 Found 2895 hits with Last Name = 'sata?a' and Initial = 'g'
Found 2895 hits with Last Name = 'sata?a' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50010044
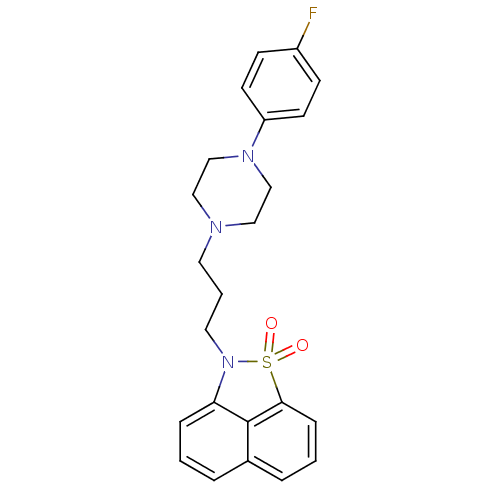
(2-{3-[4-(4-Fluoro-phenyl)-piperazin-1-yl]-propyl}-...)Show SMILES Fc1ccc(cc1)N1CCN(CCCN2c3cccc4cccc(c34)S2(=O)=O)CC1 Show InChI InChI=1S/C23H24FN3O2S/c24-19-8-10-20(11-9-19)26-16-14-25(15-17-26)12-3-13-27-21-6-1-4-18-5-2-7-22(23(18)21)30(27,28)29/h1-2,4-11H,3,12-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cracow University of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in rat cortical membranes incubated for 15 mins by liquid scintillation spectrometry |
Bioorg Med Chem 27: 3396-3407 (2019)
Article DOI: 10.1016/j.bmc.2019.06.028
BindingDB Entry DOI: 10.7270/Q20K2D06 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM22416
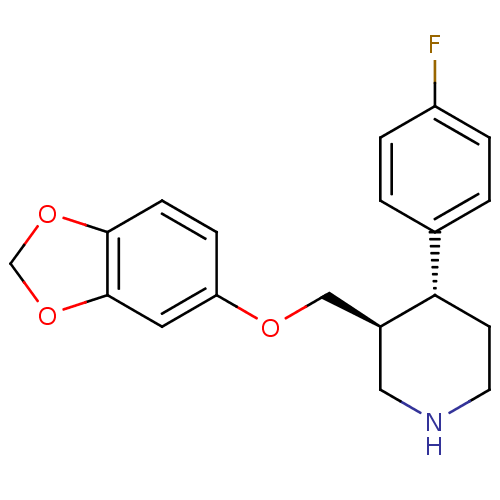
((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]RTI55 binding from human wild type SERT |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM194780
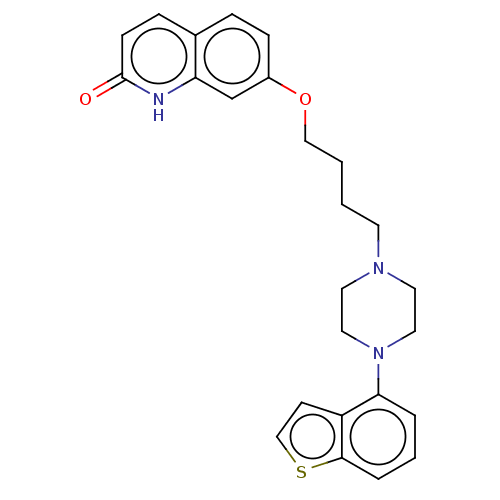
(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cracow University of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from 5HT1A receptor in rat brain hippocampus incubated for 60 mins by radioligand binding assay |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126667
BindingDB Entry DOI: 10.7270/Q2FF3WM3 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50462179
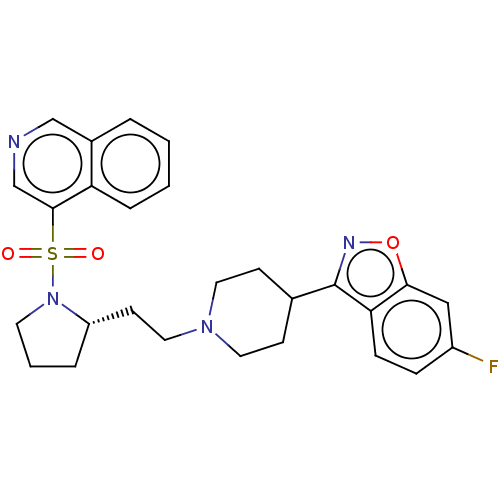
(CHEMBL4245263)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CC[C@@H]2CCCN2S(=O)(=O)c2cncc3ccccc23)CC1 |r| Show InChI InChI=1S/C27H29FN4O3S/c28-21-7-8-24-25(16-21)35-30-27(24)19-9-13-31(14-10-19)15-11-22-5-3-12-32(22)36(33,34)26-18-29-17-20-4-1-2-6-23(20)26/h1-2,4,6-8,16-19,22H,3,5,9-15H2/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50462156
