 Found 78 hits with Last Name = 'shafi' and Initial = 's'
Found 78 hits with Last Name = 'shafi' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect cell expression system using TMPD and arachidonic acid as substrate incubated for 1 min prio... |
Eur J Med Chem 70: 579-88 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.032
BindingDB Entry DOI: 10.7270/Q2N87DQ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016433
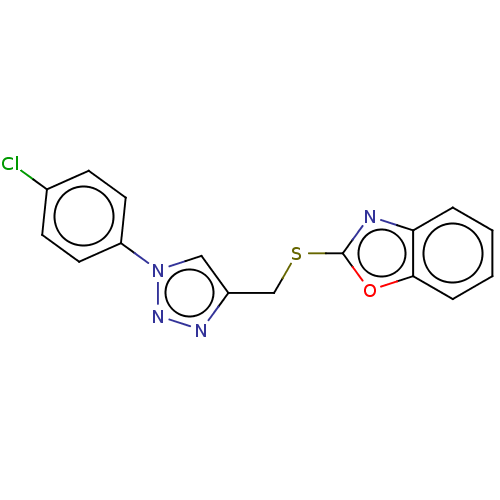
(CHEMBL3264693)Show InChI InChI=1S/C16H11ClN4OS/c17-11-5-7-13(8-6-11)21-9-12(19-20-21)10-23-16-18-14-3-1-2-4-15(14)22-16/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016431

(CHEMBL3264691)Show InChI InChI=1S/C17H14N4O2S/c1-22-14-8-6-13(7-9-14)21-10-12(19-20-21)11-24-17-18-15-4-2-3-5-16(15)23-17/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50494435

(CHEMBL3087872)Show InChI InChI=1S/C17H13FN4O2/c1-11-2-7-15-16(8-11)24-17(23)21(15)9-13-10-22(20-19-13)14-5-3-12(18)4-6-14/h2-8,10H,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect cell expression system using TMPD and arachidonic acid as substrate incubated for 1 min prio... |
Eur J Med Chem 70: 579-88 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.032
BindingDB Entry DOI: 10.7270/Q2N87DQ9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016401
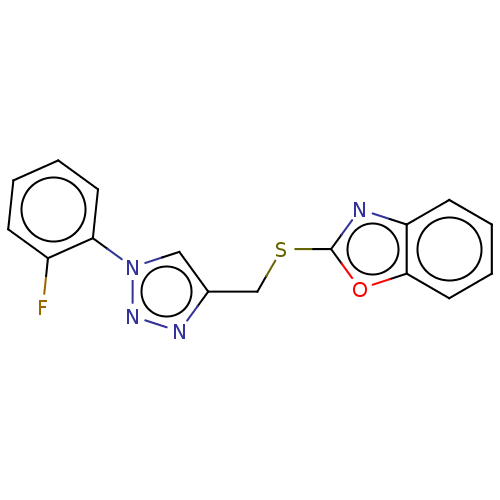
(CHEMBL3264699)Show InChI InChI=1S/C16H11FN4OS/c17-12-5-1-3-7-14(12)21-9-11(19-20-21)10-23-16-18-13-6-2-4-8-15(13)22-16/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50494436
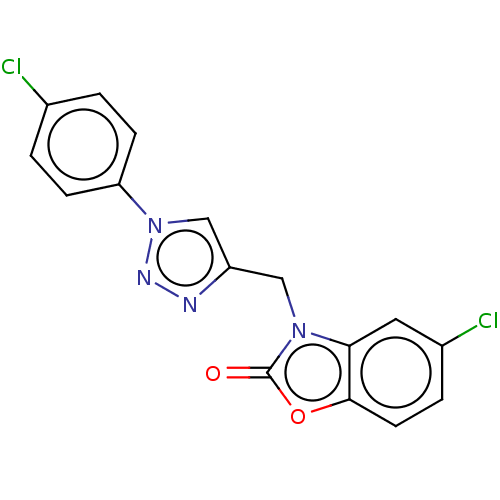
(CHEMBL3087875)Show InChI InChI=1S/C16H10Cl2N4O2/c17-10-1-4-13(5-2-10)22-9-12(19-20-22)8-21-14-7-11(18)3-6-15(14)24-16(21)23/h1-7,9H,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect cell expression system using TMPD and arachidonic acid as substrate incubated for 1 min prio... |
Eur J Med Chem 70: 579-88 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.032
BindingDB Entry DOI: 10.7270/Q2N87DQ9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016438
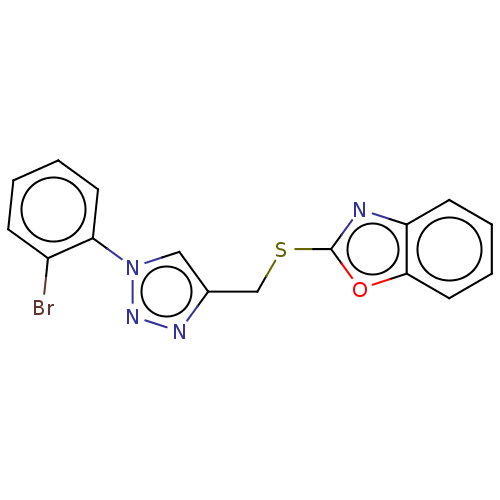
(CHEMBL3264698)Show InChI InChI=1S/C16H11BrN4OS/c17-12-5-1-3-7-14(12)21-9-11(19-20-21)10-23-16-18-13-6-2-4-8-15(13)22-16/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50494439

