 Found 32 hits with Last Name = 'saha' and Initial = 'u'
Found 32 hits with Last Name = 'saha' and Initial = 'u' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370925
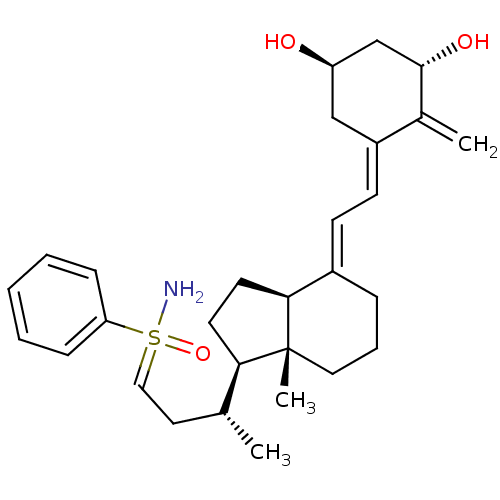
(CHEMBL1627233)Show SMILES C[C@H](CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H41NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,17,20,24,26-28,31-32H,2,7-8,13-16,18-19H2,1,3H3,(H2,30,33)/b22-11+,23-12-/t20-,24-,26-,27+,28+,29-,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411322
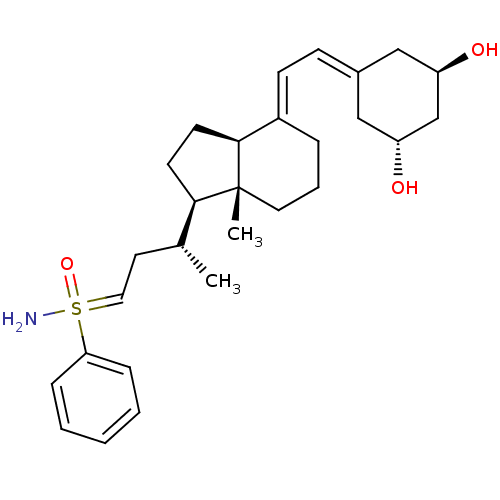
Show SMILES [#6]-[#6@H](-[#6]-[#6]=S([#7])(=O)c1ccccc1)-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6]-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C28H41NO3S/c1-20(14-16-33(29,32)25-8-4-3-5-9-25)26-12-13-27-22(7-6-15-28(26,27)2)11-10-21-17-23(30)19-24(31)18-21/h3-5,8-11,16,20,23-24,26-27,30-31H,6-7,12-15,17-19H2,1-2H3,(H2,29,32)/b22-11+/t20-,23-,24-,26-,27+,28-,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411322

Show SMILES [#6]-[#6@H](-[#6]-[#6]=S([#7])(=O)c1ccccc1)-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6]-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C28H41NO3S/c1-20(14-16-33(29,32)25-8-4-3-5-9-25)26-12-13-27-22(7-6-15-28(26,27)2)11-10-21-17-23(30)19-24(31)18-21/h3-5,8-11,16,20,23-24,26-27,30-31H,6-7,12-15,17-19H2,1-2H3,(H2,29,32)/b22-11+/t20-,23-,24-,26-,27+,28-,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411326
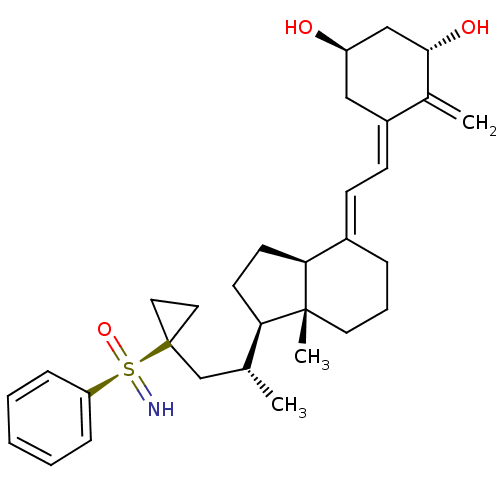
(CHEMBL2092724)Show SMILES C[C@H](CC1(CC1)[S@](=N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C31H43NO3S/c1-21(20-31(16-17-31)36(32,35)26-9-5-4-6-10-26)27-13-14-28-23(8-7-15-30(27,28)3)11-12-24-18-25(33)19-29(34)22(24)2/h4-6,9-12,21,25,27-29,32-34H,2,7-8,13-20H2,1,3H3/b23-11+,24-12-/t21-,25-,27-,28+,29+,30-,36-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50158486

