 Found 401 hits with Last Name = 'salituro' and Initial = 'gm'
Found 401 hits with Last Name = 'salituro' and Initial = 'gm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Integrase
(Human immunodeficiency virus 1) | BDBM50123449
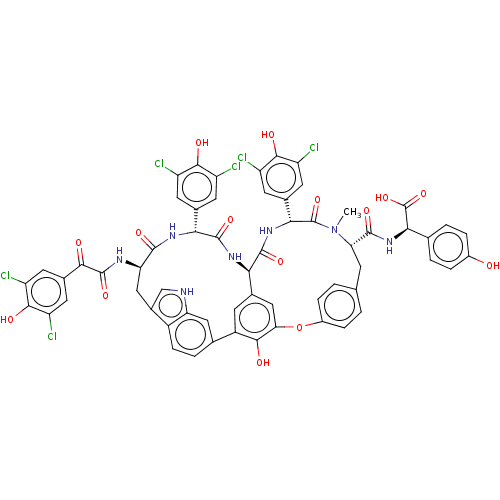
(CHEMBL437996 | Chloropeptin II | ISOCOMPLESTATIN)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-44(56(82)73-49(61(87)88)25-4-7-32(75)8-5-25)12-24-2-9-33(10-3-24)89-45-22-27-13-35(51(45)77)26-6-11-34-31(23-68-42(34)20-26)21-43(69-59(85)50(76)30-18-40(66)54(80)41(67)19-30)55(81)70-47(28-14-36(62)52(78)37(63)15-28)57(83)71-46(27)58(84)72-48(60(74)86)29-16-38(64)53(79)39(65)17-29/h2-11,13-20,22-23,43-44,46-49,68,75,77-80H,12,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146155
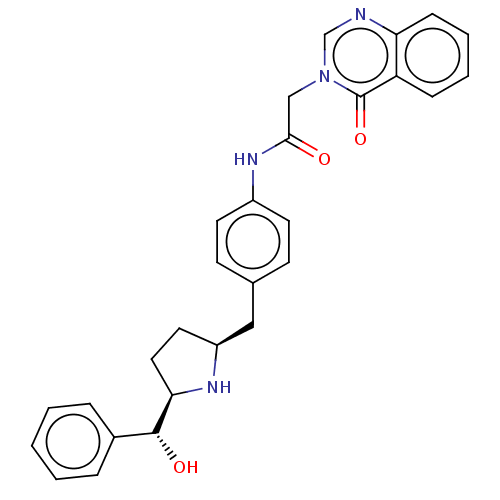
(CHEMBL3764774)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3cnc4ccccc4c3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H28N4O3/c33-26(17-32-18-29-24-9-5-4-8-23(24)28(32)35)31-21-12-10-19(11-13-21)16-22-14-15-25(30-22)27(34)20-6-2-1-3-7-20/h1-13,18,22,25,27,30,34H,14-17H2,(H,31,33)/t22-,25+,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50092645
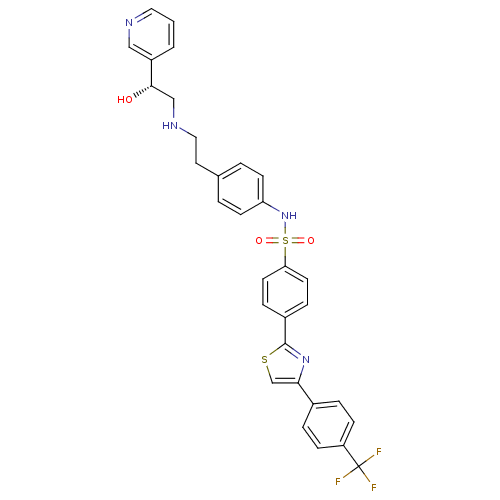
((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1 Show InChI InChI=1S/C31H27F3N4O3S2/c32-31(33,34)25-9-5-22(6-10-25)28-20-42-30(37-28)23-7-13-27(14-8-23)43(40,41)38-26-11-3-21(4-12-26)15-17-36-19-29(39)24-2-1-16-35-18-24/h1-14,16,18,20,29,36,38-39H,15,17,19H2/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG channel |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338820
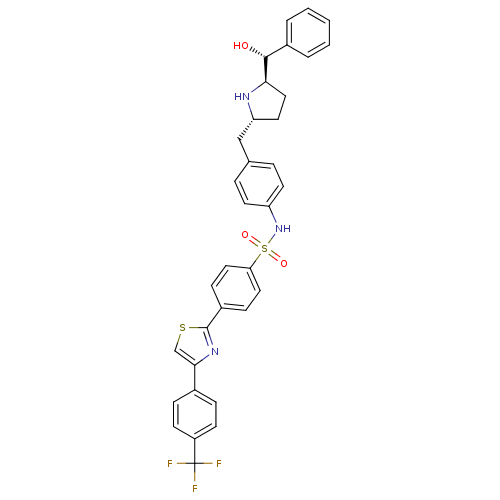
(CHEMBL1684584 | trans-N-(4-(((2S,5R)-5-((R)-hydrox...)Show SMILES O[C@@H]([C@H]1CC[C@H](Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28-,30-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338820