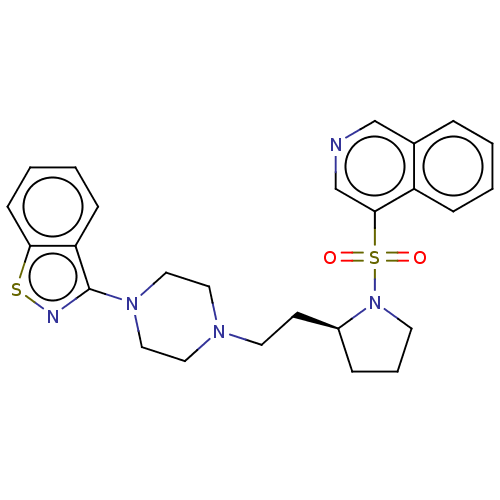
(CHEMBL4246655)Show SMILES O=S(=O)(N1CCC[C@H]1CCN1CCN(CC1)c1nsc2ccccc12)c1cncc2ccccc12 |r| Show InChI InChI=1S/C26H29N5O2S2/c32-35(33,25-19-27-18-20-6-1-2-8-22(20)25)31-12-5-7-21(31)11-13-29-14-16-30(17-15-29)26-23-9-3-4-10-24(23)34-28-26/h1-4,6,8-10,18-19,21H,5,7,11-17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50291284
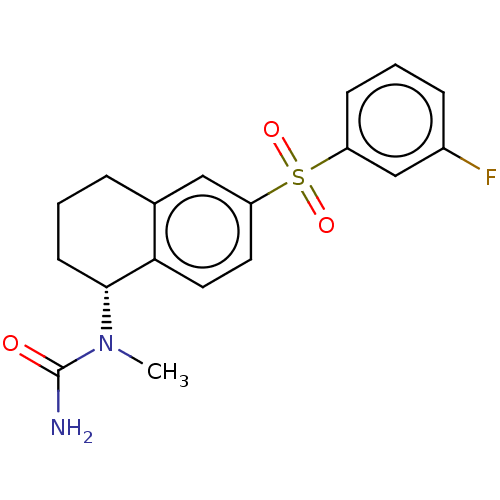
(CHEMBL4170220)Show SMILES CN([C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1)C(N)=O |r| Show InChI InChI=1S/C18H19FN2O3S/c1-21(18(20)22)17-7-2-4-12-10-15(8-9-16(12)17)25(23,24)14-6-3-5-13(19)11-14/h3,5-6,8-11,17H,2,4,7H2,1H3,(H2,20,22)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(RAT) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]Lu AE60157 from rat brain 5-HT6 receptor |
ACS Med Chem Lett 7: 618-22 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00056
BindingDB Entry DOI: 10.7270/Q2ST7T9N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(RAT) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]Lu AE60157 from rat brain 5-HT6 receptor |
ACS Med Chem Lett 7: 618-22 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00056
BindingDB Entry DOI: 10.7270/Q2ST7T9N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| DrugBank
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50291286
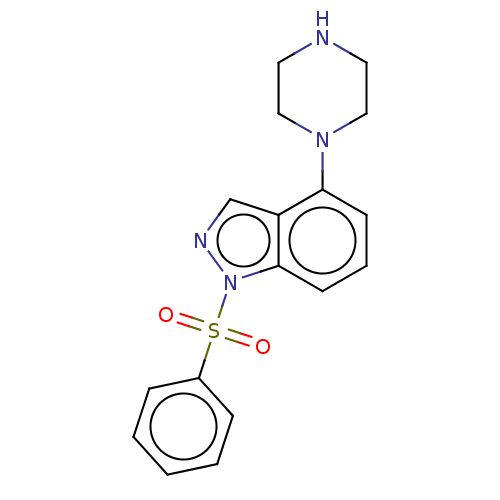
(CHEMBL4169827)Show InChI InChI=1S/C17H18N4O2S/c22-24(23,14-5-2-1-3-6-14)21-17-8-4-7-16(15(17)13-19-21)20-11-9-18-10-12-20/h1-8,13,18H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50462155
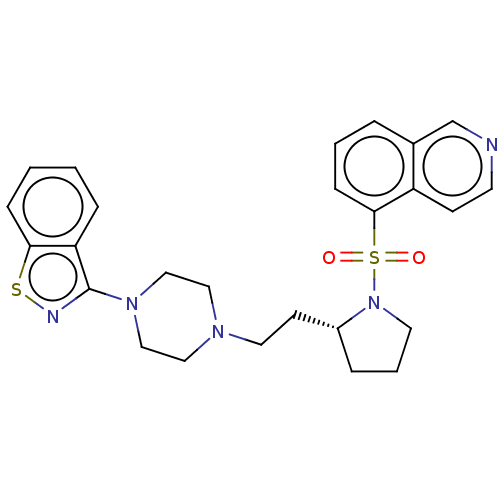
(CHEMBL4239091)Show SMILES O=S(=O)(N1CCC[C@@H]1CCN1CCN(CC1)c1nsc2ccccc12)c1cccc2cnccc12 |r| Show InChI InChI=1S/C26H29N5O2S2/c32-35(33,25-9-3-5-20-19-27-12-10-22(20)25)31-13-4-6-21(31)11-14-29-15-17-30(18-16-29)26-23-7-1-2-8-24(23)34-28-26/h1-3,5,7-10,12,19,21H,4,6,11,13-18H2/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by microbeta plate reader method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112765