(CHEMBL3087873)Show SMILES Cc1ccc2oc(=O)n(Cc3cn(nn3)-c3ccc(Br)cc3)c2c1 Show InChI InChI=1S/C17H13BrN4O2/c1-11-2-7-16-15(8-11)21(17(23)24-16)9-13-10-22(20-19-13)14-5-3-12(18)4-6-14/h2-8,10H,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect cell expression system using TMPD and arachidonic acid as substrate incubated for 1 min prio... |
Eur J Med Chem 70: 579-88 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.032
BindingDB Entry DOI: 10.7270/Q2N87DQ9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016435

(CHEMBL3264695)Show InChI InChI=1S/C16H11BrN4OS/c17-11-5-7-13(8-6-11)21-9-12(19-20-21)10-23-16-18-14-3-1-2-4-15(14)22-16/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50494437
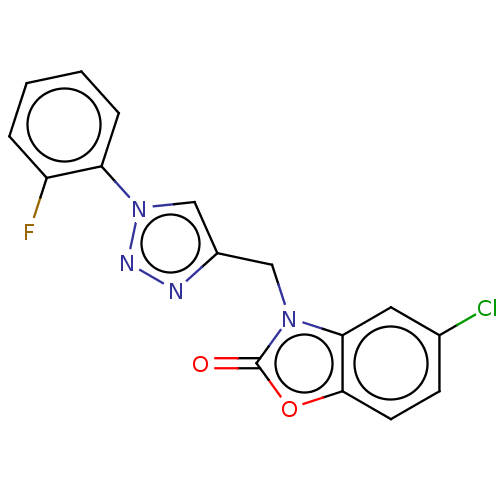
(CHEMBL3087876)Show InChI InChI=1S/C16H10ClFN4O2/c17-10-5-6-15-14(7-10)21(16(23)24-15)8-11-9-22(20-19-11)13-4-2-1-3-12(13)18/h1-7,9H,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect cell expression system using TMPD and arachidonic acid as substrate incubated for 1 min prio... |
Eur J Med Chem 70: 579-88 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.032
BindingDB Entry DOI: 10.7270/Q2N87DQ9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016428
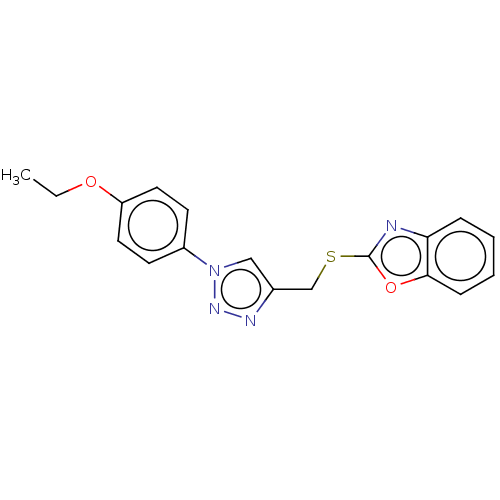
(CHEMBL3264702)Show InChI InChI=1S/C18H16N4O2S/c1-2-23-15-9-7-14(8-10-15)22-11-13(20-21-22)12-25-18-19-16-5-3-4-6-17(16)24-18/h3-11H,2,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016434
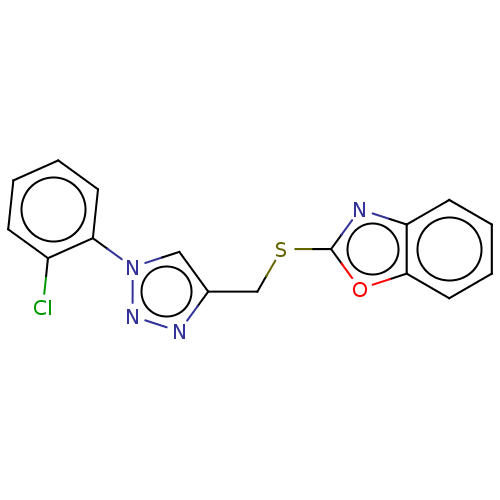
(CHEMBL3264694)Show InChI InChI=1S/C16H11ClN4OS/c17-12-5-1-3-7-14(12)21-9-11(19-20-21)10-23-16-18-13-6-2-4-8-15(13)22-16/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50494440