(CHEMBL1684584 | trans-N-(4-(((2S,5R)-5-((R)-hydrox...)Show SMILES O[C@@H]([C@H]1CC[C@H](Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28-,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 297 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50366855
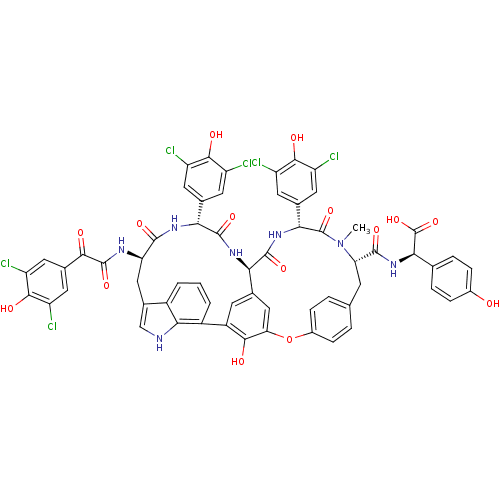
(CHEMBL525803 | Chloropeptin)Show SMILES CN1[C@@H](Cc2ccc(Oc3cc4cc(c3O)-c3cccc5c(C[C@@H](NC(=O)C(=O)c6cc(Cl)c(O)c(Cl)c6)C(=O)N[C@H](c6cc(Cl)c(O)c(Cl)c6)C(=O)N[C@H]4C(=O)N[C@H](c4cc(Cl)c(O)c(Cl)c4)C1=O)c[nH]c35)cc2)C(=O)N[C@@H](C(O)=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-43(56(82)73-48(61(87)88)25-7-9-31(75)10-8-25)13-24-5-11-32(12-6-24)89-44-22-26-14-35(51(44)77)34-4-2-3-33-30(23-68-49(33)34)21-42(69-59(85)50(76)29-19-40(66)54(80)41(67)20-29)55(81)70-46(27-15-36(62)52(78)37(63)16-27)57(83)71-45(26)58(84)72-47(60(74)86)28-17-38(64)53(79)39(65)18-28/h2-12,14-20,22-23,42-43,45-48,68,75,77-80H,13,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88)/t42-,43+,45-,46-,47-,48-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50448787

(CHEMBL3128178)Show SMILES O[C@@H]([C@H]1CC[C@@H](Cc2ccc(NC(=O)[C@@H]3CCc4scnc34)cc2)N1)c1cccnc1 |r| Show InChI InChI=1S/C24H26N4O2S/c29-23(16-2-1-11-25-13-16)20-9-7-18(27-20)12-15-3-5-17(6-4-15)28-24(30)19-8-10-21-22(19)26-14-31-21/h1-6,11,13-14,18-20,23,27,29H,7-10,12H2,(H,28,30)/t18-,19+,20+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123449

(CHEMBL437996 | Chloropeptin II | ISOCOMPLESTATIN)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-44(56(82)73-49(61(87)88)25-4-7-32(75)8-5-25)12-24-2-9-33(10-3-24)89-45-22-27-13-35(51(45)77)26-6-11-34-31(23-68-42(34)20-26)21-43(69-59(85)50(76)30-18-40(66)54(80)41(67)19-30)55(81)70-47(28-14-36(62)52(78)37(63)15-28)57(83)71-46(27)58(84)72-48(60(74)86)29-16-38(64)53(79)39(65)17-29/h2-11,13-20,22-23,43-44,46-49,68,75,77-80H,12,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of disintegration activity of HIV1 intact integrase |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123449