BindingDB Entry DOI: 10.7270/Q2ZC86K7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2BR (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50462179

(CHEMBL4245263)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CC[C@@H]2CCCN2S(=O)(=O)c2cncc3ccccc23)CC1 |r| Show InChI InChI=1S/C27H29FN4O3S/c28-21-7-8-24-25(16-21)35-30-27(24)19-9-13-31(14-10-19)15-11-22-5-3-12-32(22)36(33,34)26-18-29-17-20-4-1-2-6-23(20)26/h1-2,4,6-8,16-19,22H,3,5,9-15H2/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Ketanserin from human 5-HT2A receptor expressed in rat cortex tissue incubated for 30 mins by liquid scintillation counting meth... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50443101
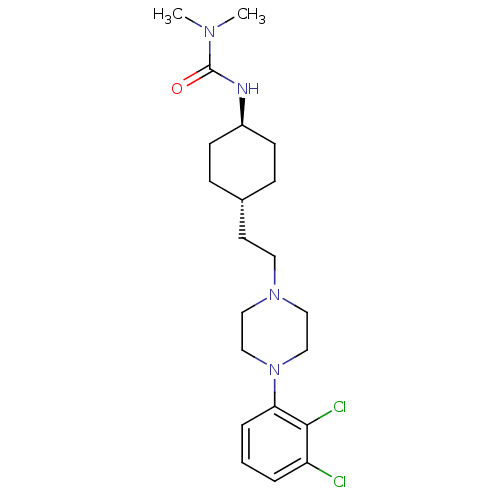
(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ketanserin from human 5-HT2AR expressed in CHO-K1 cell membranes after 1.5 hrs by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50462148

(CHEMBL4242392)Show SMILES O=S(=O)(N1CCC[C@H]1CCN1CCN(CC1)c1nsc2ccccc12)c1cccc2cnccc12 |r| Show InChI InChI=1S/C26H29N5O2S2/c32-35(33,25-9-3-5-20-19-27-12-10-22(20)25)31-13-4-6-21(31)11-14-29-15-17-30(18-16-29)26-23-7-1-2-8-24(23)34-28-26/h1-3,5,7-10,12,19,21H,4,6,11,13-18H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM85222
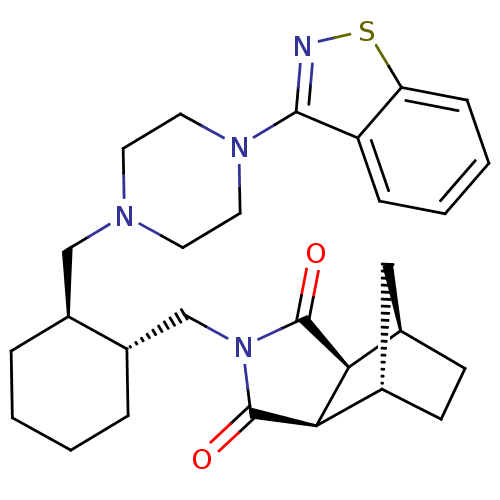
(CAS_441351-20-8 | Lurasidone | SM 13496)Show SMILES O=C1[C@H]2[C@@H]3CC[C@@H](C3)[C@H]2C(=O)N1C[C@@H]1CCCC[C@H]1CN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C28H36N4O2S/c33-27-24-18-9-10-19(15-18)25(24)28(34)32(27)17-21-6-2-1-5-20(21)16-30-11-13-31(14-12-30)26-22-7-3-4-8-23(22)35-29-26/h3-4,7-8,18-21,24-25H,1-2,5-6,9-17H2/t18-,19+,20-,21-,24+,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5HT7 receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM85222