(CHEMBL3087874)Show InChI InChI=1S/C17H13ClN4O2/c1-11-6-7-15-16(8-11)24-17(23)21(15)9-12-10-22(20-19-12)14-5-3-2-4-13(14)18/h2-8,10H,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect cell expression system using TMPD and arachidonic acid as substrate incubated for 1 min prio... |
Eur J Med Chem 70: 579-88 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.032
BindingDB Entry DOI: 10.7270/Q2N87DQ9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016442
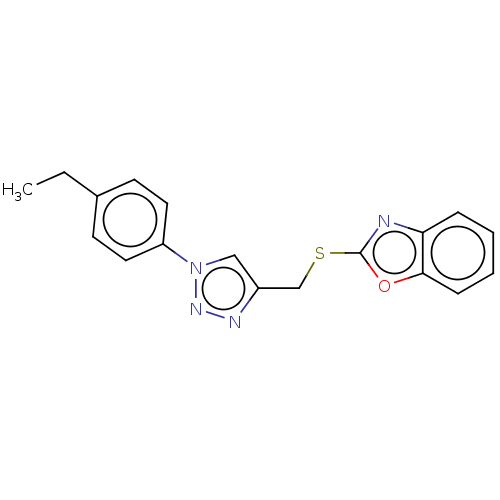
(CHEMBL3264690)Show InChI InChI=1S/C18H16N4OS/c1-2-13-7-9-15(10-8-13)22-11-14(20-21-22)12-24-18-19-16-5-3-4-6-17(16)23-18/h3-11H,2,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016427
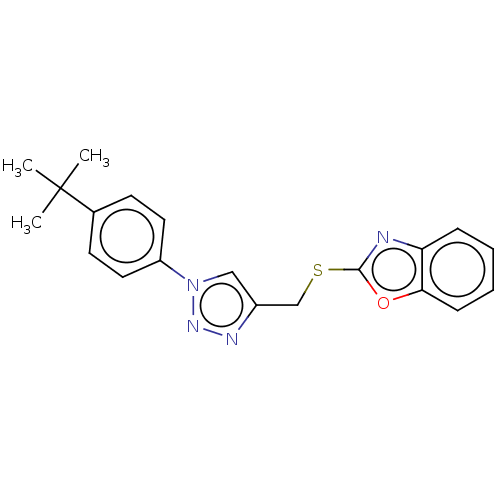
(CHEMBL3264701)Show SMILES CC(C)(C)c1ccc(cc1)-n1cc(CSc2nc3ccccc3o2)nn1 Show InChI InChI=1S/C20H20N4OS/c1-20(2,3)14-8-10-16(11-9-14)24-12-15(22-23-24)13-26-19-21-17-6-4-5-7-18(17)25-19/h4-12H,13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016429
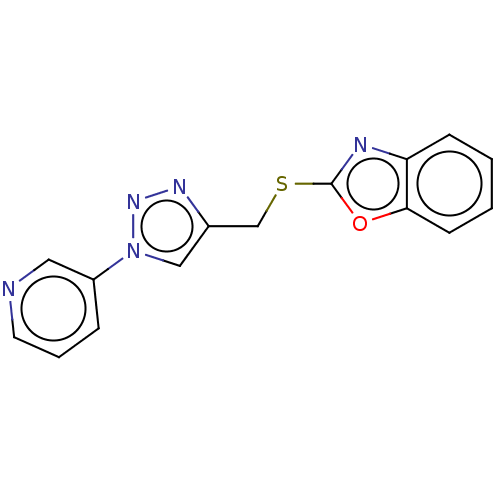
(CHEMBL3264703)Show InChI InChI=1S/C15H11N5OS/c1-2-6-14-13(5-1)17-15(21-14)22-10-11-9-20(19-18-11)12-4-3-7-16-8-12/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016419
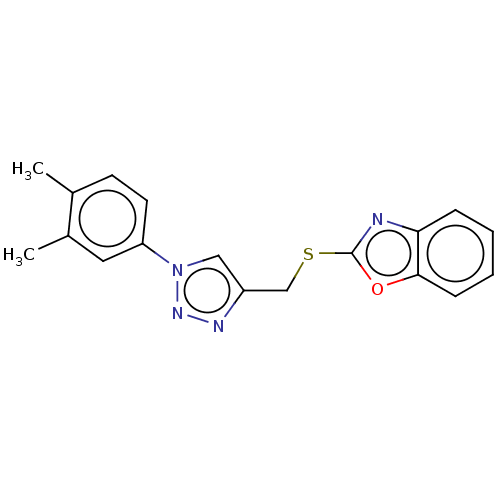
(CHEMBL3264700)Show InChI InChI=1S/C18H16N4OS/c1-12-7-8-15(9-13(12)2)22-10-14(20-21-22)11-24-18-19-16-5-3-4-6-17(16)23-18/h3-10H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016432
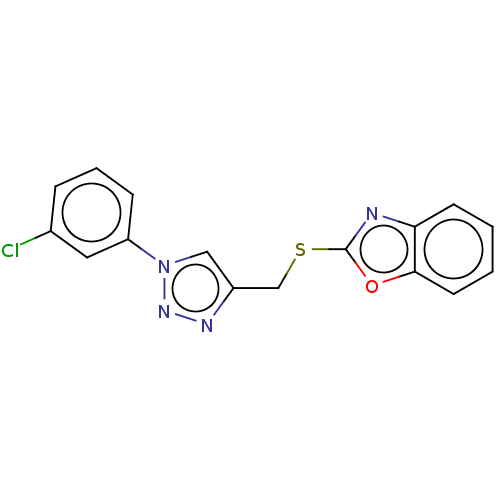
(CHEMBL3264692)Show InChI InChI=1S/C16H11ClN4OS/c17-11-4-3-5-13(8-11)21-9-12(19-20-21)10-23-16-18-14-6-1-2-7-15(14)22-16/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016437
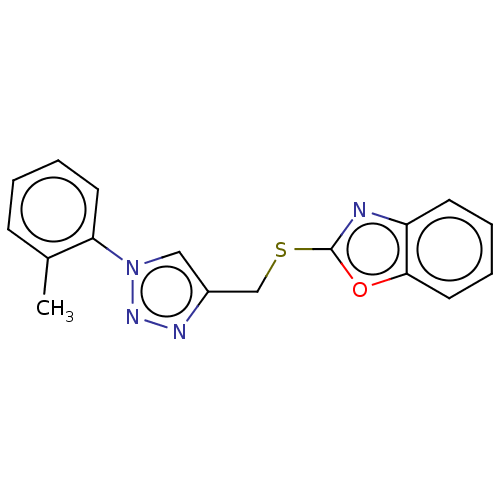
(CHEMBL3264697)Show InChI InChI=1S/C17H14N4OS/c1-12-6-2-4-8-15(12)21-10-13(19-20-21)11-23-17-18-14-7-3-5-9-16(14)22-17/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016430
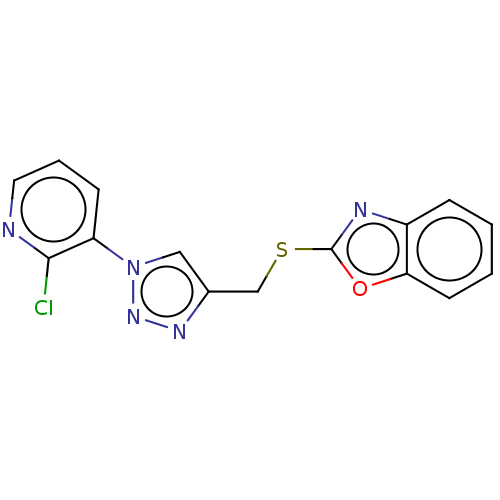
(CHEMBL3264704)Show InChI InChI=1S/C15H10ClN5OS/c16-14-12(5-3-7-17-14)21-8-10(19-20-21)9-23-15-18-11-4-1-2-6-13(11)22-15/h1-8H,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM175278
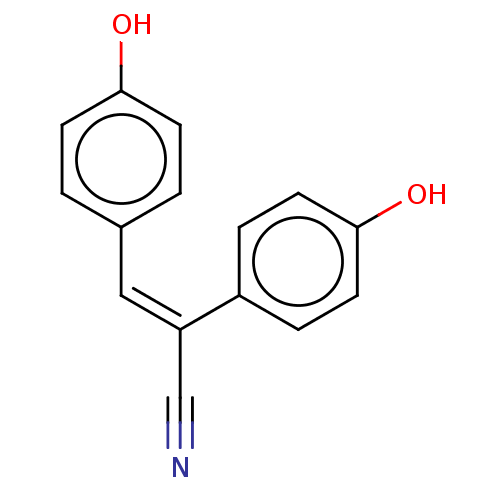
((E)-2,3-bis(4-Hydroxyphenyl)acrylonitrile (21))Show InChI InChI=1S/C15H11NO2/c16-10-13(12-3-7-15(18)8-4-12)9-11-1-5-14(17)6-2-11/h1-9,17-18H/b13-9- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.06E+3 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Indian Institute of Integrative Medicine
