 Found 104 hits with Last Name = 'sano' and Initial = 'h'
Found 104 hits with Last Name = 'sano' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85790
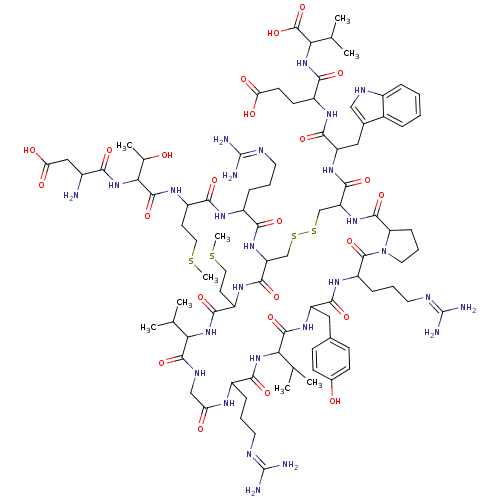
(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85789
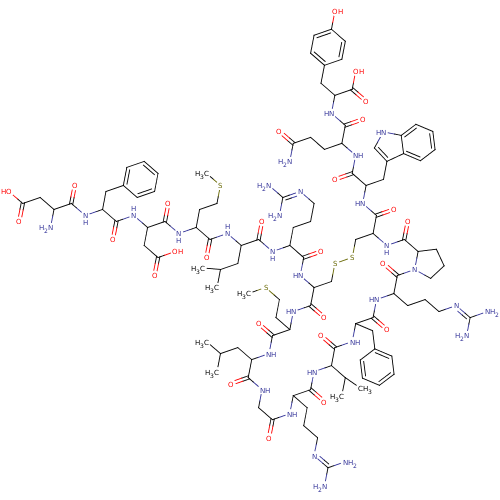
([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85789

([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85788
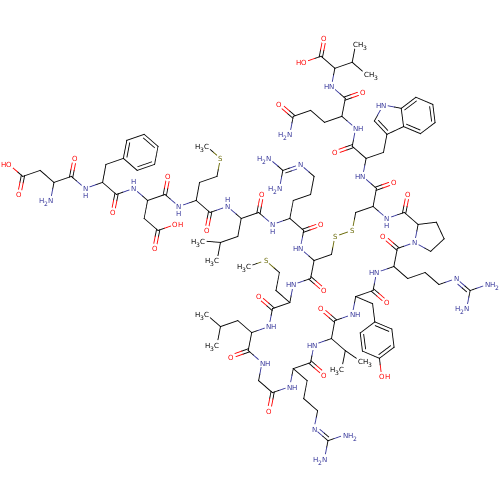
(MCH | hMCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C105H160N30O26S4/c1-53(2)42-70-86(144)118-50-80(138)119-64(24-16-36-114-103(108)109)90(148)133-83(55(5)6)100(158)130-73(45-58-28-30-60(136)31-29-58)93(151)124-69(26-18-38-116-105(112)113)101(159)135-39-19-27-78(135)99(157)132-77(98(156)128-74(46-59-49-117-63-23-15-14-22-61(59)63)95(153)121-66(32-33-79(107)137)91(149)134-84(56(7)8)102(160)161)52-165-164-51-76(97(155)123-68(35-41-163-10)88(146)126-70)131-87(145)65(25-17-37-115-104(110)111)120-92(150)71(43-54(3)4)127-89(147)67(34-40-162-9)122-96(154)75(48-82(141)142)129-94(152)72(44-57-20-12-11-13-21-57)125-85(143)62(106)47-81(139)140/h11-15,20-23,28-31,49,53-56,62,64-78,83-84,117,136H,16-19,24-27,32-48,50-52,106H2,1-10H3,(H2,107,137)(H,118,144)(H,119,138)(H,120,150)(H,121,153)(H,122,154)(H,123,155)(H,124,151)(H,125,143)(H,126,146)(H,127,147)(H,128,156)(H,129,152)(H,130,158)(H,131,145)(H,132,157)(H,133,148)(H,134,149)(H,139,140)(H,141,142)(H,160,161)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85790

(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Gallus gallus) | BDBM50145539