(CAS_441351-20-8 | Lurasidone | SM 13496)Show SMILES O=C1[C@H]2[C@@H]3CC[C@@H](C3)[C@H]2C(=O)N1C[C@@H]1CCCC[C@H]1CN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C28H36N4O2S/c33-27-24-18-9-10-19(15-18)25(24)28(34)32(27)17-21-6-2-1-5-20(21)16-30-11-13-31(14-12-30)26-22-7-3-4-8-23(22)35-29-26/h3-4,7-8,18-21,24-25H,1-2,5-6,9-17H2/t18-,19+,20-,21-,24+,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cracow University of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human recombinant 5HT7b receptor measured after 2 hrs |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115459
BindingDB Entry DOI: 10.7270/Q2D50RJB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044615

(CHEMBL3329435)Show InChI InChI=1S/C18H20N4O2S/c1-14-20-18-16(21-12-10-19-11-13-21)8-5-9-17(18)22(14)25(23,24)15-6-3-2-4-7-15/h2-9,19H,10-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02009
BindingDB Entry DOI: 10.7270/Q2JQ14RQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM82517
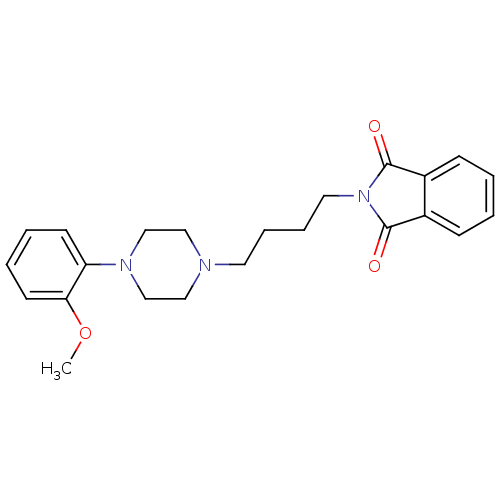
(2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)c3ccccc3C2=O)CC1 Show InChI InChI=1S/C23H27N3O3/c1-29-21-11-5-4-10-20(21)25-16-14-24(15-17-25)12-6-7-13-26-22(27)18-8-2-3-9-19(18)23(26)28/h2-5,8-11H,6-7,12-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113931
BindingDB Entry DOI: 10.7270/Q2PR810V |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50570641
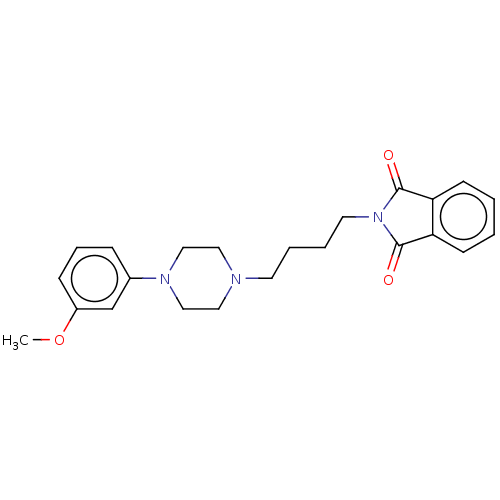
(CHEMBL4875097)Show SMILES COc1cccc(c1)N1CCN(CCCCN2C(=O)c3ccccc3C2=O)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-OH-DPAT from human 5-HT1AR expressed in human HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128028
BindingDB Entry DOI: 10.7270/Q2XK8KBD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