| Assay Description
The reaction mixture contained 50 mM phosphate buffer, pH 6.8, 0.05% L-DOPA and the supernatant (tyrosinase). After incubation in the absence or pres... |
Bioorg Chem 64: 97-102 (2016)
Article DOI: 10.1016/j.bioorg.2016.01.001
BindingDB Entry DOI: 10.7270/Q28C9V13 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016436
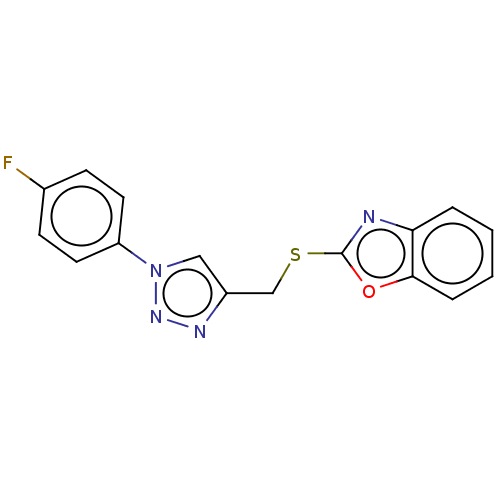
(CHEMBL3264696)Show InChI InChI=1S/C16H11FN4OS/c17-11-5-7-13(8-6-11)21-9-12(19-20-21)10-23-16-18-14-3-1-2-4-15(14)22-16/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50494438
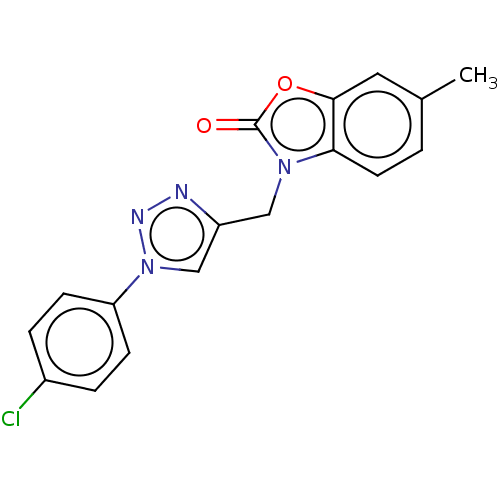
(CHEMBL3087871)Show SMILES Cc1ccc2n(Cc3cn(nn3)-c3ccc(Cl)cc3)c(=O)oc2c1 Show InChI InChI=1S/C17H13ClN4O2/c1-11-2-7-15-16(8-11)24-17(23)21(15)9-13-10-22(20-19-13)14-5-3-12(18)4-6-14/h2-8,10H,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect cell expression system using TMPD and arachidonic acid as substrate incubated for 1 min prio... |
Eur J Med Chem 70: 579-88 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.032
BindingDB Entry DOI: 10.7270/Q2N87DQ9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016441
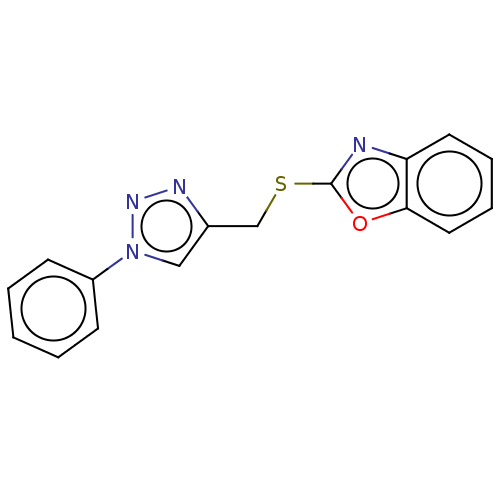
(CHEMBL3264689)Show InChI InChI=1S/C16H12N4OS/c1-2-6-13(7-3-1)20-10-12(18-19-20)11-22-16-17-14-8-4-5-9-15(14)21-16/h1-10H,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016439
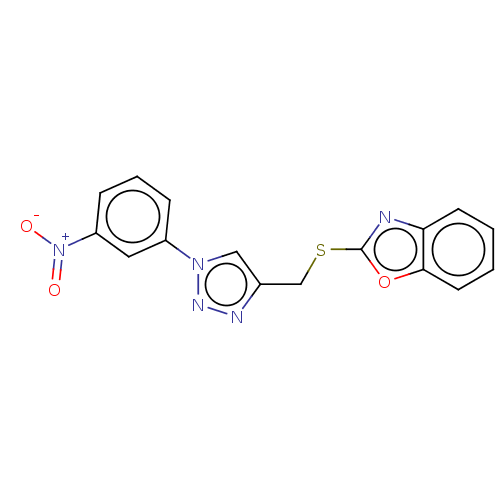
(CHEMBL3264687)Show SMILES [O-][N+](=O)c1cccc(c1)-n1cc(CSc2nc3ccccc3o2)nn1 Show InChI InChI=1S/C16H11N5O3S/c22-21(23)13-5-3-4-12(8-13)20-9-11(18-19-20)10-25-16-17-14-6-1-2-7-15(14)24-16/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50016440
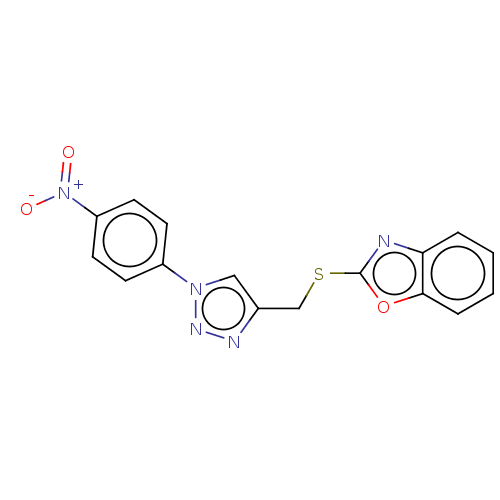
(CHEMBL3264688)Show SMILES [O-][N+](=O)c1ccc(cc1)-n1cc(CSc2nc3ccccc3o2)nn1 Show InChI InChI=1S/C16H11N5O3S/c22-21(23)13-7-5-12(6-8-13)20-9-11(18-19-20)10-25-16-17-14-3-1-2-4-15(14)24-16/h1-9H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Indian Institute of Integrative Medicine
| Assay Description
The reaction mixture contained 50 mM phosphate buffer, pH 6.8, 0.05% L-DOPA and the supernatant (tyrosinase). After incubation in the absence or pres... |
Bioorg Chem 64: 97-102 (2016)
Article DOI: 10.1016/j.bioorg.2016.01.001
BindingDB Entry DOI: 10.7270/Q28C9V13 |
More data for this
Ligand-Target Pair | |
Chloroquine resistance transporter
(Plasmodium falciparum) | BDBM50563441