((2S,4R)-2,4-Dihydroxy-6-[2-[(R)-1-((R)-5-hydroxy-1...)Show SMILES C[C@H](CCCC(C)(C)O)C1CCC2C(CCC[C@]12C)=CCC1C[C@@H](O)CC(=O)C1=O |w:19.21| Show InChI InChI=1S/C26H42O4/c1-17(7-5-13-25(2,3)30)21-11-12-22-18(8-6-14-26(21,22)4)9-10-19-15-20(27)16-23(28)24(19)29/h9,17,19-22,27,30H,5-8,10-16H2,1-4H3/t17-,19?,20-,21?,22?,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 |
Bioorg Med Chem Lett 14: 2579-83 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.076
BindingDB Entry DOI: 10.7270/Q20C4V6Z |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Gallus gallus) | BDBM50145538

((S)-1-Benzyl-5-((R)-2-{(R)-4-[2-[(3S,5R)-3,5-dihyd...)Show SMILES C[C@H](C[C@H]1C[C@](C)(O)C(=O)N1Cc1ccccc1)C1CCC2\C(CCC[C@]12C)=C/C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C34H47NO4/c1-22(17-27-20-34(4,39)32(38)35(27)21-24-9-6-5-7-10-24)29-14-15-30-25(11-8-16-33(29,30)3)12-13-26-18-28(36)19-31(37)23(26)2/h5-7,9-10,12-13,22,27-31,36-37,39H,2,8,11,14-21H2,1,3-4H3/b25-12-,26-13+/t22-,27+,28-,29?,30?,31+,33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 |
Bioorg Med Chem Lett 14: 2579-83 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.076
BindingDB Entry DOI: 10.7270/Q20C4V6Z |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062250
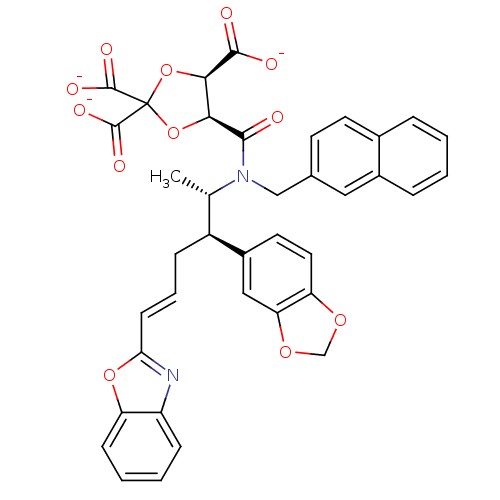
(CHEMBL36407 | trisodium 5-[2-benzo[d][1,3]dioxol-5...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@H]1OC(O[C@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062251
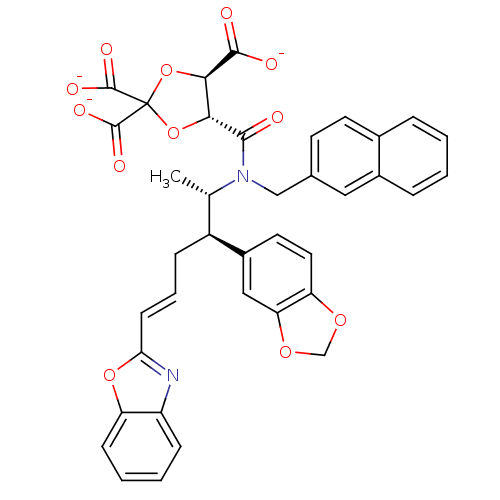
(CHEMBL285263 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249
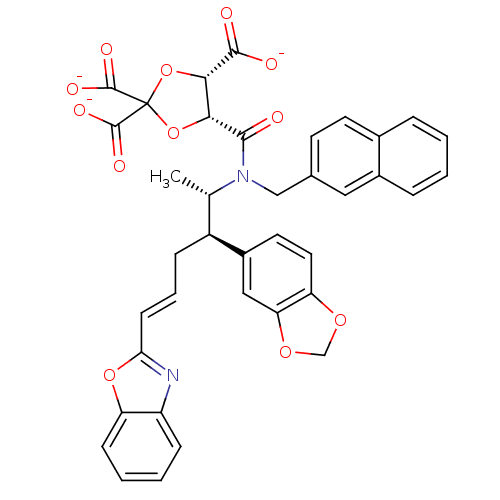
(CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249

(CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase in competitive manner with respect to FPP (farnesyl diphosphate) at 0.6 microM FPP and 0.36 microM Ras peptide |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062252
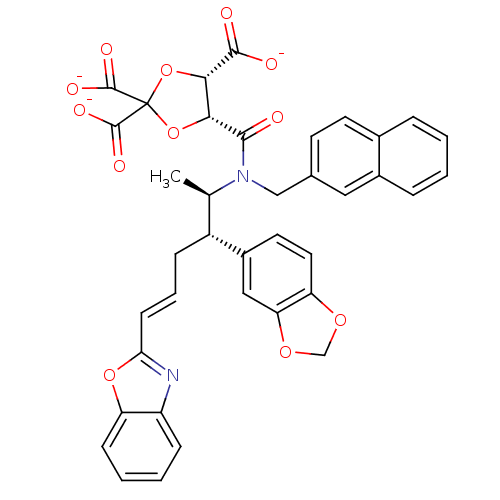
(CHEMBL36339 | trisodium 5-[2-benzo[d][1,3]dioxol-5...)Show SMILES C[C@H]([C@@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32-,33+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062248
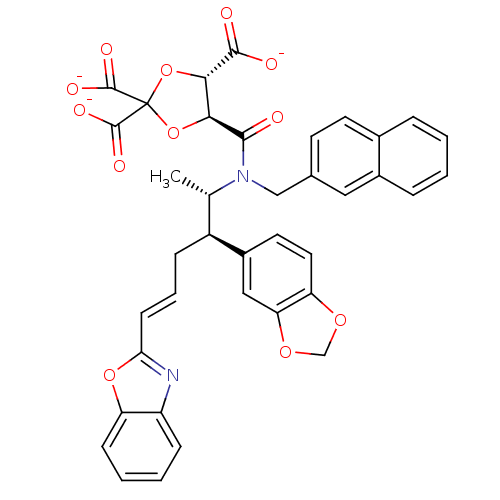
(CHEMBL36515 | trisodium 5-[2-benzo[d][1,3]dioxol-5...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32-,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249

(CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase in competitive manner with respect to FPP (farnesyl diphosphate) at 0.6 microM FPP and 3.6 microM Ras peptide. |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062256
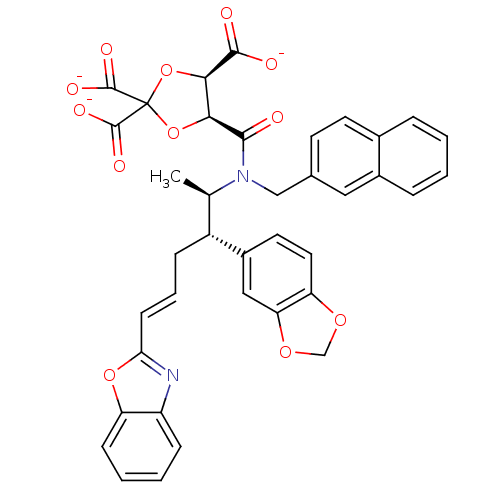
(CHEMBL284073 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@H]([C@@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@H]1OC(O[C@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Gallus gallus) | BDBM50145540
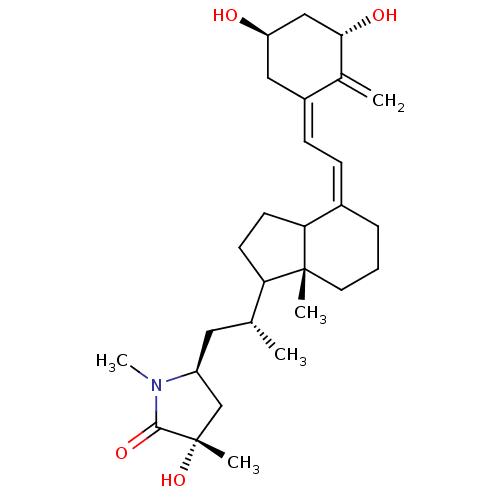
((S)-5-((R)-2-{(R)-4-[2-[(3S,5R)-3,5-Dihydroxy-2-me...)Show SMILES C[C@H](C[C@H]1C[C@](C)(O)C(=O)N1C)C1CCC2\C(CCC[C@]12C)=C/C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C28H43NO4/c1-17(13-21-16-28(4,33)26(32)29(21)5)23-10-11-24-19(7-6-12-27(23,24)3)8-9-20-14-22(30)15-25(31)18(20)2/h8-9,17,21-25,30-31,33H,2,6-7,10-16H2,1,3-5H3/b19-8-,20-9+/t17-,21+,22-,23?,24?,25+,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 |
Bioorg Med Chem Lett 14: 2579-83 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.076
BindingDB Entry DOI: 10.7270/Q20C4V6Z |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062258
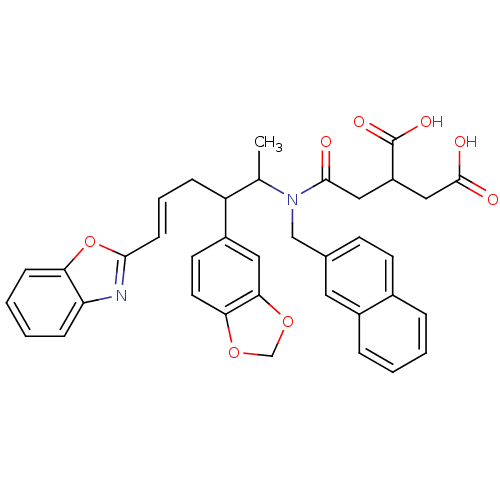
(2-{[((E)-2-Benzo[1,3]dioxol-5-yl-5-benzooxazol-2-y...)Show SMILES CC(C(C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)CC(CC(O)=O)C(O)=O Show InChI InChI=1S/C37H34N2O8/c1-23(39(35(40)19-28(37(43)44)20-36(41)42)21-24-13-14-25-7-2-3-8-26(25)17-24)29(27-15-16-32-33(18-27)46-22-45-32)9-6-12-34-38-30-10-4-5-11-31(30)47-34/h2-8,10-18,23,28-29H,9,19-22H2,1H3,(H,41,42)(H,43,44)/b12-6+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062257
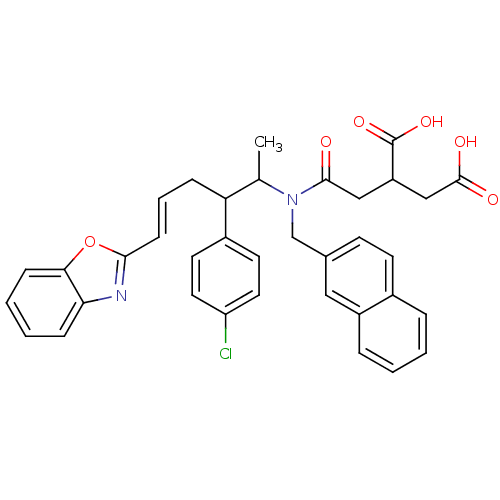
(2-({[(E)-5-Benzooxazol-2-yl-2-(4-chloro-phenyl)-1-...)Show SMILES CC(C(C\C=C\c1nc2ccccc2o1)c1ccc(Cl)cc1)N(Cc1ccc2ccccc2c1)C(=O)CC(CC(O)=O)C(O)=O Show InChI InChI=1S/C36H33ClN2O6/c1-23(30(26-15-17-29(37)18-16-26)9-6-12-33-38-31-10-4-5-11-32(31)45-33)39(34(40)20-28(36(43)44)21-35(41)42)22-24-13-14-25-7-2-3-8-27(25)19-24/h2-8,10-19,23,28,30H,9,20-22H2,1H3,(H,41,42)(H,43,44)/b12-6+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Gallus gallus) | BDBM50404242

(CHEMBL2114211)Show SMILES C[C@H](C[C@@H]1C[C@@](C)(O)C(=O)N1Cc1ccccc1)C1CCC2\C(CCC[C@]12C)=C/C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C34H47NO4/c1-22(17-27-20-34(4,39)32(38)35(27)21-24-9-6-5-7-10-24)29-14-15-30-25(11-8-16-33(29,30)3)12-13-26-18-28(36)19-31(37)23(26)2/h5-7,9-10,12-13,22,27-31,36-37,39H,2,8,11,14-21H2,1,3-4H3/b25-12-,26-13+/t22-,27-,28-,29?,30?,31+,33-,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 |
Bioorg Med Chem Lett 14: 2579-83 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.076
BindingDB Entry DOI: 10.7270/Q20C4V6Z |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249

(CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of FTase in competitive manner with respect to FPP (farnesyl diphosphate) at 6.0 microM FPP and 0.36 microM Ras peptide. |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Gallus gallus) | BDBM50404243
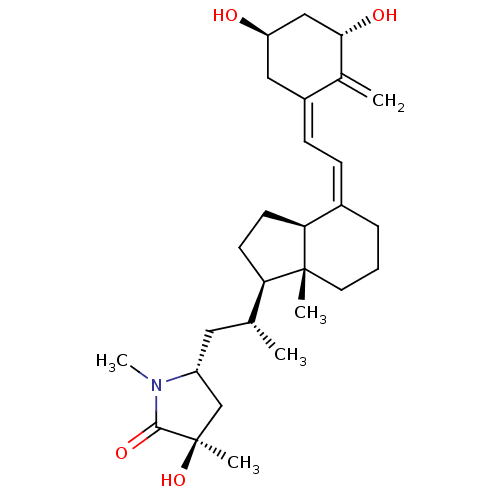
(CHEMBL2114212 | CHEMBL3350688)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C/C=C1\C[C@@H](O)C[C@H](O)C1=C)[C@H](C)C[C@@H]1C[C@@](C)(O)C(=O)N1C Show InChI InChI=1S/C28H43NO4/c1-17(13-21-16-28(4,33)26(32)29(21)5)23-10-11-24-19(7-6-12-27(23,24)3)8-9-20-14-22(30)15-25(31)18(20)2/h8-9,17,21-25,30-31,33H,2,6-7,10-16H2,1,3-5H3/b19-8-,20-9+/t17-,21-,22-,23?,24?,25+,27-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 |
Bioorg Med Chem Lett 14: 2579-83 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.076
BindingDB Entry DOI: 10.7270/Q20C4V6Z |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062253
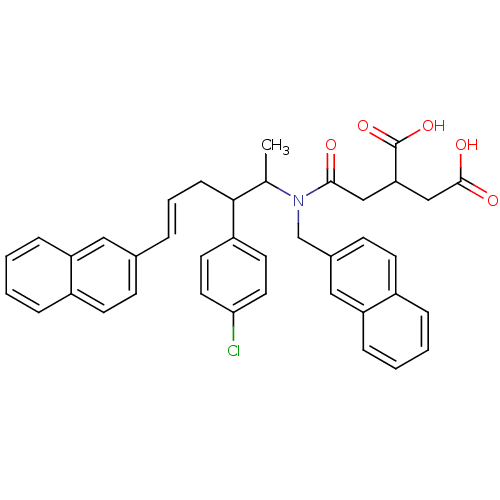
(2-({[(E)-2-(4-Chloro-phenyl)-1-methyl-5-naphthalen...)Show SMILES CC(C(C\C=C\c1ccc2ccccc2c1)c1ccc(Cl)cc1)N(Cc1ccc2ccccc2c1)C(=O)CC(CC(O)=O)C(O)=O Show InChI InChI=1S/C39H36ClNO5/c1-26(36(31-17-19-35(40)20-18-31)12-6-7-27-13-15-29-8-2-4-10-32(29)21-27)41(37(42)23-34(39(45)46)24-38(43)44)25-28-14-16-30-9-3-5-11-33(30)22-28/h2-11,13-22,26,34,36H,12,23-25H2,1H3,(H,43,44)(H,45,46)/b7-6+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Gallus gallus) | BDBM50223417
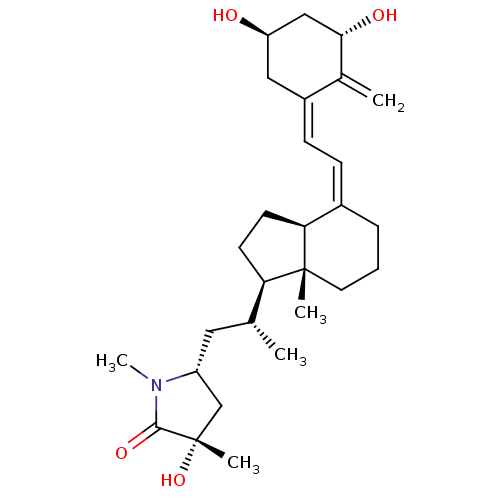