(CHEMBL437996 | Chloropeptin II | ISOCOMPLESTATIN)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-44(56(82)73-49(61(87)88)25-4-7-32(75)8-5-25)12-24-2-9-33(10-3-24)89-45-22-27-13-35(51(45)77)26-6-11-34-31(23-68-42(34)20-26)21-43(69-59(85)50(76)30-18-40(66)54(80)41(67)19-30)55(81)70-47(28-14-36(62)52(78)37(63)15-28)57(83)71-46(27)58(84)72-48(60(74)86)29-16-38(64)53(79)39(65)17-29/h2-11,13-20,22-23,43-44,46-49,68,75,77-80H,12,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of disintegration activity of HIV1 integrase catalytic core domain (50 to 212) |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478734
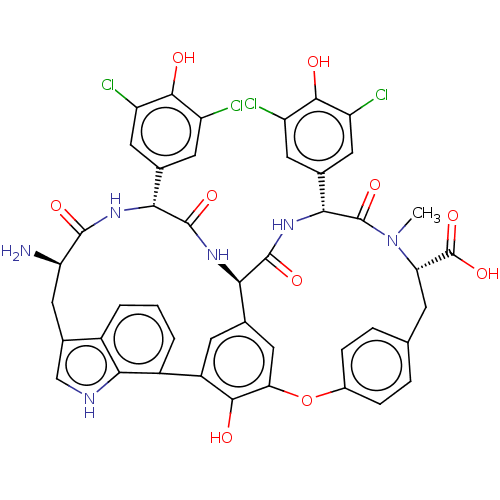
(CHEMBL448564)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@H](N)Cc3c[nH]c4c(cccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(O)=O)cc1)c3O)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C45H36Cl4N6O10/c1-55-32(45(63)64)9-18-5-7-23(8-6-18)65-33-16-19-10-26(38(33)56)25-4-2-3-24-22(17-51-37(24)25)15-31(50)41(59)52-35(20-11-27(46)39(57)28(47)12-20)42(60)53-34(19)43(61)54-36(44(55)62)21-13-29(48)40(58)30(49)14-21/h2-8,10-14,16-17,31-32,34-36,51,56-58H,9,15,50H2,1H3,(H,52,59)(H,53,60)(H,54,61)(H,63,64)/t31-,32+,34-,35-,36-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478738
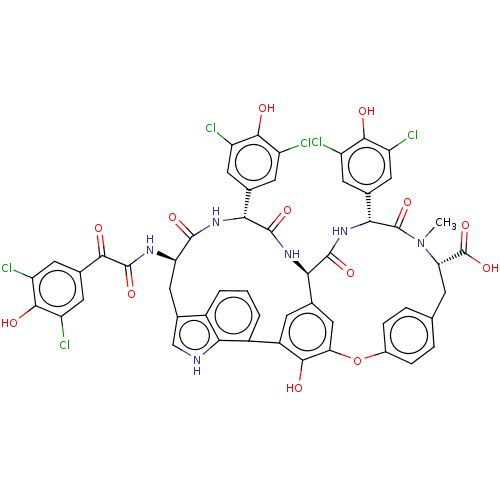
(CHEMBL507121)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4c(cccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(O)=O)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C53H38Cl6N6O13/c1-65-37(53(76)77)9-20-5-7-26(8-6-20)78-38-18-21-10-29(44(38)67)28-4-2-3-27-25(19-60-42(27)28)17-36(61-51(74)43(66)24-15-34(58)47(70)35(59)16-24)48(71)62-40(22-11-30(54)45(68)31(55)12-22)49(72)63-39(21)50(73)64-41(52(65)75)23-13-32(56)46(69)33(57)14-23/h2-8,10-16,18-19,36-37,39-41,60,67-70H,9,17H2,1H3,(H,61,74)(H,62,71)(H,63,72)(H,64,73)(H,76,77)/t36-,37+,39-,40-,41-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50092645

((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1 Show InChI InChI=1S/C31H27F3N4O3S2/c32-31(33,34)25-9-5-22(6-10-25)28-20-42-30(37-28)23-7-13-27(14-8-23)43(40,41)38-26-11-3-21(4-12-26)15-17-36-19-29(39)24-2-1-16-35-18-24/h1-14,16,18,20,29,36,38-39H,15,17,19H2/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146157
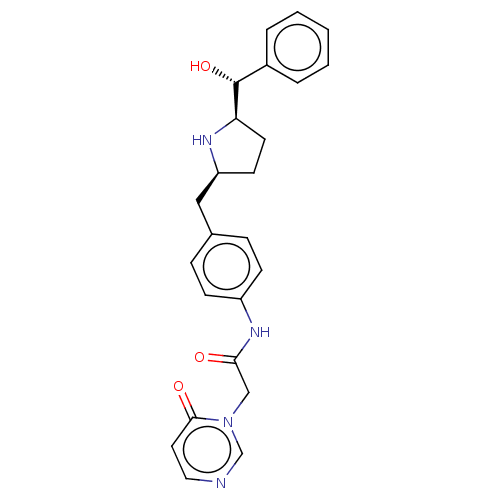
(CHEMBL3764950)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3cnccc3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C24H26N4O3/c29-22(15-28-16-25-13-12-23(28)30)27-19-8-6-17(7-9-19)14-20-10-11-21(26-20)24(31)18-4-2-1-3-5-18/h1-9,12-13,16,20-21,24,26,31H,10-11,14-15H2,(H,27,29)/t20-,21+,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338814
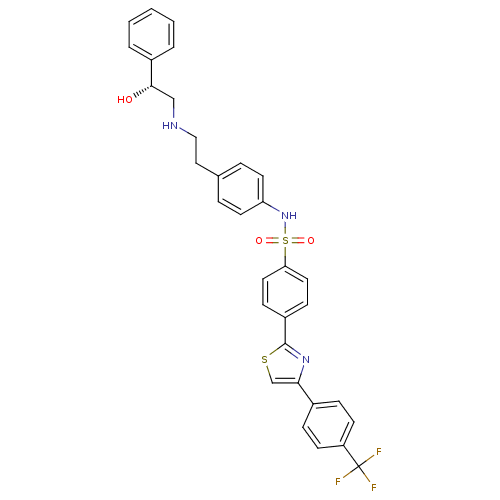
((R)-N-(4-(2-(2-hydroxy-2-phenylethylamino)ethyl)ph...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1ccccc1 |r| Show InChI InChI=1S/C32H28F3N3O3S2/c33-32(34,35)26-12-8-23(9-13-26)29-21-42-31(37-29)25-10-16-28(17-11-25)43(40,41)38-27-14-6-22(7-15-27)18-19-36-20-30(39)24-4-2-1-3-5-24/h1-17,21,30,36,38-39H,18-20H2/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146161
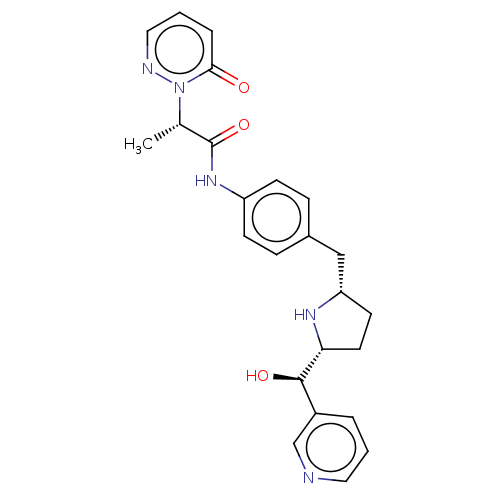
(CHEMBL3763998)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)[C@H](C)n3ncccc3=O)cc2)N1)[C@H](O)c1cccnc1 |r| Show InChI InChI=1S/C24H27N5O3/c1-16(29-22(30)5-3-13-26-29)24(32)28-19-8-6-17(7-9-19)14-20-10-11-21(27-20)23(31)18-4-2-12-25-15-18/h2-9,12-13,15-16,20-21,23,27,31H,10-11,14H2,1H3,(H,28,32)/t16-,20-,21+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478735
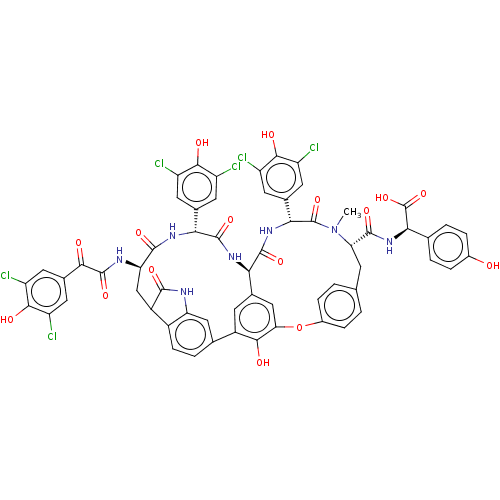
(CHEBI:65655 | COMPLESTATINS A)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](CC3C(=O)Nc4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O16/c1-74-43(56(83)73-48(61(88)89)24-4-7-30(75)8-5-24)12-23-2-9-31(10-3-23)90-44-21-26-13-33(50(44)77)25-6-11-32-34(54(81)68-41(32)20-25)22-42(69-59(86)49(76)29-18-39(66)53(80)40(67)19-29)55(82)70-46(27-14-35(62)51(78)36(63)15-27)57(84)71-45(26)58(85)72-47(60(74)87)28-16-37(64)52(79)38(65)17-28/h2-11,13-21,34,42-43,45-48,75,77-80H,12,22H2,1H3,(H,68,81)(H,69,86)(H,70,82)(H,71,84)(H,72,85)(H,73,83)(H,88,89)/t34?,42-,43+,45-,46-,47-,48-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50448789