(CHEMBL4745810)Show SMILES COc1cc(NC(c2ccc(CN3CCNCC3)cc2)c2cccc(Cl)c2)n2nc(C)nc2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum Dd2 CRT expressed in Xenopus laevis oocyte assessed as inhibition of [3H]-CQ transport incubated for 1.5 hrs by m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113227
BindingDB Entry DOI: 10.7270/Q2R49VH9 |
More data for this
Ligand-Target Pair | |
Chloroquine resistance transporter
(Plasmodium falciparum) | BDBM50563442
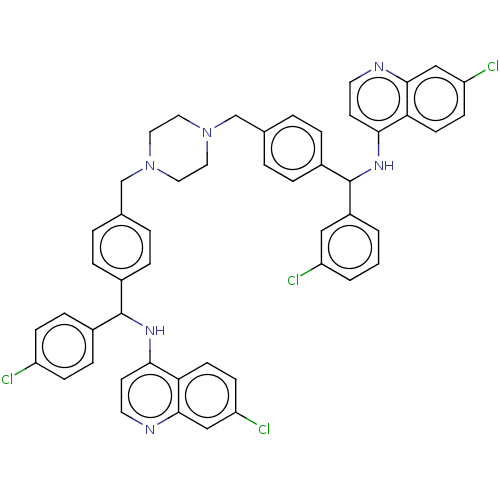
(CHEMBL4754685)Show SMILES Clc1ccc(cc1)C(Nc1ccnc2cc(Cl)ccc12)c1ccc(CN2CCN(Cc3ccc(cc3)C(Nc3ccnc4cc(Cl)ccc34)c3cccc(Cl)c3)CC2)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum Dd2 CRT expressed in Xenopus laevis oocyte assessed as inhibition of [3H]-CQ transport incubated for 1.5 hrs by m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113227
BindingDB Entry DOI: 10.7270/Q2R49VH9 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM175269
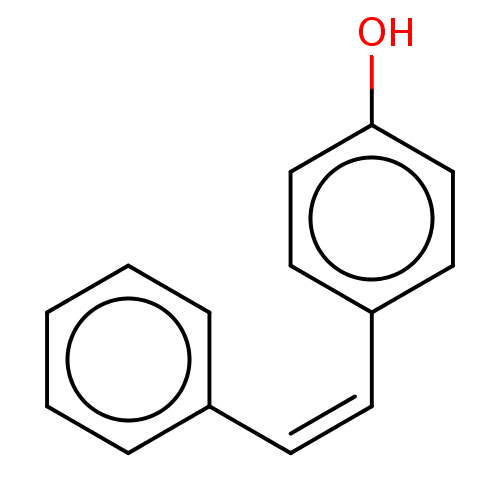
(Trans-4-Styryl-phenol (8))Show InChI InChI=1S/C14H12O/c15-14-10-8-13(9-11-14)7-6-12-4-2-1-3-5-12/h1-11,15H/b7-6- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Indian Institute of Integrative Medicine
| Assay Description
The reaction mixture contained 50 mM phosphate buffer, pH 6.8, 0.05% L-DOPA and the supernatant (tyrosinase). After incubation in the absence or pres... |
Bioorg Chem 64: 97-102 (2016)
Article DOI: 10.1016/j.bioorg.2016.01.001
BindingDB Entry DOI: 10.7270/Q28C9V13 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50016401