(CHEMBL3350292)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C/C=C1\C[C@@H](O)C[C@H](O)C1=C)[C@H](C)C[C@@H]1C[C@](C)(O)C(=O)N1C Show InChI InChI=1S/C28H43NO4/c1-17(13-21-16-28(4,33)26(32)29(21)5)23-10-11-24-19(7-6-12-27(23,24)3)8-9-20-14-22(30)15-25(31)18(20)2/h8-9,17,21-25,30-31,33H,2,6-7,10-16H2,1,3-5H3/b19-8-,20-9+/t17-,21-,22-,23-,24+,25+,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 |
Bioorg Med Chem Lett 14: 2579-83 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.076
BindingDB Entry DOI: 10.7270/Q20C4V6Z |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062255
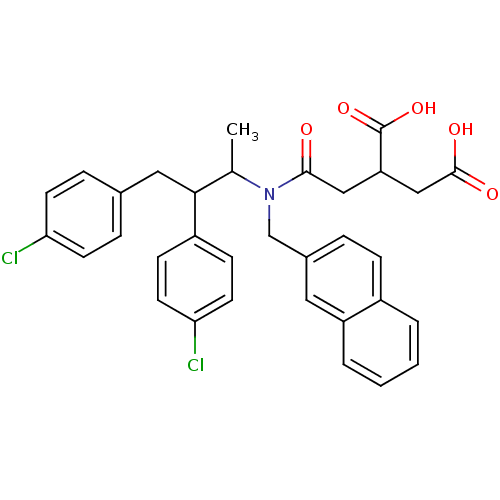
(2-({[2,3-Bis-(4-chloro-phenyl)-1-methyl-propyl]-na...)Show SMILES CC(C(Cc1ccc(Cl)cc1)c1ccc(Cl)cc1)N(Cc1ccc2ccccc2c1)C(=O)CC(CC(O)=O)C(O)=O Show InChI InChI=1S/C33H31Cl2NO5/c1-21(30(25-10-14-29(35)15-11-25)17-22-7-12-28(34)13-8-22)36(31(37)18-27(33(40)41)19-32(38)39)20-23-6-9-24-4-2-3-5-26(24)16-23/h2-16,21,27,30H,17-20H2,1H3,(H,38,39)(H,40,41) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062254
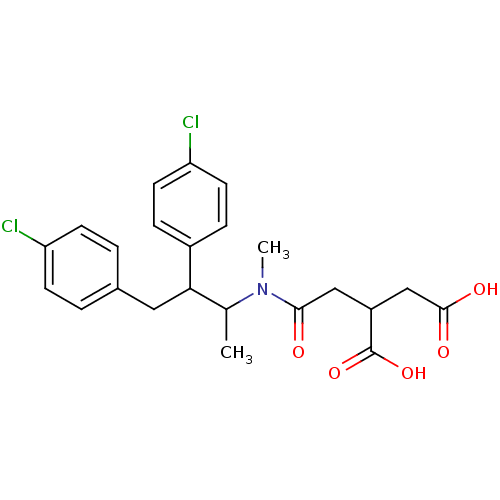
(2-({[2,3-Bis-(4-chloro-phenyl)-1-methyl-propyl]-me...)Show SMILES CC(C(Cc1ccc(Cl)cc1)c1ccc(Cl)cc1)N(C)C(=O)CC(CC(O)=O)C(O)=O Show InChI InChI=1S/C23H25Cl2NO5/c1-14(26(2)21(27)12-17(23(30)31)13-22(28)29)20(16-5-9-19(25)10-6-16)11-15-3-7-18(24)8-4-15/h3-10,14,17,20H,11-13H2,1-2H3,(H,28,29)(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620495
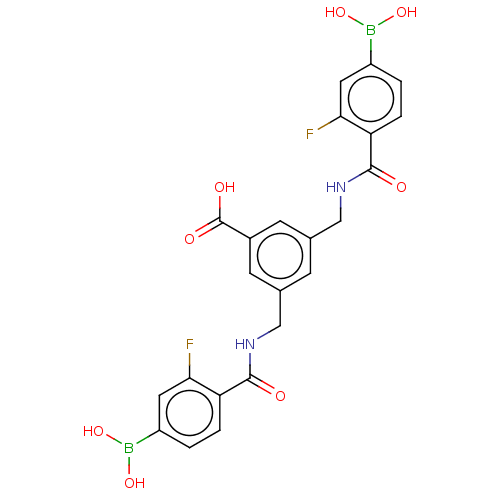
(3,5-Bis((4-borono-2-fluorobenzamido)methyl)benzoic...)Show SMILES OB(O)c1ccc(C(=O)NCc2cc(CNC(=O)c3ccc(cc3F)B(O)O)cc(c2)C(O)=O)c(F)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 3.10E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620495