((1R,3S,5Z)-5-{2-[(1R,3aS,4E,7aR)-1-[(2R)-4-(benzen...)Show SMILES C[C@H](CCS(=O)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H40O4S/c1-20(15-17-34(32,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(30)19-28(31)21(23)2/h4-6,9-12,20,24,26-28,30-31H,2,7-8,13-19H2,1,3H3/b22-11+,23-12-/t20-,24-,26-,27+,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411324
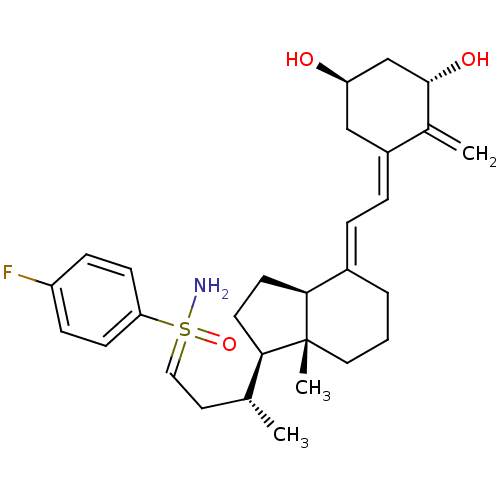
Show SMILES C[C@H](CC=S(N)(=O)c1ccc(F)cc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H40FNO3S/c1-19(14-16-35(31,34)25-10-8-23(30)9-11-25)26-12-13-27-21(5-4-15-29(26,27)3)6-7-22-17-24(32)18-28(33)20(22)2/h6-11,16,19,24,26-28,32-33H,2,4-5,12-15,17-18H2,1,3H3,(H2,31,34)/b21-6+,22-7-/t19-,24-,26-,27+,28+,29-,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370925

(CHEMBL1627233)Show SMILES C[C@H](CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H41NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,17,20,24,26-28,31-32H,2,7-8,13-16,18-19H2,1,3H3,(H2,30,33)/b22-11+,23-12-/t20-,24-,26-,27+,28+,29-,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411324

Show SMILES C[C@H](CC=S(N)(=O)c1ccc(F)cc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H40FNO3S/c1-19(14-16-35(31,34)25-10-8-23(30)9-11-25)26-12-13-27-21(5-4-15-29(26,27)3)6-7-22-17-24(32)18-28(33)20(22)2/h6-11,16,19,24,26-28,32-33H,2,4-5,12-15,17-18H2,1,3H3,(H2,31,34)/b21-6+,22-7-/t19-,24-,26-,27+,28+,29-,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411323
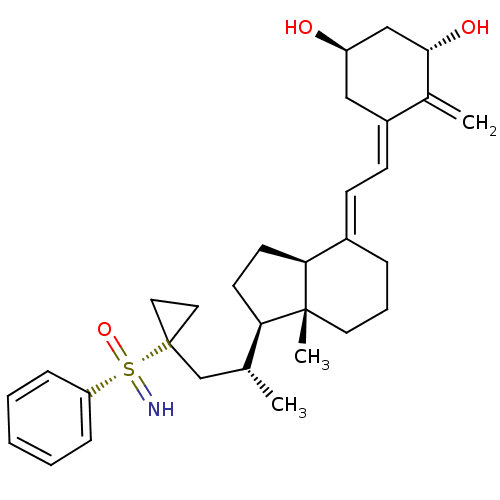
(CHEMBL2093103)Show SMILES C[C@H](CC1(CC1)[S@@](=N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C31H43NO3S/c1-21(20-31(16-17-31)36(32,35)26-9-5-4-6-10-26)27-13-14-28-23(8-7-15-30(27,28)3)11-12-24-18-25(33)19-29(34)22(24)2/h4-6,9-12,21,25,27-29,32-34H,2,7-8,13-20H2,1,3H3/b23-11+,24-12-/t21-,25-,27-,28+,29+,30-,36+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370926
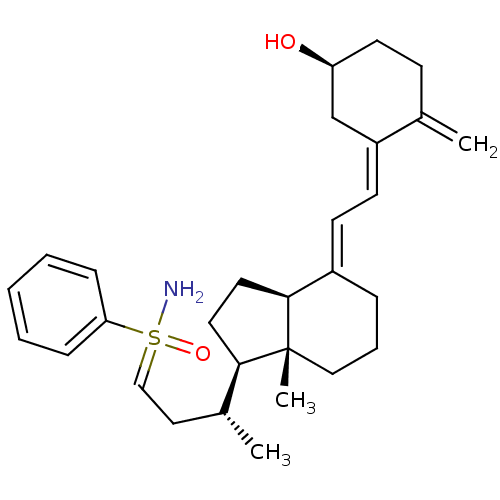
(CHEMBL1627204)Show SMILES C[C@H](CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)CCC1=C |r| Show InChI InChI=1S/C29H41NO2S/c1-21-11-14-25(31)20-24(21)13-12-23-8-7-18-29(3)27(15-16-28(23)29)22(2)17-19-33(30,32)26-9-5-4-6-10-26/h4-6,9-10,12-13,19,22,25,27-28,31H,1,7-8,11,14-18,20H2,2-3H3,(H2,30,32)/b23-12+,24-13-/t22-,25+,27-,28+,29-,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26B1
(Homo sapiens (Human)) | BDBM557292

