isopropyl-2-propyl-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50022775 ((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mysore Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman colorimetric assay | Bioorg Med Chem 15: 7391-8 (2007) Article DOI: 10.1016/j.bmc.2007.07.014 BindingDB Entry DOI: 10.7270/Q2FB53R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049113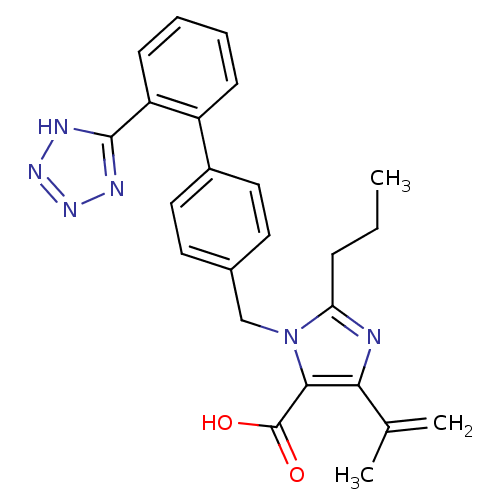 (5-Isopropenyl-2-propyl-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 221 total ) | Next | Last >> |