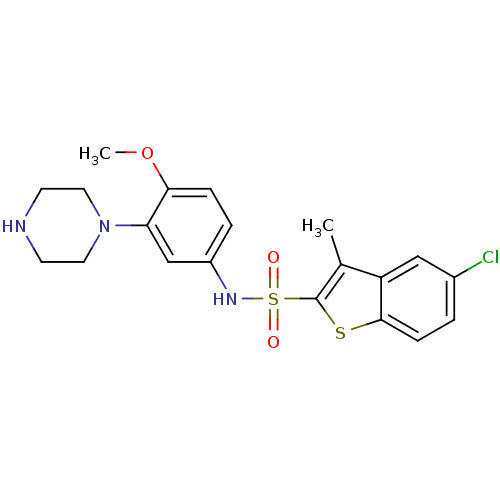
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Technology and Biotechnology of Drugs, Jagiellonian University, Medical College, Medyczna 9, PL 30-688 Kraków, Poland. Electronic address: dlazewska@cm-uj.krakow.pl.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in human HeLa cells after 1 hr by liquid scintillation counting method |
Eur J Med Chem 135: 117-124 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.033
BindingDB Entry DOI: 10.7270/Q2QN696C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50462142
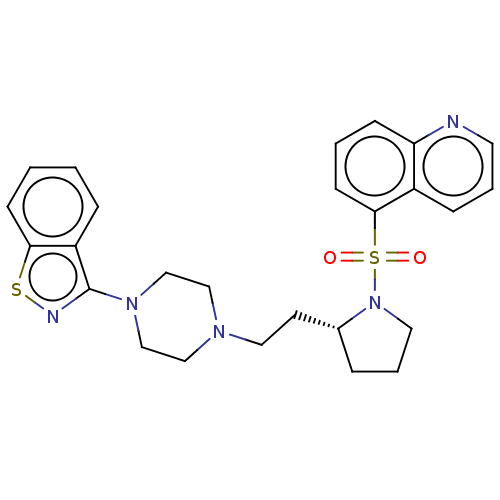
(CHEMBL4242858)Show SMILES O=S(=O)(N1CCC[C@@H]1CCN1CCN(CC1)c1nsc2ccccc12)c1cccc2ncccc12 |r| Show InChI InChI=1S/C26H29N5O2S2/c32-35(33,25-11-3-9-23-21(25)8-4-13-27-23)31-14-5-6-20(31)12-15-29-16-18-30(19-17-29)26-22-7-1-2-10-24(22)34-28-26/h1-4,7-11,13,20H,5-6,12,14-19H2/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50419052

(SB-399885)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)Nc1cc(Cl)cc(Cl)c1OC Show InChI InChI=1S/C18H21Cl2N3O4S/c1-26-17-4-3-13(11-16(17)23-7-5-21-6-8-23)28(24,25)22-15-10-12(19)9-14(20)18(15)27-2/h3-4,9-11,21-22H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00224
BindingDB Entry DOI: 10.7270/Q2X3528B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50419052

(SB-399885)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)Nc1cc(Cl)cc(Cl)c1OC Show InChI InChI=1S/C18H21Cl2N3O4S/c1-26-17-4-3-13(11-16(17)23-7-5-21-6-8-23)28(24,25)22-15-10-12(19)9-14(20)18(15)27-2/h3-4,9-11,21-22H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Technology and Biotechnology of Drugs, Jagiellonian University, Medical College, Medyczna 9, PL 30-688 Kraków, Poland. Electronic address: dlazewska@cm-uj.krakow.pl.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in human HeLa cells |