(CHEMBL3264699)Show InChI InChI=1S/C16H11FN4OS/c17-12-5-1-3-7-14(12)21-9-11(19-20-21)10-23-16-18-13-6-2-4-8-15(13)22-16/h1-9H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Eur J Med Chem 70: 579-88 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.032
BindingDB Entry DOI: 10.7270/Q2N87DQ9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50028529
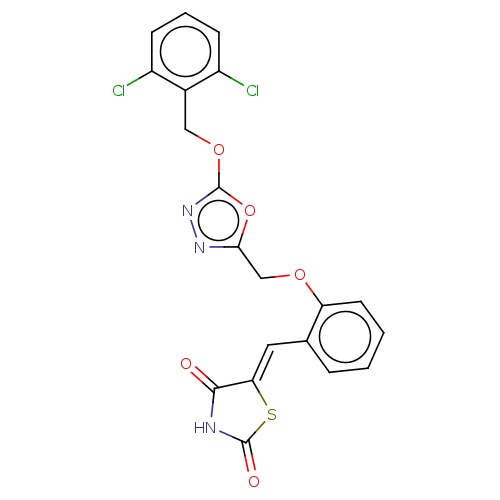
(CHEMBL3355597)Show SMILES Clc1cccc(Cl)c1COc1nnc(COc2ccccc2\C=C2\SC(=O)NC2=O)o1 Show InChI InChI=1S/C20H13Cl2N3O5S/c21-13-5-3-6-14(22)12(13)9-29-20-25-24-17(30-20)10-28-15-7-2-1-4-11(15)8-16-18(26)23-19(27)31-16/h1-8H,9-10H2,(H,23,26,27)/b16-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 87: 175-85 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.010
BindingDB Entry DOI: 10.7270/Q2T72K08 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50016438