(US11358933, Compound 026)Show SMILES CC(C)(C)c1cccc(c1)-c1cc2cc(\C=C\C(O)=O)ccc2[nH]1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26B1
(Homo sapiens (Human)) | BDBM557299

(US11358933, Compound 034)Show SMILES Cc1ccc(cc1-c1cc2cc(\C=C\C(O)=O)ccc2[nH]1)C(C)(C)C | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370927
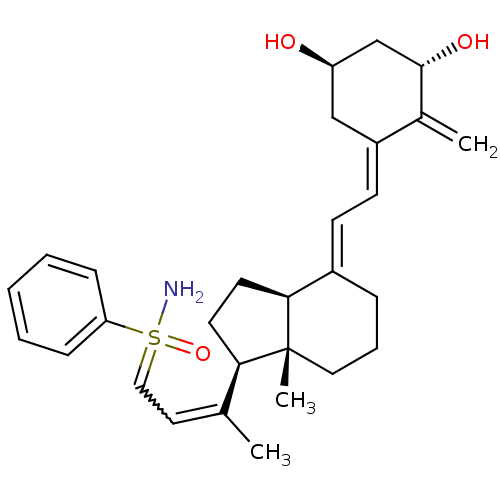
(CHEMBL1627205 | CHEMBL1627234)Show SMILES CC(=CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r,w:2.2| Show InChI InChI=1S/C29H39NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,15,17,24,26-28,31-32H,2,7-8,13-14,16,18-19H2,1,3H3,(H2,30,33)/b20-15?,22-11+,23-12-/t24-,26-,27+,28+,29-,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 549 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370925

(CHEMBL1627233)Show SMILES C[C@H](CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H41NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,17,20,24,26-28,31-32H,2,7-8,13-16,18-19H2,1,3H3,(H2,30,33)/b22-11+,23-12-/t20-,24-,26-,27+,28+,29-,34?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 554 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP27B hydroxylase expressed in SW900 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370927

(CHEMBL1627205 | CHEMBL1627234)Show SMILES CC(=CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r,w:2.2| Show InChI InChI=1S/C29H39NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,15,17,24,26-28,31-32H,2,7-8,13-14,16,18-19H2,1,3H3,(H2,30,33)/b20-15?,22-11+,23-12-/t24-,26-,27+,28+,29-,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
Sterol 26-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370925