Eur J Med Chem 135: 117-124 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.033
BindingDB Entry DOI: 10.7270/Q2QN696C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM30708
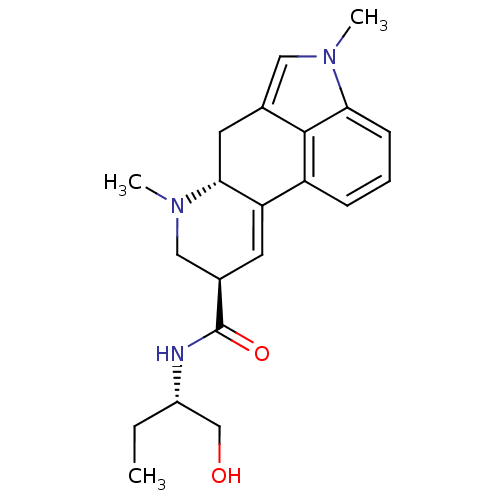
((6aR,9R)-4,7-dimethyl-N-[(1S)-1-methylolpropyl]-6,...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3cn(C)c4cccc(C2=C1)c34 |c:24| Show InChI InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2CR (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803
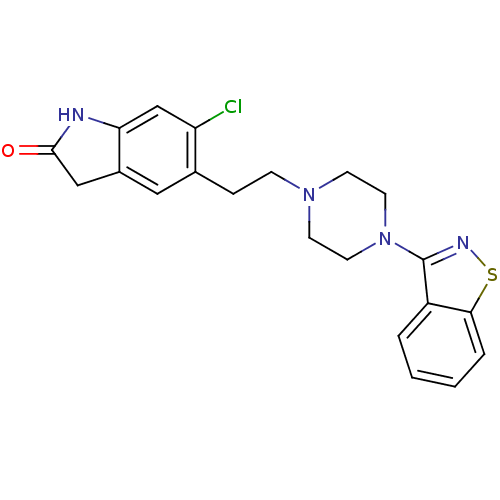
(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50019754
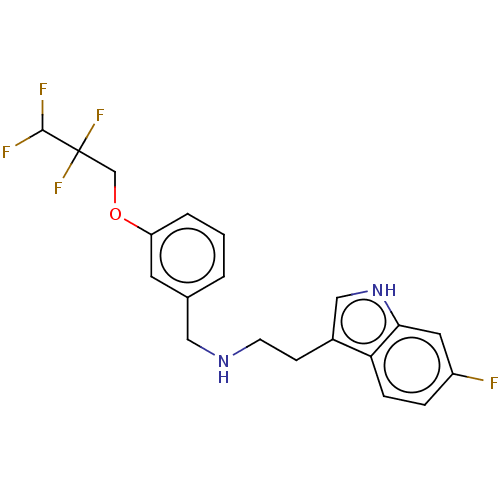
(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(RAT) | BDBM50019754

(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]Lu AE60157 from rat brain 5-HT6 receptor |
ACS Med Chem Lett 7: 618-22 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00056
BindingDB Entry DOI: 10.7270/Q2ST7T9N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(RAT) | BDBM50019754

(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]Lu AE60157 from rat brain 5-HT6 receptor |
ACS Med Chem Lett 7: 618-22 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00056
BindingDB Entry DOI: 10.7270/Q2ST7T9N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methylspiperone from human D2 long receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method |
Eur J Med Chem 179: 1-15 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.001
BindingDB Entry DOI: 10.7270/Q2S185TZ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methylspiperone from human D2 long receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method |
Eur J Med Chem 179: 1-15 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.001