(CHEMBL3264698)Show InChI InChI=1S/C16H11BrN4OS/c17-12-5-1-3-7-14(12)21-9-11(19-20-21)10-23-16-18-13-6-2-4-8-15(13)22-16/h1-9H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50028526

(CHEMBL3338895)Show SMILES O=C1NC(=O)\C(S1)=C/c1ccccc1OCc1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C19H13N3O4S/c23-17-15(27-19(24)20-17)10-13-8-4-5-9-14(13)25-11-16-21-22-18(26-16)12-6-2-1-3-7-12/h1-10H,11H2,(H,20,23,24)/b15-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 87: 175-85 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.010
BindingDB Entry DOI: 10.7270/Q2T72K08 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50016434

(CHEMBL3264694)Show InChI InChI=1S/C16H11ClN4OS/c17-12-5-1-3-7-14(12)21-9-11(19-20-21)10-23-16-18-13-6-2-4-8-15(13)22-16/h1-9H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM175267
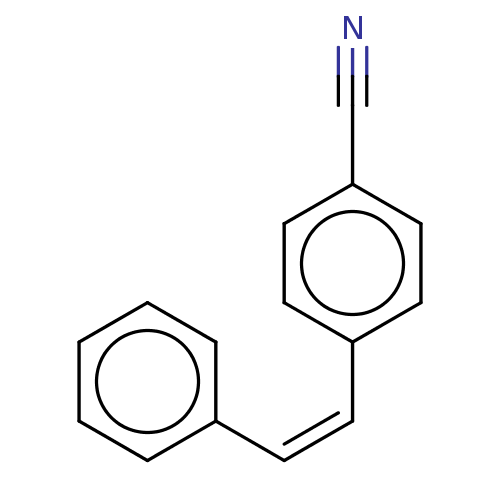
(Trans-4-Styrylbenzonitrile (6))Show InChI InChI=1S/C15H11N/c16-12-15-10-8-14(9-11-15)7-6-13-4-2-1-3-5-13/h1-11H/b7-6- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Indian Institute of Integrative Medicine
| Assay Description
The reaction mixture contained 50 mM phosphate buffer, pH 6.8, 0.05% L-DOPA and the supernatant (tyrosinase). After incubation in the absence or pres... |
Bioorg Chem 64: 97-102 (2016)
Article DOI: 10.1016/j.bioorg.2016.01.001
BindingDB Entry DOI: 10.7270/Q28C9V13 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM175271
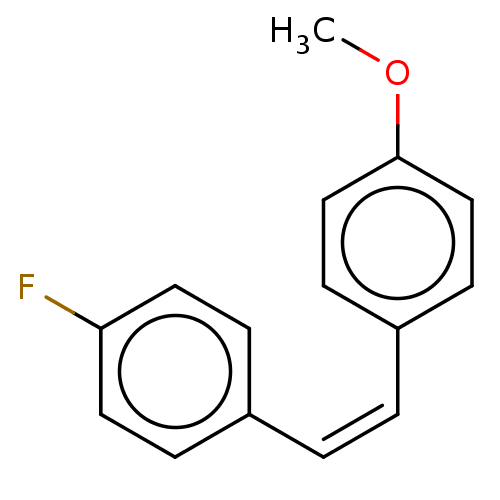
(Trans-1-fluoro-4-(4-methoxystyryl)benzene (10))Show InChI InChI=1S/C15H13FO/c1-17-15-10-6-13(7-11-15)3-2-12-4-8-14(16)9-5-12/h2-11H,1H3/b3-2- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Indian Institute of Integrative Medicine
| Assay Description
The reaction mixture contained 50 mM phosphate buffer, pH 6.8, 0.05% L-DOPA and the supernatant (tyrosinase). After incubation in the absence or pres... |
Bioorg Chem 64: 97-102 (2016)
Article DOI: 10.1016/j.bioorg.2016.01.001
BindingDB Entry DOI: 10.7270/Q28C9V13 |
More data for this
Ligand-Target Pair | |
Chloroquine resistance transporter
(Plasmodium falciparum) | BDBM50563440
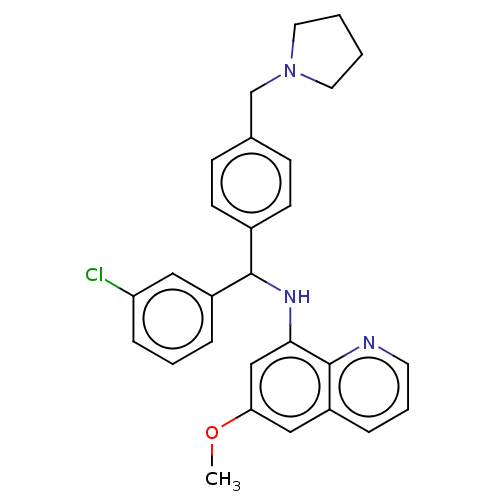
(CHEMBL4784051)Show SMILES COc1cc(NC(c2ccc(CN3CCCC3)cc2)c2cccc(Cl)c2)c2ncccc2c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum Dd2 CRT expressed in Xenopus laevis oocyte assessed as inhibition of [3H]-CQ transport incubated for 1.5 hrs by m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113227
BindingDB Entry DOI: 10.7270/Q2R49VH9 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM175272
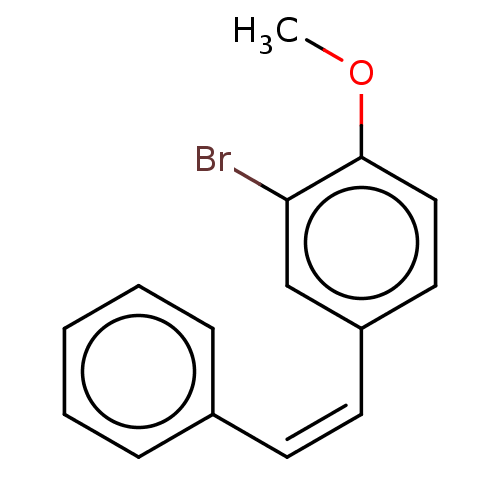
(Trans-2-Bromo-1-methoxy-4-styryl-benzene (11))Show InChI InChI=1S/C15H13BrO/c1-17-15-10-9-13(11-14(15)16)8-7-12-5-3-2-4-6-12/h2-11H,1H3/b8-7- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.62E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Indian Institute of Integrative Medicine
| Assay Description
The reaction mixture contained 50 mM phosphate buffer, pH 6.8, 0.05% L-DOPA and the supernatant (tyrosinase). After incubation in the absence or pres... |
Bioorg Chem 64: 97-102 (2016)
Article DOI: 10.1016/j.bioorg.2016.01.001
BindingDB Entry DOI: 10.7270/Q28C9V13 |
More data for this
Ligand-Target Pair | |
Chloroquine resistance transporter
(Plasmodium falciparum) | BDBM50563439