(CHEMBL3128188)Show SMILES O[C@@H]([C@H]1CC[C@@H](Cc2ccc(NC(=O)[C@@H]3CCc4ccnn34)cc2)N1)c1cccnc1 |r| Show InChI InChI=1S/C24H27N5O2/c30-23(17-2-1-12-25-15-17)21-9-7-19(27-21)14-16-3-5-18(6-4-16)28-24(31)22-10-8-20-11-13-26-29(20)22/h1-6,11-13,15,19,21-23,27,30H,7-10,14H2,(H,28,31)/t19-,21+,22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50092645

((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1 Show InChI InChI=1S/C31H27F3N4O3S2/c32-31(33,34)25-9-5-22(6-10-25)28-20-42-30(37-28)23-7-13-27(14-8-23)43(40,41)38-26-11-3-21(4-12-26)15-17-36-19-29(39)24-2-1-16-35-18-24/h1-14,16,18,20,29,36,38-39H,15,17,19H2/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338814

((R)-N-(4-(2-(2-hydroxy-2-phenylethylamino)ethyl)ph...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1ccccc1 |r| Show InChI InChI=1S/C32H28F3N3O3S2/c33-32(34,35)26-12-8-23(9-13-26)29-21-42-31(37-29)25-10-16-28(17-11-25)43(40,41)38-27-14-6-22(7-15-27)18-19-36-20-30(39)24-4-2-1-3-5-24/h1-17,21,30,36,38-39H,18-20H2/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146159
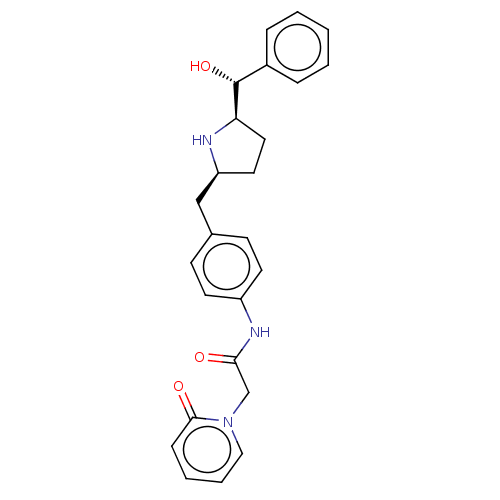
(CHEMBL3764592)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3ccccc3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C25H27N3O3/c29-23(17-28-15-5-4-8-24(28)30)27-20-11-9-18(10-12-20)16-21-13-14-22(26-21)25(31)19-6-2-1-3-7-19/h1-12,15,21-22,25-26,31H,13-14,16-17H2,(H,27,29)/t21-,22+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50338821
(2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...)Show SMILES Nc1nc(CC(=O)Nc2ccc(C[C@@H]3CC[C@@H](N3)[C@H](O)c3ccccc3)cc2)cs1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-21(28)26-17-8-6-15(7-9-17)12-18-10-11-20(25-18)22(29)16-4-2-1-3-5-16/h1-9,14,18,20,22,25,29H,10-13H2,(H2,24,27)(H,26,28)/t18-,20+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338818