(CHEMBL1627233)Show SMILES C[C@H](CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H41NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,17,20,24,26-28,31-32H,2,7-8,13-16,18-19H2,1,3H3,(H2,30,33)/b22-11+,23-12-/t20-,24-,26-,27+,28+,29-,34?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP27A1 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370932
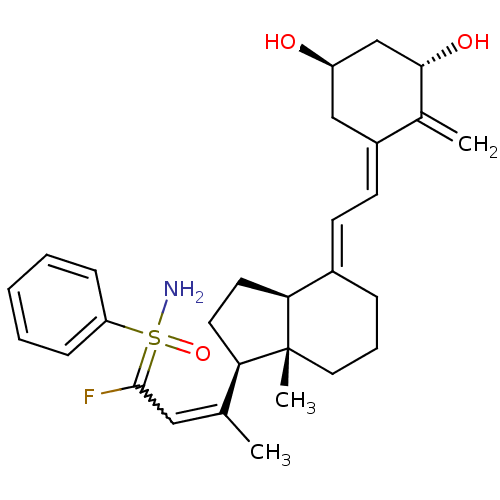
(CHEMBL1627208)Show SMILES CC(=CC(F)=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r,w:2.2| Show InChI InChI=1S/C29H38FNO3S/c1-19(16-28(30)35(31,34)24-9-5-4-6-10-24)25-13-14-26-21(8-7-15-29(25,26)3)11-12-22-17-23(32)18-27(33)20(22)2/h4-6,9-12,16,23,25-27,32-33H,2,7-8,13-15,17-18H2,1,3H3,(H2,31,34)/b19-16?,21-11+,22-12-/t23-,25-,26+,27+,29-,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26B1
(Homo sapiens (Human)) | BDBM557298
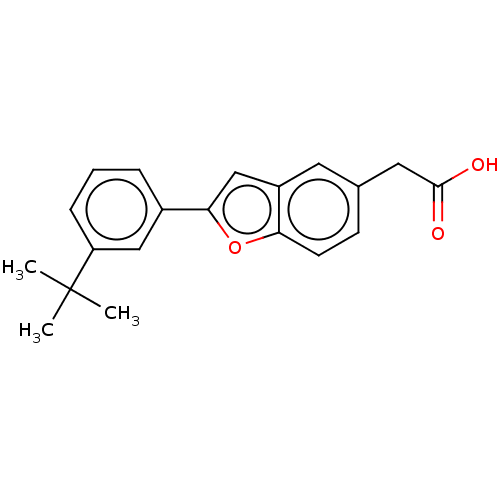
(US11358933, Compound 032) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM557292

(US11358933, Compound 026)Show SMILES CC(C)(C)c1cccc(c1)-c1cc2cc(\C=C\C(O)=O)ccc2[nH]1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM557293
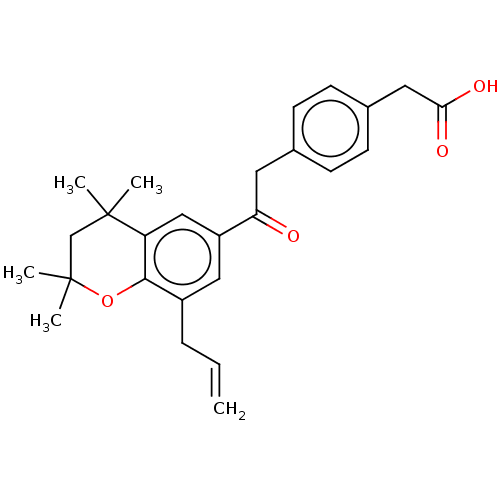
(US11358933, Compound 027)Show SMILES CC1(C)CC(C)(C)c2cc(cc(CC=C)c2O1)C(=O)Cc1ccc(CC(O)=O)cc1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM557298

(US11358933, Compound 032) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26B1
(Homo sapiens (Human)) | BDBM557293

(US11358933, Compound 027)Show SMILES CC1(C)CC(C)(C)c2cc(cc(CC=C)c2O1)C(=O)Cc1ccc(CC(O)=O)cc1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM557299