(CHEMBL4748649)Show SMILES COc1cc(NC(c2ccc(CN3CCNCC3)cc2)c2cccc(Cl)c2)c2ncccc2c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum Dd2 CRT expressed in Xenopus laevis oocyte assessed as inhibition of [3H]-CQ transport incubated for 1.5 hrs by m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113227
BindingDB Entry DOI: 10.7270/Q2R49VH9 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM175263
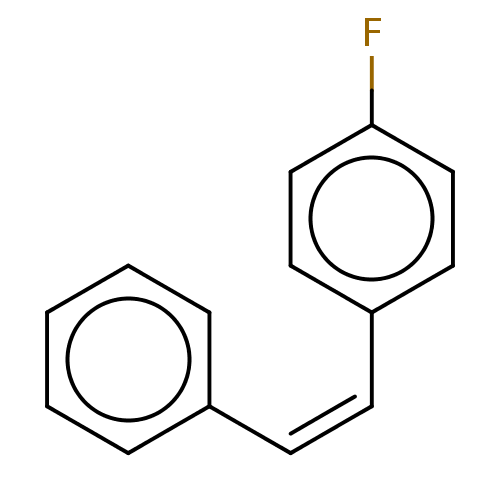
(Trans-1-Fluoro-4-styryl-benzene (2))Show InChI InChI=1S/C14H11F/c15-14-10-8-13(9-11-14)7-6-12-4-2-1-3-5-12/h1-11H/b7-6- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.94E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Indian Institute of Integrative Medicine
| Assay Description
The reaction mixture contained 50 mM phosphate buffer, pH 6.8, 0.05% L-DOPA and the supernatant (tyrosinase). After incubation in the absence or pres... |
Bioorg Chem 64: 97-102 (2016)
Article DOI: 10.1016/j.bioorg.2016.01.001
BindingDB Entry DOI: 10.7270/Q28C9V13 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50016433

(CHEMBL3264693)Show InChI InChI=1S/C16H11ClN4OS/c17-11-5-7-13(8-6-11)21-9-12(19-20-21)10-23-16-18-14-3-1-2-4-15(14)22-16/h1-9H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50016432

(CHEMBL3264692)Show InChI InChI=1S/C16H11ClN4OS/c17-11-4-3-5-13(8-11)21-9-12(19-20-21)10-23-16-18-14-6-1-2-7-15(14)22-16/h1-9H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50016431

(CHEMBL3264691)Show InChI InChI=1S/C17H14N4O2S/c1-22-14-8-6-13(7-9-14)21-10-12(19-20-21)11-24-17-18-15-4-2-3-5-16(15)23-17/h2-10H,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50016429

(CHEMBL3264703)Show InChI InChI=1S/C15H11N5OS/c1-2-6-14-13(5-1)17-15(21-14)22-10-11-9-20(19-18-11)12-4-3-7-16-8-12/h1-9H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50016430

(CHEMBL3264704)Show InChI InChI=1S/C15H10ClN5OS/c16-14-12(5-3-7-17-14)21-8-10(19-20-21)9-23-15-18-11-4-1-2-6-13(11)22-15/h1-8H,9H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50016439

(CHEMBL3264687)Show SMILES [O-][N+](=O)c1cccc(c1)-n1cc(CSc2nc3ccccc3o2)nn1 Show InChI InChI=1S/C16H11N5O3S/c22-21(23)13-5-3-4-12(8-13)20-9-11(18-19-20)10-25-16-17-14-6-1-2-7-15(14)24-16/h1-9H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 (unknown origin) |
Eur J Med Chem 81: 204-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.012
BindingDB Entry DOI: 10.7270/Q2GM88VQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data