(CHEMBL1684582 | N-(4-(((5R)-5-((S)-hydroxy(phenyl)...)Show SMILES O[C@H]([C@H]1CCC(Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28?,30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50092645

((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1 Show InChI InChI=1S/C31H27F3N4O3S2/c32-31(33,34)25-9-5-22(6-10-25)28-20-42-30(37-28)23-7-13-27(14-8-23)43(40,41)38-26-11-3-21(4-12-26)15-17-36-19-29(39)24-2-1-16-35-18-24/h1-14,16,18,20,29,36,38-39H,15,17,19H2/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diltiazem from human Cav1.2 channel |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478740
(CHEBI:65656 | COMPLESTATIN B)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](CC3(O)C(=O)Nc4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O17/c1-74-42(54(82)73-47(59(87)88)24-4-7-30(75)8-5-24)12-23-2-9-31(10-3-23)91-43-21-26-13-32(49(43)77)25-6-11-33-40(20-25)69-60(89)61(33,90)22-41(68-57(85)48(76)29-18-38(66)52(80)39(67)19-29)53(81)70-45(27-14-34(62)50(78)35(63)15-27)55(83)71-44(26)56(84)72-46(58(74)86)28-16-36(64)51(79)37(65)17-28/h2-11,13-21,41-42,44-47,75,77-80,90H,12,22H2,1H3,(H,68,85)(H,69,89)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88)/t41-,42+,44-,45-,46-,47-,61?/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338816
(CHEMBL1684580 | N-(4-(((5S)-5-((S)-hydroxy(phenyl)...)Show SMILES O[C@H]([C@@H]1CCC(Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28?,30-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50146155

(CHEMBL3764774)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3cnc4ccccc4c3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H28N4O3/c33-26(17-32-18-29-24-9-5-4-8-23(24)28(32)35)31-21-12-10-19(11-13-21)16-22-14-15-25(30-22)27(34)20-6-2-1-3-7-20/h1-13,18,22,25,27,30,34H,14-17H2,(H,31,33)/t22-,25+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG channel |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50232623

(CHEMBL4081159)Show SMILES Clc1cnc(nc1)N1CCC(CC1)[C@H]1C[C@H]1CCOc1ccc(CC(=O)N2CCC2)cn1 |r| Show InChI InChI=1S/C24H30ClN5O2/c25-20-15-27-24(28-16-20)30-9-4-18(5-10-30)21-13-19(21)6-11-32-22-3-2-17(14-26-22)12-23(31)29-7-1-8-29/h2-3,14-16,18-19,21H,1,4-13H2/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1124-1128 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.091
BindingDB Entry DOI: 10.7270/Q2XK8HSD |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338817

(CHEMBL1684581 | N-(4-(((5S)-5-((R)-hydroxy(phenyl)...)Show SMILES O[C@@H]([C@@H]1CCC(Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28?,30-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338815
(CHEMBL1684577 | N-(4-(((5R)-5-((R)-hydroxy(phenyl)...)Show SMILES O[C@@H]([C@H]1CCC(Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28?,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338819
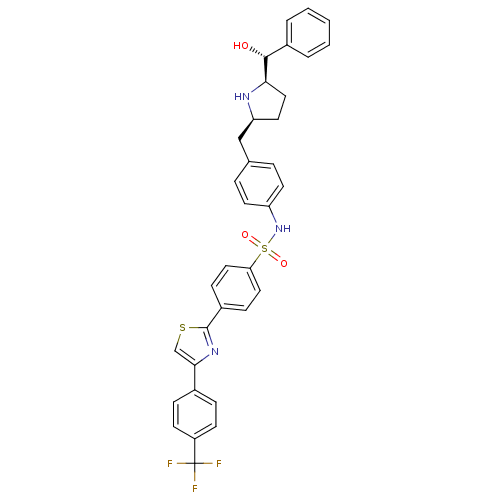
(CHEMBL1684583 | cis-N-(4-(((2S,5R)-5-((R)-hydroxy(...)Show SMILES O[C@@H]([C@H]1CC[C@@H](Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28-,30+,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50338821

(2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...)Show SMILES Nc1nc(CC(=O)Nc2ccc(C[C@@H]3CC[C@@H](N3)[C@H](O)c3ccccc3)cc2)cs1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-21(28)26-17-8-6-15(7-9-17)12-18-10-11-20(25-18)22(29)16-4-2-1-3-5-16/h1-9,14,18,20,22,25,29H,10-13H2,(H2,24,27)(H,26,28)/t18-,20+,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes assessed as dextromethorphan O-demethylation |
J Med Chem 57: 1437-53 (2014)
Article DOI: 10.1021/jm4017224
BindingDB Entry DOI: 10.7270/Q25M6767 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50146155