(US11358933, Compound 034)Show SMILES Cc1ccc(cc1-c1cc2cc(\C=C\C(O)=O)ccc2[nH]1)C(C)(C)C | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26B1
(Homo sapiens (Human)) | BDBM557291
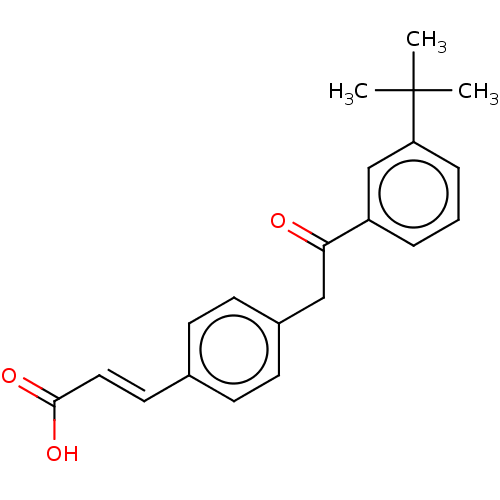
(US11358933, Compound 025)Show SMILES CC(C)(C)c1cccc(c1)C(=O)Cc1ccc(\C=C\C(O)=O)cc1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26B1
(Homo sapiens (Human)) | BDBM557297
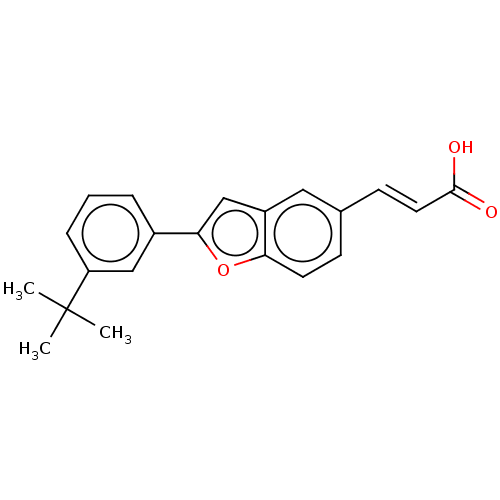
(US11358933, Compound 031)Show SMILES CC(C)(C)c1cccc(c1)-c1cc2cc(\C=C\C(O)=O)ccc2o1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM557296
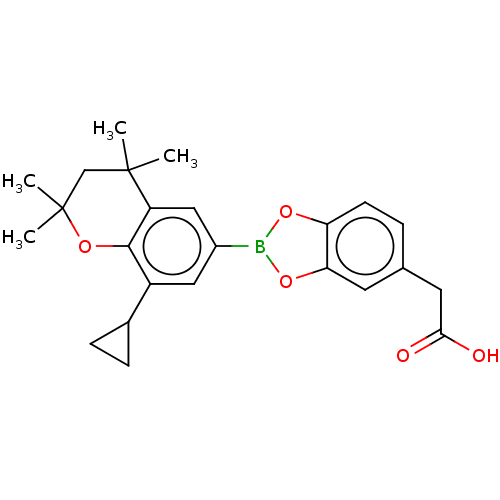
(US11358933, Compound 030)Show SMILES CC1(C)CC(C)(C)c2cc(cc(C3CC3)c2O1)B1Oc2ccc(CC(O)=O)cc2O1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM557291

(US11358933, Compound 025)Show SMILES CC(C)(C)c1cccc(c1)C(=O)Cc1ccc(\C=C\C(O)=O)cc1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26B1
(Homo sapiens (Human)) | BDBM557295
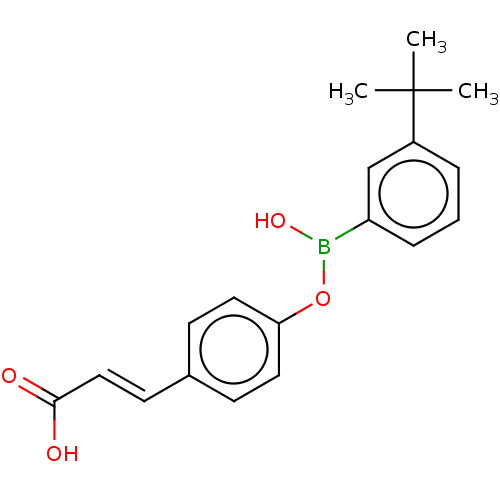
(US11358933, Compound 029)Show SMILES CC(C)(C)c1cccc(c1)B(O)Oc1ccc(\C=C\C(O)=O)cc1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26B1
(Homo sapiens (Human)) | BDBM557296

(US11358933, Compound 030)Show SMILES CC1(C)CC(C)(C)c2cc(cc(C3CC3)c2O1)B1Oc2ccc(CC(O)=O)cc2O1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26B1
(Homo sapiens (Human)) | BDBM557294
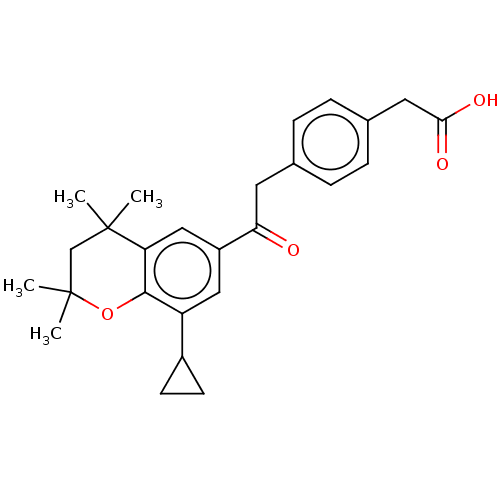
(US11358933, Compound 028)Show SMILES CC1(C)CC(C)(C)c2cc(cc(C3CC3)c2O1)C(=O)Cc1ccc(CC(O)=O)cc1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM557294

(US11358933, Compound 028)Show SMILES CC1(C)CC(C)(C)c2cc(cc(C3CC3)c2O1)C(=O)Cc1ccc(CC(O)=O)cc1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CYP26 inhibitory activities of various compounds. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CC13XJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data