(3,5-Bis((4-borono-2-fluorobenzamido)methyl)benzoic...)Show SMILES OB(O)c1ccc(C(=O)NCc2cc(CNC(=O)c3ccc(cc3F)B(O)O)cc(c2)C(O)=O)c(F)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 8.60E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620496

(3,5-Bis((4-borono-3-fluorobenzamido)methyl)benzoic...)Show SMILES OB(O)c1ccc(cc1F)C(=O)NCc1cc(CNC(=O)c2ccc(B(O)O)c(F)c2)cc(c1)C(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 3.40E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620496

(3,5-Bis((4-borono-3-fluorobenzamido)methyl)benzoic...)Show SMILES OB(O)c1ccc(cc1F)C(=O)NCc1cc(CNC(=O)c2ccc(B(O)O)c(F)c2)cc(c1)C(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 5.40E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620497

(N,N′-bis(4-borono-3-fluorobenzamido)-N-ethyl...)Show SMILES NC(=O)CN(CCNC(=O)c1ccc(B(O)O)c(F)c1)C(=O)c1ccc(B(O)O)c(F)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 2.70E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620497

(N,N′-bis(4-borono-3-fluorobenzamido)-N-ethyl...)Show SMILES NC(=O)CN(CCNC(=O)c1ccc(B(O)O)c(F)c1)C(=O)c1ccc(B(O)O)c(F)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 1.60E+7 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620498

((S)-2,4-bis(4-borono-3-fluorobenzamido)butanoic ac...)Show SMILES OB(O)c1ccc(cc1F)C(=O)NCC[C@H](NC(=O)c1ccc(B(O)O)c(F)c1)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 2.60E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620498

((S)-2,4-bis(4-borono-3-fluorobenzamido)butanoic ac...)Show SMILES OB(O)c1ccc(cc1F)C(=O)NCC[C@H](NC(=O)c1ccc(B(O)O)c(F)c1)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 6.50E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620499
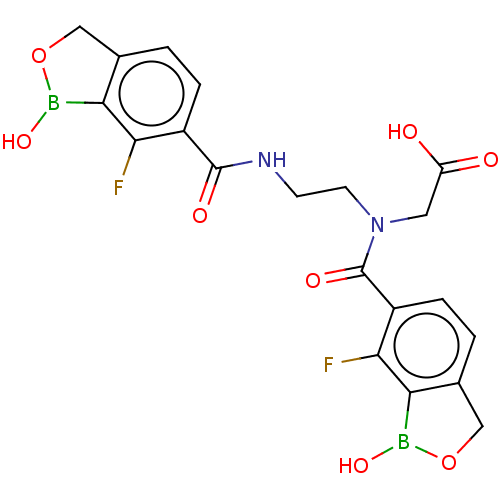
(N-(7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxab...)Show SMILES OB1OCc2ccc(C(=O)NCCN(CC(O)=O)C(=O)c3ccc4COB(O)c4c3F)c(F)c12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 8.00E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620499

(N-(7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxab...)Show SMILES OB1OCc2ccc(C(=O)NCCN(CC(O)=O)C(=O)c3ccc4COB(O)c4c3F)c(F)c12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 4.00E+7 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620500

(3,5-Bis((7-fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,...)Show SMILES OB1OCc2ccc(C(=O)NCc3cc(CNC(=O)c4ccc5COB(O)c5c4F)cc(c3)C(O)=O)c(F)c12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620501

(N-(5-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxab...)Show SMILES OB1OCc2cc(F)c(cc12)C(=O)NCCN(CC(O)=O)C(=O)c1cc2B(O)OCc2cc1F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 1.50E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620501

(N-(5-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxab...)Show SMILES OB1OCc2cc(F)c(cc12)C(=O)NCCN(CC(O)=O)C(=O)c1cc2B(O)OCc2cc1F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 2.41E+7 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620502
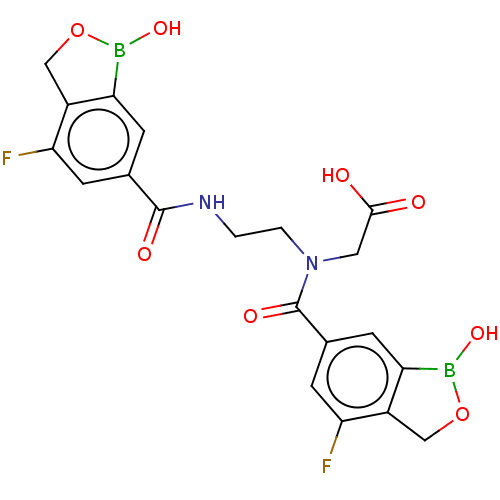
(N-(4-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxab...)Show SMILES OB1OCc2c1cc(cc2F)C(=O)NCCN(CC(O)=O)C(=O)c1cc2B(O)OCc2c(F)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 2.70E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620502