(CHEMBL3764774)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3cnc4ccccc4c3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H28N4O3/c33-26(17-32-18-29-24-9-5-4-8-23(24)28(32)35)31-21-12-10-19(11-13-21)16-22-14-15-25(30-22)27(34)20-6-2-1-3-7-20/h1-13,18,22,25,27,30,34H,14-17H2,(H,31,33)/t22-,25+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50338821

(2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...)Show SMILES Nc1nc(CC(=O)Nc2ccc(C[C@@H]3CC[C@@H](N3)[C@H](O)c3ccccc3)cc2)cs1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-21(28)26-17-8-6-15(7-9-17)12-18-10-11-20(25-18)22(29)16-4-2-1-3-5-16/h1-9,14,18,20,22,25,29H,10-13H2,(H2,24,27)(H,26,28)/t18-,20+,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes assessed as dextraomethorphan O-demethylation |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146158
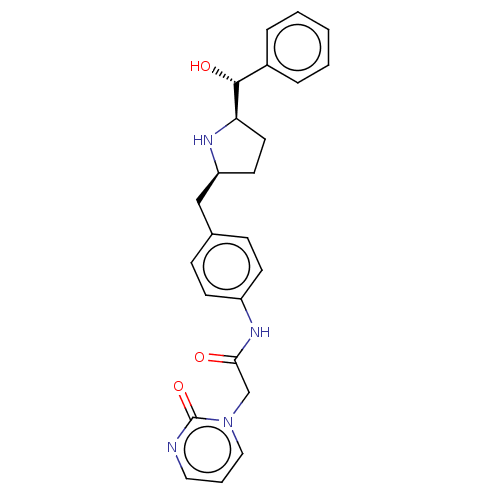
(CHEMBL3763594)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3cccnc3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C24H26N4O3/c29-22(16-28-14-4-13-25-24(28)31)27-19-9-7-17(8-10-19)15-20-11-12-21(26-20)23(30)18-5-2-1-3-6-18/h1-10,13-14,20-21,23,26,30H,11-12,15-16H2,(H,27,29)/t20-,21+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338819

(CHEMBL1684583 | cis-N-(4-(((2S,5R)-5-((R)-hydroxy(...)Show SMILES O[C@@H]([C@H]1CC[C@@H](Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28-,30+,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338818

(CHEMBL1684582 | N-(4-(((5R)-5-((S)-hydroxy(phenyl)...)Show SMILES O[C@H]([C@H]1CCC(Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28?,30-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338815

(CHEMBL1684577 | N-(4-(((5R)-5-((R)-hydroxy(phenyl)...)Show SMILES O[C@@H]([C@H]1CCC(Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28?,30-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123449

(CHEMBL437996 | Chloropeptin II | ISOCOMPLESTATIN)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-44(56(82)73-49(61(87)88)25-4-7-32(75)8-5-25)12-24-2-9-33(10-3-24)89-45-22-27-13-35(51(45)77)26-6-11-34-31(23-68-42(34)20-26)21-43(69-59(85)50(76)30-18-40(66)54(80)41(67)19-30)55(81)70-47(28-14-36(62)52(78)37(63)15-28)57(83)71-46(27)58(84)72-48(60(74)86)29-16-38(64)53(79)39(65)17-29/h2-11,13-20,22-23,43-44,46-49,68,75,77-80H,12,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of integration activity of HIV1 intact integrase |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50232627
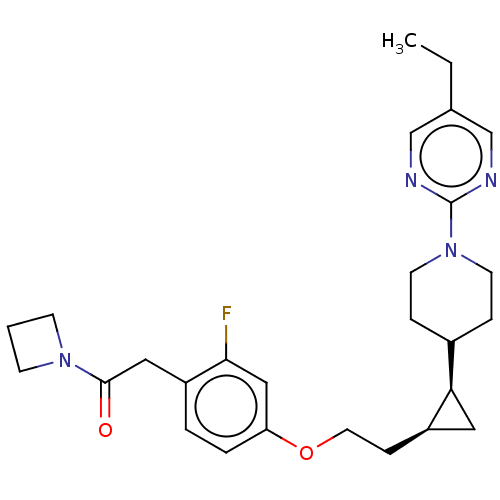
(CHEMBL4098051)Show SMILES CCc1cnc(nc1)N1CCC(CC1)[C@H]1C[C@H]1CCOc1ccc(CC(=O)N2CCC2)c(F)c1 |r| Show InChI InChI=1S/C27H35FN4O2/c1-2-19-17-29-27(30-18-19)32-11-6-20(7-12-32)24-14-21(24)8-13-34-23-5-4-22(25(28)16-23)15-26(33)31-9-3-10-31/h4-5,16-18,20-21,24H,2-3,6-15H2,1H3/t21-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1124-1128 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.091
BindingDB Entry DOI: 10.7270/Q2XK8HSD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50232626
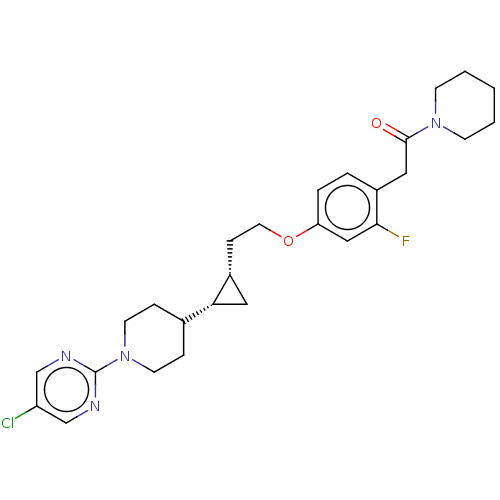
(CHEMBL4070965)Show SMILES Fc1cc(OCC[C@@H]2C[C@@H]2C2CCN(CC2)c2ncc(Cl)cn2)ccc1CC(=O)N1CCCCC1 |r| Show InChI InChI=1S/C27H34ClFN4O2/c28-22-17-30-27(31-18-22)33-11-6-19(7-12-33)24-14-20(24)8-13-35-23-5-4-21(25(29)16-23)15-26(34)32-9-2-1-3-10-32/h4-5,16-20,24H,1-3,6-15H2/t20-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1124-1128 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.091
BindingDB Entry DOI: 10.7270/Q2XK8HSD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50232631