BindingDB Entry DOI: 10.7270/Q2S185TZ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H] raclopride from human recombinant D2L receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting... |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50462152
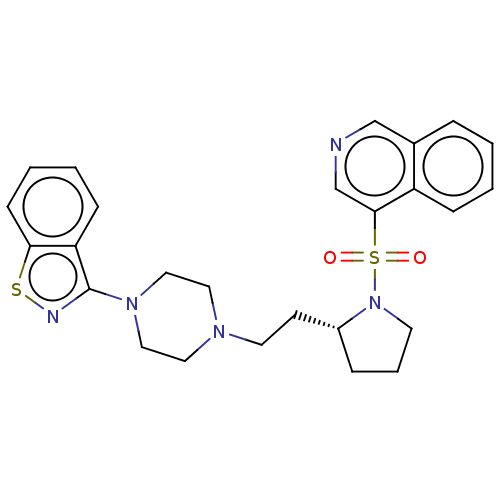
(CHEMBL4238679)Show SMILES O=S(=O)(N1CCC[C@@H]1CCN1CCN(CC1)c1nsc2ccccc12)c1cncc2ccccc12 |r| Show InChI InChI=1S/C26H29N5O2S2/c32-35(33,25-19-27-18-20-6-1-2-8-22(20)25)31-12-5-7-21(31)11-13-29-14-16-30(17-15-29)26-23-9-3-4-10-24(23)34-28-26/h1-4,6,8-10,18-19,21H,5,7,11-17H2/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50579346

(CHEMBL4867565)Show SMILES Fc1cccc(c1)S(=O)(=O)n1ccc2c(nc3ccccc3c12)N1CCNCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00224
BindingDB Entry DOI: 10.7270/Q2X3528B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50604800
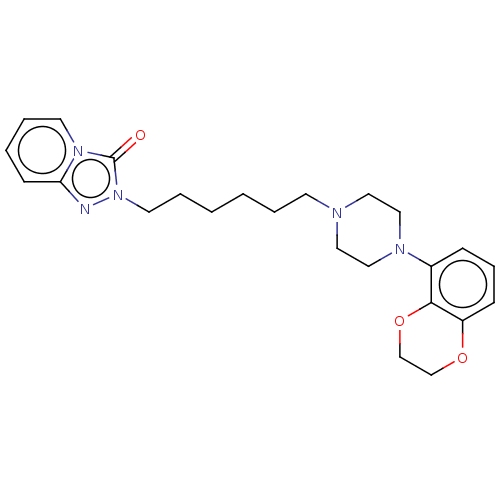
(CHEMBL5189101)Show SMILES O=c1n(CCCCCCN2CCN(CC2)c2cccc3OCCOc23)nc2ccccn12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114319
BindingDB Entry DOI: 10.7270/Q21V5K1X |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50545435
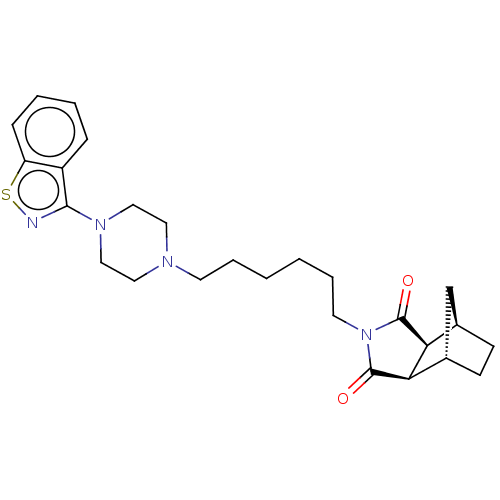
(CHEMBL4641609)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@]1([H])C(=O)N(CCCCCCN3CCN(CC3)c3nsc4ccccc34)C(=O)[C@]21[H] |r| Show InChI InChI=1S/C26H34N4O2S/c31-25-22-18-9-10-19(17-18)23(22)26(32)30(25)12-6-2-1-5-11-28-13-15-29(16-14-28)24-20-7-3-4-8-21(20)33-27-24/h3-4,7-8,18-19,22-23H,1-2,5-6,9-17H2/t18-,19+,22+,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cracow University of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115459
BindingDB Entry DOI: 10.7270/Q2D50RJB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50462155

(CHEMBL4239091)Show SMILES O=S(=O)(N1CCC[C@@H]1CCN1CCN(CC1)c1nsc2ccccc12)c1cccc2cnccc12 |r| Show InChI InChI=1S/C26H29N5O2S2/c32-35(33,25-9-3-5-20-19-27-12-10-22(20)25)31-13-4-6-21(31)11-14-29-15-17-30(18-16-29)26-23-7-1-2-8-24(23)34-28-26/h1-3,5,7-10,12,19,21H,4,6,11,13-18H2/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method |
