

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50580260 (CHEMBL5093347) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50580262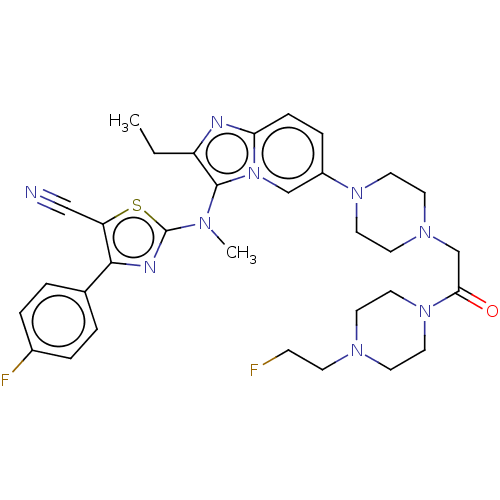 (CHEMBL5085520) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50580256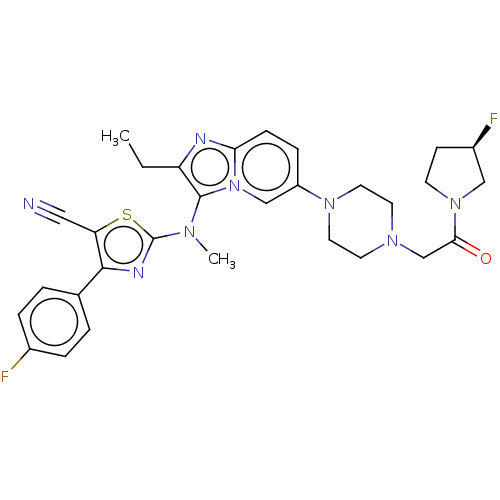 (CHEMBL5091502) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50580261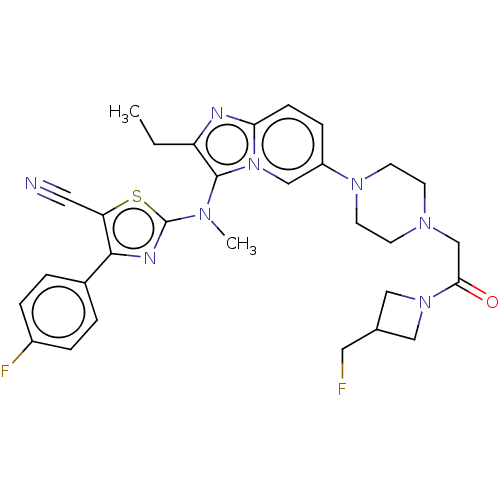 (CHEMBL5084424) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50580258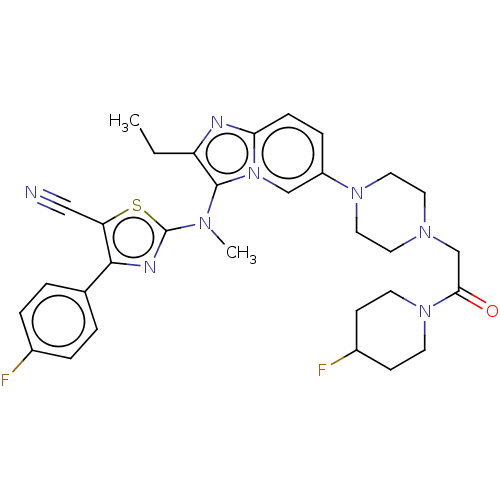 (CHEMBL5076928) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50580255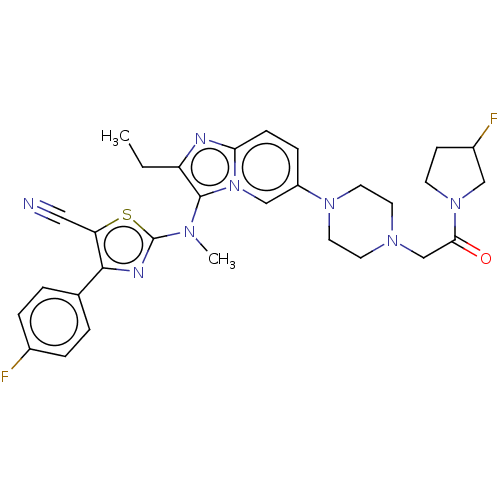 (CHEMBL5084058) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50580259 (CHEMBL5088520) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50580263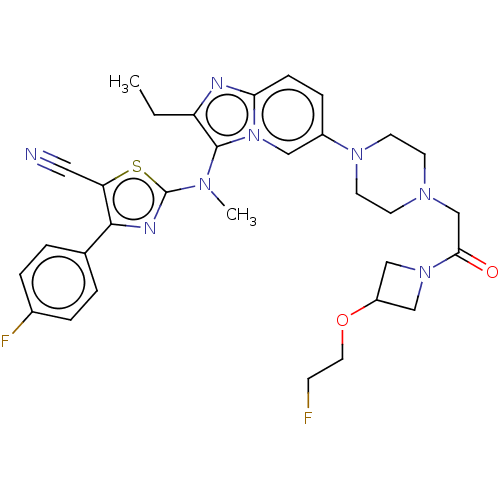 (CHEMBL5088371) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50580262 (CHEMBL5085520) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM192943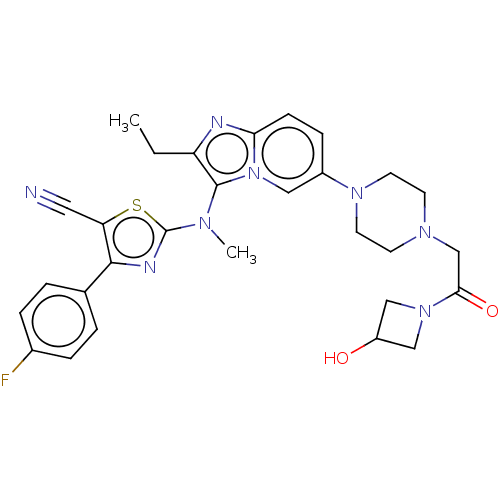 (US10526329, Compound 139 | US9670204, 138 2-((2-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50580257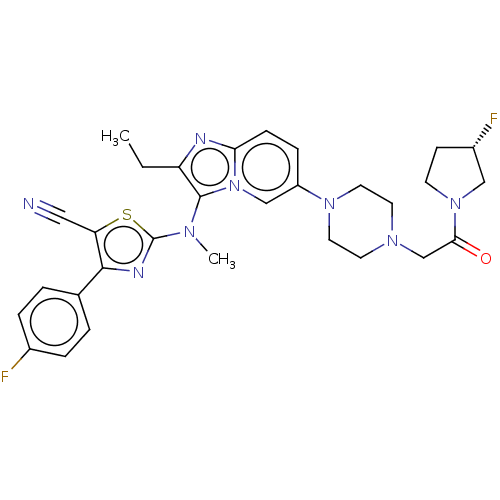 (CHEMBL5078471) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50580254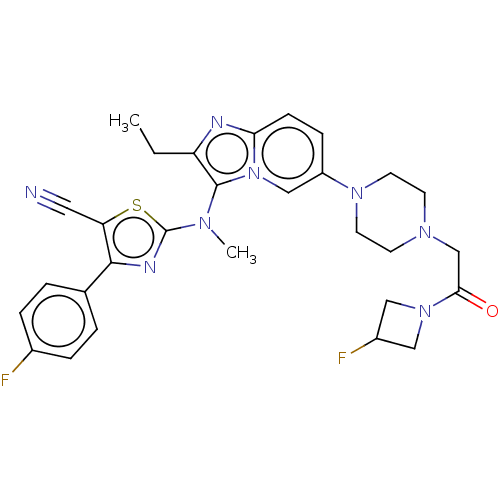 (CHEMBL5088215) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00913 BindingDB Entry DOI: 10.7270/Q20P13WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol 24-hydroxylase (Homo sapiens (Human)) | BDBM50545375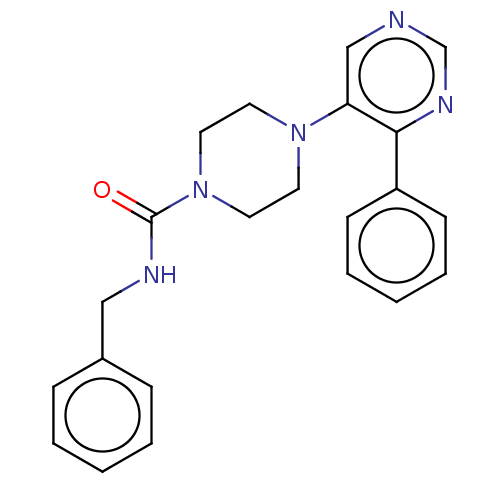 (CHEMBL4527162) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity to full length human CYP46A1 expressed in Escherichia coli DH5alpha as cholesterol-24 hydroxylation using cholesterol as substrate i... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127068 BindingDB Entry DOI: 10.7270/Q2PK0KRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM150300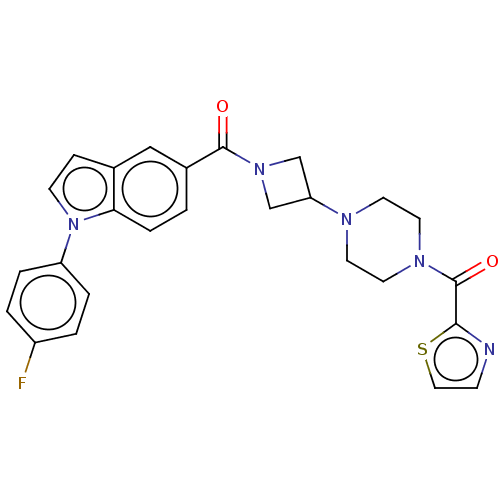 (US8987247, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of carbonic anhydrase 2 assessed as inhibition of carbon dioxide hydration | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50411265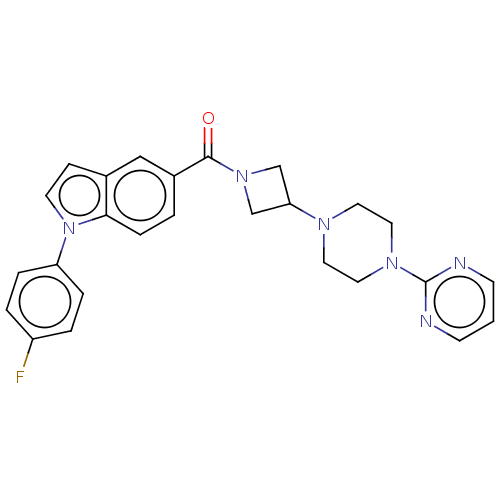 (CHEMBL5274306) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of carbonic anhydrase 2 assessed as inhibition of carbon dioxide hydration | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50411264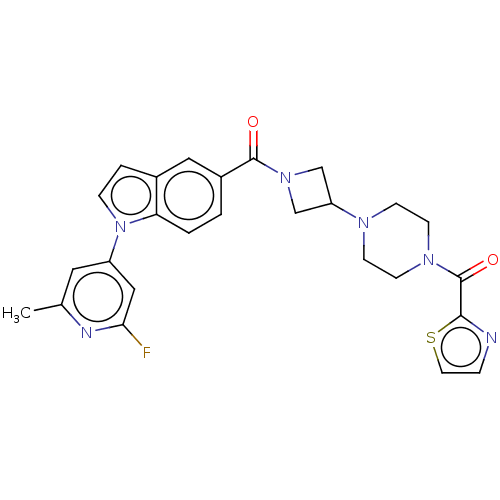 (CHEMBL5290125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of carbonic anhydrase 2 assessed as inhibition of carbon dioxide hydration | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50543946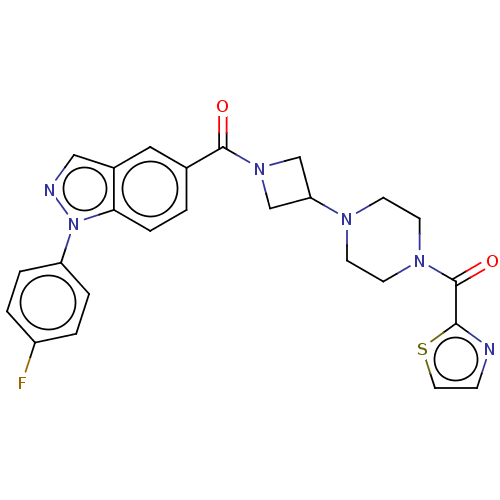 (CHEMBL4640364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of carbonic anhydrase 2 assessed as inhibition of carbon dioxide hydration | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50501822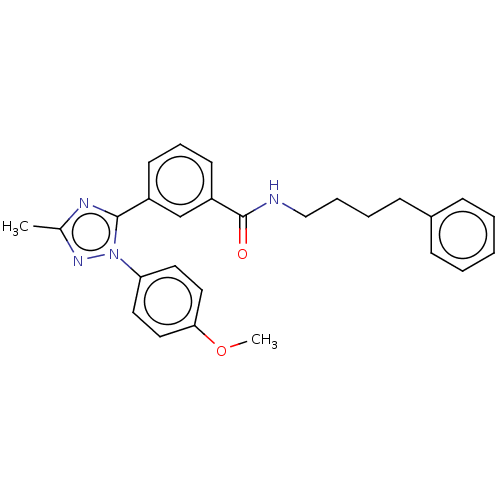 (CHEMBL4587127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate preincubated for 5 mins followed by NADPH-generating system addition an... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50501820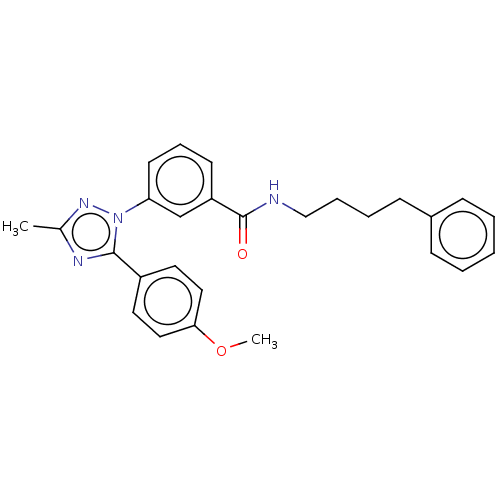 (CHEMBL4528087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate preincubated for 5 mins followed by NADPH-generating system addition an... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50501823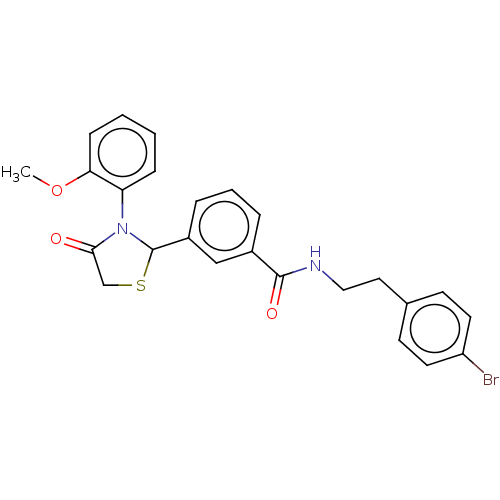 (CHEMBL3261573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate preincubated for 5 mins followed by NADPH-generating system addition an... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50501822 (CHEMBL4587127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 5 mins followed by NADPH-generating system addition and... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50501823 (CHEMBL3261573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 5 mins followed by NADPH-generating system addition and ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50501821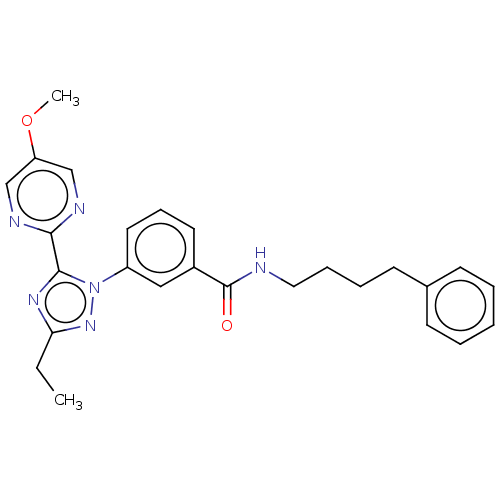 (CHEMBL4582168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate preincubated for 5 mins followed by NADPH-generating system addition an... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50501823 (CHEMBL3261573) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using tolbutamide as substrate preincubated for 5 mins followed by NADPH-generating system addition an... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50501822 (CHEMBL4587127) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate preincubated for 5 mins followed by NADPH-generating system addition and ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50501820 (CHEMBL4528087) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using tolbutamide as substrate preincubated for 5 mins followed by NADPH-generating system addition an... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50501820 (CHEMBL4528087) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate preincubated for 5 mins followed by NADPH-generating system addition and ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50501823 (CHEMBL3261573) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate preincubated for 5 mins followed by NADPH-generating system addition and ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50501820 (CHEMBL4528087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 5 mins followed by NADPH-generating system addition and ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50501822 (CHEMBL4587127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 5 mins followed by NADPH-generating system addition and ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50501822 (CHEMBL4587127) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using tolbutamide as substrate preincubated for 5 mins followed by NADPH-generating system addition an... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50501820 (CHEMBL4528087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 5 mins followed by NADPH-generating system addition and... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50501821 (CHEMBL4582168) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using tolbutamide as substrate preincubated for 5 mins followed by NADPH-generating system addition an... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50501823 (CHEMBL3261573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 5 mins followed by NADPH-generating system addition and... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50501823 (CHEMBL3261573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by NADPH-generating system addition ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50501821 (CHEMBL4582168) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate preincubated for 5 mins followed by NADPH-generating system addition and ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50501822 (CHEMBL4587127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by NADPH-generating system addition ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50501820 (CHEMBL4528087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by NADPH-generating system addition ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50501821 (CHEMBL4582168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 5 mins followed by NADPH-generating system addition and... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50501821 (CHEMBL4582168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 5 mins followed by NADPH-generating system addition and ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50501821 (CHEMBL4582168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by NADPH-generating system addition ... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myoferlin (Homo sapiens) | BDBM50501821 (CHEMBL4582168) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Reversible binding affinity to recombinant human GST-tagged MYOF C2 domain (3445 to 3912 residues) expressed in Escherichia coli BL21 (DE3) by surfac... | J Med Chem 62: 4949-4966 (2019) Article DOI: 10.1021/acs.jmedchem.9b00059 BindingDB Entry DOI: 10.7270/Q2XS5ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||