(CHEMBL4104464)Show SMILES Fc1cc(OCC[C@@H]2C[C@@H]2C2CCN(CC2)c2ncc(Cl)cn2)ccc1CC(=O)N1CCC1 |r| Show InChI InChI=1S/C25H30ClFN4O2/c26-20-15-28-25(29-16-20)31-9-4-17(5-10-31)22-12-18(22)6-11-33-21-3-2-19(23(27)14-21)13-24(32)30-7-1-8-30/h2-3,14-18,22H,1,4-13H2/t18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1124-1128 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.091
BindingDB Entry DOI: 10.7270/Q2XK8HSD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50232624
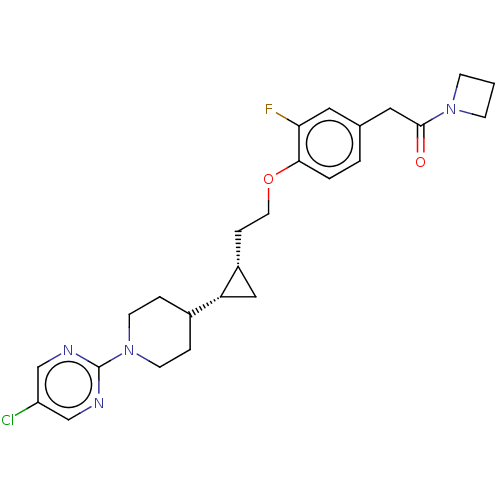
(CHEMBL4099186)Show SMILES Fc1cc(CC(=O)N2CCC2)ccc1OCC[C@@H]1C[C@@H]1C1CCN(CC1)c1ncc(Cl)cn1 |r| Show InChI InChI=1S/C25H30ClFN4O2/c26-20-15-28-25(29-16-20)31-9-4-18(5-10-31)21-14-19(21)6-11-33-23-3-2-17(12-22(23)27)13-24(32)30-7-1-8-30/h2-3,12,15-16,18-19,21H,1,4-11,13-14H2/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1124-1128 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.091
BindingDB Entry DOI: 10.7270/Q2XK8HSD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338817

(CHEMBL1684581 | N-(4-(((5S)-5-((R)-hydroxy(phenyl)...)Show SMILES O[C@@H]([C@@H]1CCC(Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28?,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338816

(CHEMBL1684580 | N-(4-(((5S)-5-((S)-hydroxy(phenyl)...)Show SMILES O[C@H]([C@@H]1CCC(Cc2ccc(NS(=O)(=O)c3ccc(cc3)-c3nc(cs3)-c3ccc(cc3)C(F)(F)F)cc2)N1)c1ccccc1 |r| Show InChI InChI=1S/C34H30F3N3O3S2/c35-34(36,37)26-12-8-23(9-13-26)31-21-44-33(39-31)25-10-17-29(18-11-25)45(42,43)40-27-14-6-22(7-15-27)20-28-16-19-30(38-28)32(41)24-4-2-1-3-5-24/h1-15,17-18,21,28,30,32,38,40-41H,16,19-20H2/t28?,30-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 21: 1865-70 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.087
BindingDB Entry DOI: 10.7270/Q27081R3 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478736
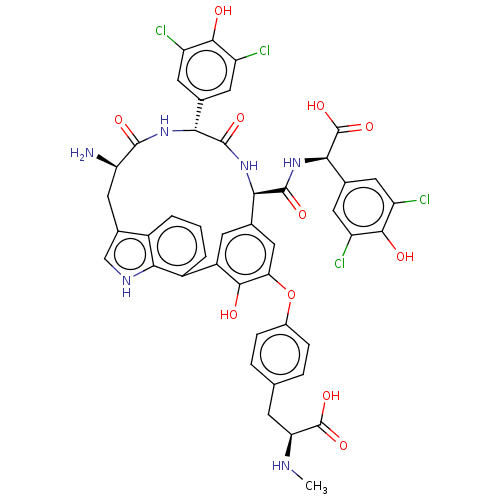
(CHEMBL505739)Show SMILES CN[C@@H](Cc1ccc(Oc2cc3cc(c2O)-c2cccc4c(C[C@@H](N)C(=O)N[C@H](c5cc(Cl)c(O)c(Cl)c5)C(=O)N[C@H]3C(=O)N[C@@H](C(O)=O)c3cc(Cl)c(O)c(Cl)c3)c[nH]c24)cc1)C(O)=O |r| Show InChI InChI=1S/C45H38Cl4N6O11/c1-51-32(44(62)63)9-18-5-7-23(8-6-18)66-33-16-19-10-26(38(33)56)25-4-2-3-24-22(17-52-37(24)25)15-31(50)41(59)53-35(20-11-27(46)39(57)28(47)12-20)42(60)54-34(19)43(61)55-36(45(64)65)21-13-29(48)40(58)30(49)14-21/h2-8,10-14,16-17,31-32,34-36,51-52,56-58H,9,15,50H2,1H3,(H,53,59)(H,54,60)(H,55,61)(H,62,63)(H,64,65)/t31-,32+,34-,35-,36-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123449