Eur J Med Chem 145: 790-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.002
BindingDB Entry DOI: 10.7270/Q27947BS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50570644
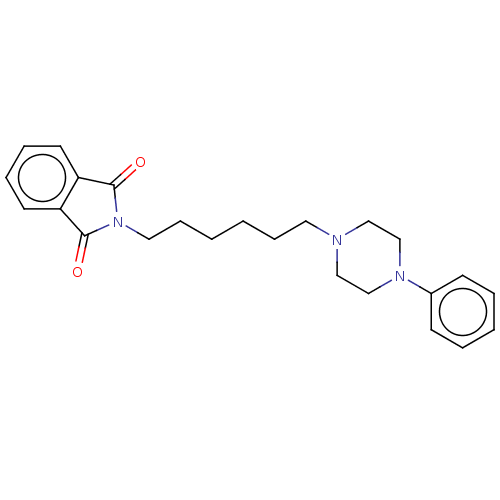
(CHEMBL4849844)Show SMILES O=C1N(CCCCCCN2CCN(CC2)c2ccccc2)C(=O)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-OH-DPAT from human 5-HT1AR expressed in human HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128028
BindingDB Entry DOI: 10.7270/Q2XK8KBD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50570657
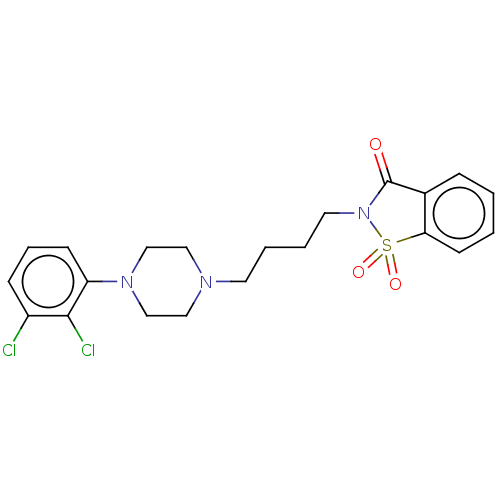
(CHEMBL4860626)Show SMILES Clc1cccc(N2CCN(CCCCN3C(=O)c4ccccc4S3(=O)=O)CC2)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-OH-DPAT from human 5-HT1AR expressed in human HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128028
BindingDB Entry DOI: 10.7270/Q2XK8KBD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50502805
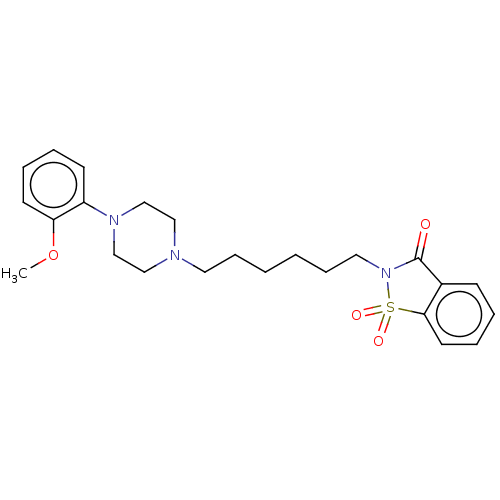
(CHEMBL4565558)Show SMILES COc1ccccc1N1CCN(CCCCCCN2C(=O)c3ccccc3S2(=O)=O)CC1 Show InChI InChI=1S/C24H31N3O4S/c1-31-22-12-6-5-11-21(22)26-18-16-25(17-19-26)14-8-2-3-9-15-27-24(28)20-10-4-7-13-23(20)32(27,29)30/h4-7,10-13H,2-3,8-9,14-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-raclopride from human D2R expressed in human HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128028
BindingDB Entry DOI: 10.7270/Q2XK8KBD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562710

(CHEMBL4759730)Show SMILES CN1CCN(CC1)c1cccc2n(ccc12)S(=O)(=O)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5-HT6 receptor incubated for 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112916
BindingDB Entry DOI: 10.7270/Q22R3WD3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data