(N-(4-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxab...)Show SMILES OB1OCc2c1cc(cc2F)C(=O)NCCN(CC(O)=O)C(=O)c1cc2B(O)OCc2c(F)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 3.40E+7 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620503

(N-(6-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxab...)Show SMILES OB1OCc2cc(C(=O)NCCN(CC(O)=O)C(=O)c3cc4COB(O)c4cc3F)c(F)cc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 2.60E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620503

(N-(6-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxab...)Show SMILES OB1OCc2cc(C(=O)NCCN(CC(O)=O)C(=O)c3cc4COB(O)c4cc3F)c(F)cc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 6.79E+7 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620504

(N2,N6-Bis(6-fluoro-1-hydroxy-1,3-dihydrobenzo[c][1...)Show SMILES OB1OCc2cc(C(=O)NCCCC[C@H](NC(=O)c3cc4COB(O)c4cc3F)C(O)=O)c(F)cc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 3.20E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620504

(N2,N6-Bis(6-fluoro-1-hydroxy-1,3-dihydrobenzo[c][1...)Show SMILES OB1OCc2cc(C(=O)NCCCC[C@H](NC(=O)c3cc4COB(O)c4cc3F)C(O)=O)c(F)cc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 3.52E+8 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620505
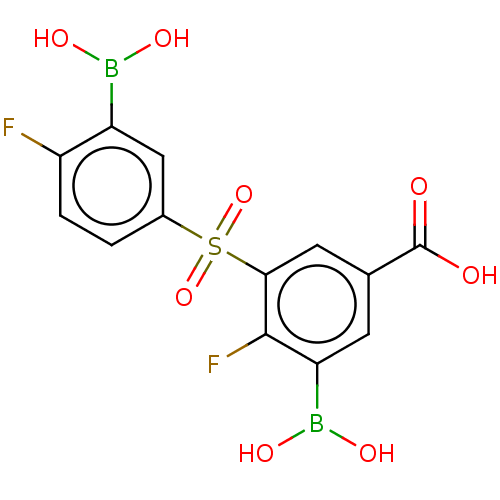
(3-Borono-5-((3-borono-4-fluorophenyl)sulfonyl)-4-f...)Show SMILES OB(O)c1cc(ccc1F)S(=O)(=O)c1cc(cc(B(O)O)c1F)C(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 1.80E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620505

(3-Borono-5-((3-borono-4-fluorophenyl)sulfonyl)-4-f...)Show SMILES OB(O)c1cc(ccc1F)S(=O)(=O)c1cc(cc(B(O)O)c1F)C(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 1.70E+7 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620506

(3-Borono-5-(3-borono-5-fluorobenzoyl)benzoic acid ...)Show SMILES OB(O)c1cc(F)cc(c1)C(=O)c1cc(cc(c1)C(O)=O)B(O)O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620506

(3-Borono-5-(3-borono-5-fluorobenzoyl)benzoic acid ...)Show SMILES OB(O)c1cc(F)cc(c1)C(=O)c1cc(cc(c1)C(O)=O)B(O)O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 1.80E+7 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620507
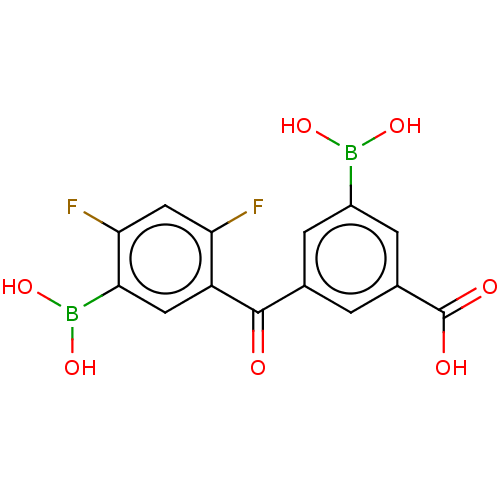
(3-Borono-5-(5-borono-2,4-difluorobenzoyl)benzoic a...)Show SMILES OB(O)c1cc(cc(c1)C(=O)c1cc(B(O)O)c(F)cc1F)C(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM620507

(3-Borono-5-(5-borono-2,4-difluorobenzoyl)benzoic a...)Show SMILES OB(O)c1cc(cc(c1)C(=O)c1cc(B(O)O)c(F)cc1F)C(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 4.00E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data