(CHEMBL437996 | Chloropeptin II | ISOCOMPLESTATIN)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-44(56(82)73-49(61(87)88)25-4-7-32(75)8-5-25)12-24-2-9-33(10-3-24)89-45-22-27-13-35(51(45)77)26-6-11-34-31(23-68-42(34)20-26)21-43(69-59(85)50(76)30-18-40(66)54(80)41(67)19-30)55(81)70-47(28-14-36(62)52(78)37(63)15-28)57(83)71-46(27)58(84)72-48(60(74)86)29-16-38(64)53(79)39(65)17-29/h2-11,13-20,22-23,43-44,46-49,68,75,77-80H,12,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146156
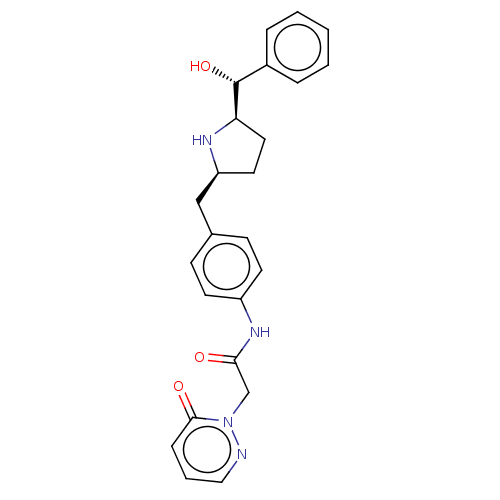
(CHEMBL3764088)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3ncccc3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C24H26N4O3/c29-22(16-28-23(30)7-4-14-25-28)27-19-10-8-17(9-11-19)15-20-12-13-21(26-20)24(31)18-5-2-1-3-6-18/h1-11,14,20-21,24,26,31H,12-13,15-16H2,(H,27,29)/t20-,21+,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50146162

(CHEMBL3763934)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)[C@@H]3CCc4cccc(=O)n34)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H29N3O3/c31-25-8-4-7-22-14-16-24(30(22)25)27(33)29-20-11-9-18(10-12-20)17-21-13-15-23(28-21)26(32)19-5-2-1-3-6-19/h1-12,21,23-24,26,28,32H,13-17H2,(H,29,33)/t21-,23+,24-,26+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine transporter |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50366855

(CHEMBL525803 | Chloropeptin)Show SMILES CN1[C@@H](Cc2ccc(Oc3cc4cc(c3O)-c3cccc5c(C[C@@H](NC(=O)C(=O)c6cc(Cl)c(O)c(Cl)c6)C(=O)N[C@H](c6cc(Cl)c(O)c(Cl)c6)C(=O)N[C@H]4C(=O)N[C@H](c4cc(Cl)c(O)c(Cl)c4)C1=O)c[nH]c35)cc2)C(=O)N[C@@H](C(O)=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-43(56(82)73-48(61(87)88)25-7-9-31(75)10-8-25)13-24-5-11-32(12-6-24)89-44-22-26-14-35(51(44)77)34-4-2-3-33-30(23-68-49(33)34)21-42(69-59(85)50(76)29-19-40(66)54(80)41(67)20-29)55(81)70-46(27-15-36(62)52(78)37(63)16-27)57(83)71-45(26)58(84)72-47(60(74)86)28-17-38(64)53(79)39(65)18-28/h2-12,14-20,22-23,42-43,45-48,68,75,77-80H,13,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88)/t42-,43+,45-,46-,47-,48-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50281513

(Acetic acid 9-((S)-1-acetoxy-3-methyl-butyl)-8-met...)Show SMILES COc1c(ccc2Oc3c(COC(=O)c12)cc(C)cc3OC(C)=O)[C@H](CC(C)C)OC(C)=O Show InChI InChI=1S/C25H28O8/c1-13(2)9-20(31-15(4)26)18-7-8-19-22(24(18)29-6)25(28)30-12-17-10-14(3)11-21(23(17)33-19)32-16(5)27/h7-8,10-11,13,20H,9,12H2,1-6H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit binding of Oxytocin to its Oxytocin receptor in rat uterine tissue |
Bioorg Med Chem Lett 3: 337-340 (1993)
Article DOI: 10.1016/S0960-894X(01)80905-7
BindingDB Entry DOI: 10.7270/Q28052J0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data