

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50012250 (CHEMBL58947 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012246 (CHEMBL58153 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012246 (CHEMBL58153 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50282993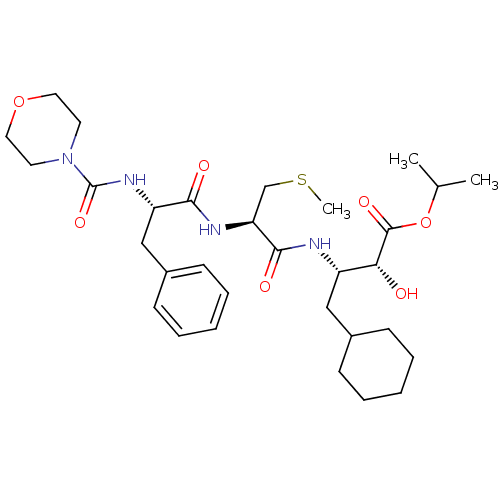 ((2S,4R)-4-Cyclohexyl-2-hydroxy-3-((R)-3-methylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012251 (2-{2-(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012246 (CHEMBL58153 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035820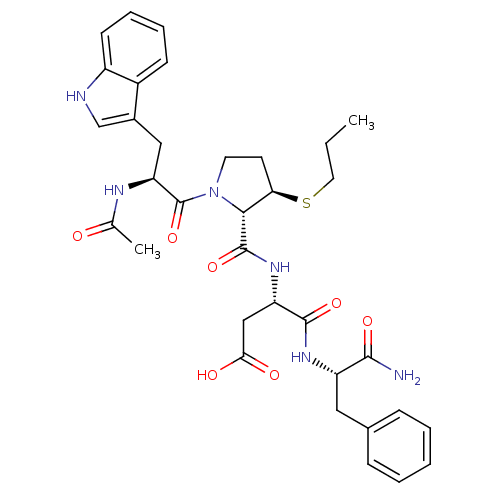 (Ac-Trp-L-3-MPt(Pr)-Asp-Phe-NH2 | CHEMBL37830) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012249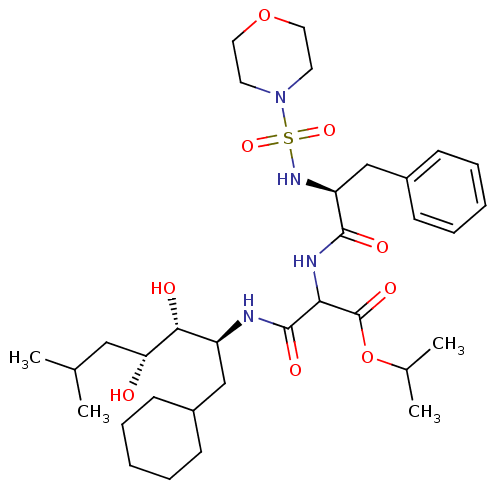 (CHEMBL58256 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006202 (3-Amino-N-[1-[1-(1-cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012257 (CHEMBL299252 | N-[1-Cyclohexylmethyl-3,3-difluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17941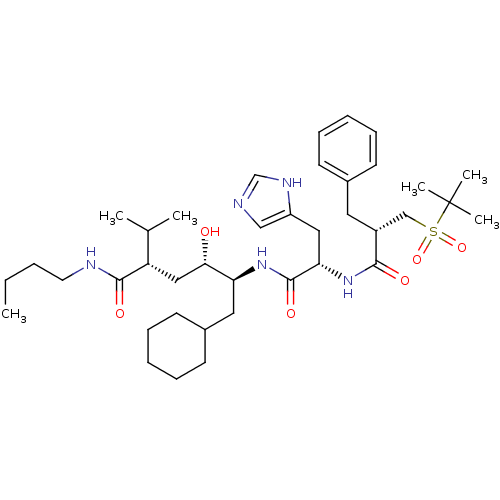 ((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012252 (CHEMBL57492 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012247 (4-(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-hexyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035832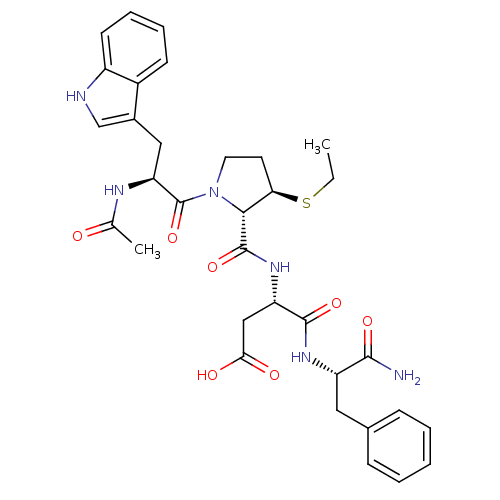 (Ac-Trp-L-3-MPt(Et)-Asp-Phe-NH2 | CHEMBL35471) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035825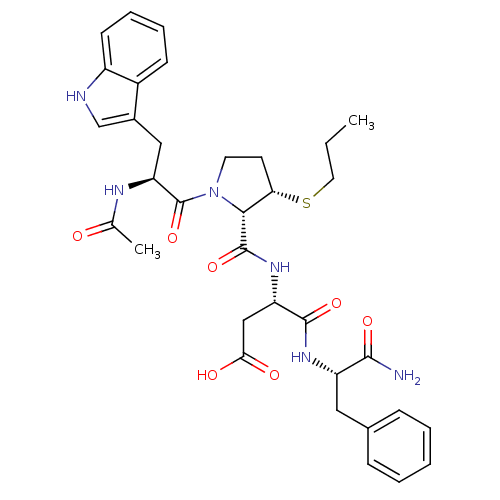 (Ac-Trp-L-3-MPt(Pr)-Asp-Phe-NH2 | CHEMBL34554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012255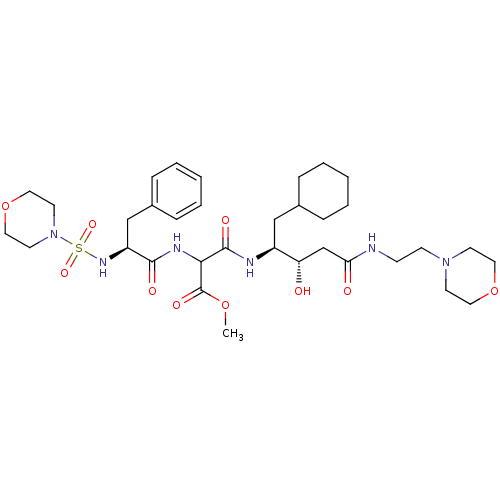 (CHEMBL293328 | N-[1-Cyclohexylmethyl-2-hydroxy-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012253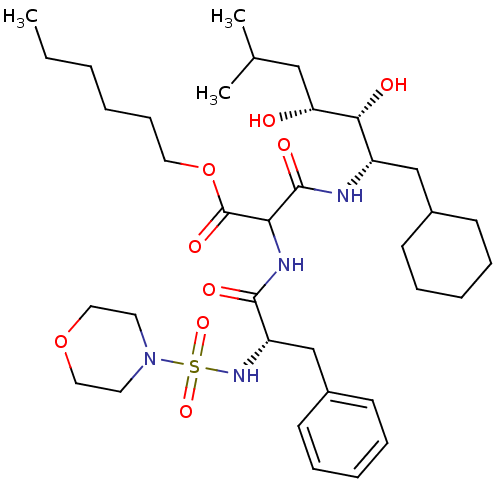 (CHEMBL56982 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50282993 ((2S,4R)-4-Cyclohexyl-2-hydroxy-3-((R)-3-methylsulf...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration on Bovine Cathepsin D | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012250 (CHEMBL58947 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Bovine Cathepsin D. | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012254 (CHEMBL60535 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035829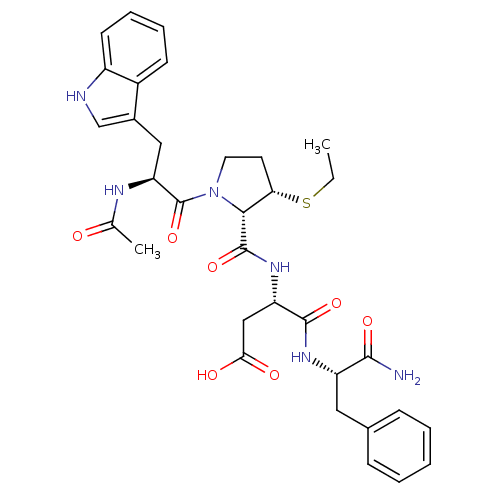 (Ac-Trp-L-3-MPt(Et)-Asp-Phe-NH2 | CHEMBL34853) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012247 (4-(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-hexyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Bovine Cathepsin D. | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012246 (CHEMBL58153 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 using [3H]-etorphine as a radioligand | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012246 (CHEMBL58153 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of monkey plasma renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012249 (CHEMBL58256 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Bovine Cathepsin D. | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012246 (CHEMBL58153 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Bovine Cathepsin D. | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035834 (Ac-Trp-L-3-MPt(Me)-Asp-Phe-NH2 | CHEMBL287753) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035833 (Ac-Trp-D-3-MPt(Pr)-Asp-Phe-NH2 | CHEMBL37984) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012253 (CHEMBL56982 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Bovine Cathepsin D. | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012252 (CHEMBL57492 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Bovine Cathepsin D. | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012256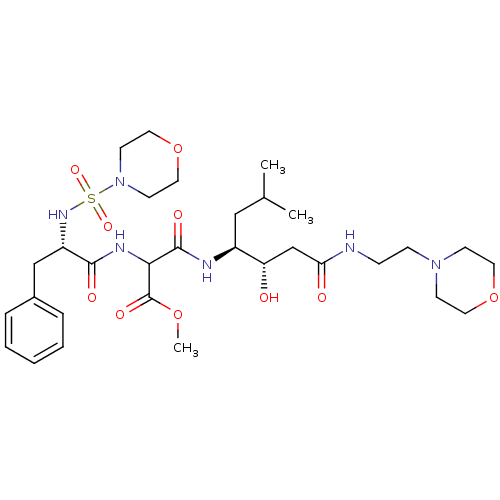 (CHEMBL431751 | N-{1-[1-Hydroxy-2-(2-morpholin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human renin | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035822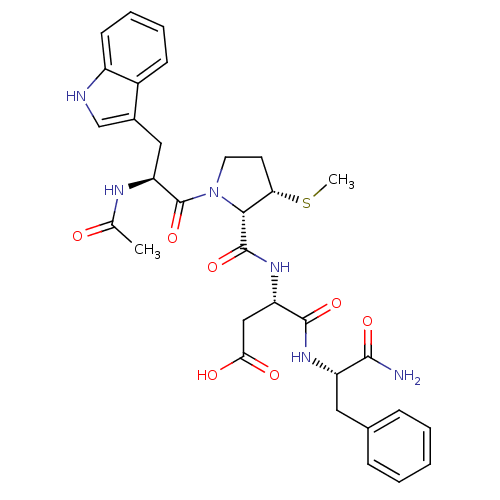 (Ac-Trp-L-3-MPc(Me)-Asp-Phe-NH2 | CHEMBL37983) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012254 (CHEMBL60535 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Bovine Cathepsin D. | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012257 (CHEMBL299252 | N-[1-Cyclohexylmethyl-3,3-difluoro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 515 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration on Bovine Cathepsin D | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035828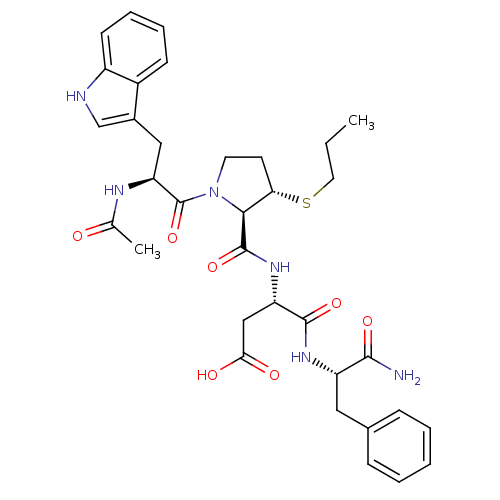 (Ac-Trp-D-3-MPt(Pr)-Asp-Phe-NH2 | CHEMBL37900) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035823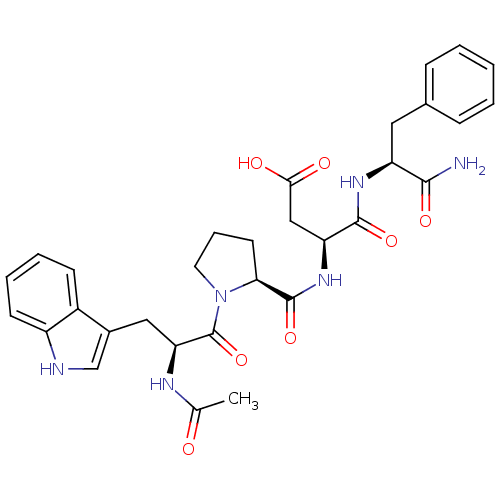 (Ac-Trp-D-Pro-Asp-Phe-NH2 | Ac-Trp-Pro-Asp-Phe-NH2 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035821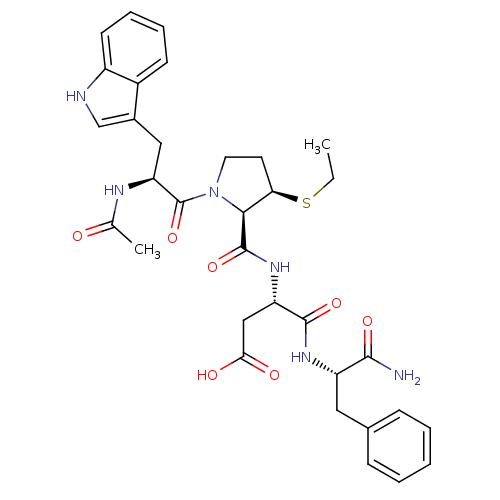 (Ac-Trp-D-3-MPt(Et)-Asp-Phe-NH2 | CHEMBL34916) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50035820 (Ac-Trp-L-3-MPt(Pr)-Asp-Phe-NH2 | CHEMBL37830) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor by displacement of [125I]-BH-CCK-8 from rat pancreatic acini | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50035833 (Ac-Trp-D-3-MPt(Pr)-Asp-Phe-NH2 | CHEMBL37984) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor by displacement of [125I]-BH-CCK-8 from rat pancreatic acini | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012255 (CHEMBL293328 | N-[1-Cyclohexylmethyl-2-hydroxy-3-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration on Bovine Cathepsin D | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50035823 (Ac-Trp-D-Pro-Asp-Phe-NH2 | Ac-Trp-Pro-Asp-Phe-NH2 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor by displacement of [125I]-BH-CCK-8 from rat pancreatic acini | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50012256 (CHEMBL431751 | N-{1-[1-Hydroxy-2-(2-morpholin-4-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration on Bovine Cathepsin D | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50006202 (3-Amino-N-[1-[1-(1-cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Bovine Cathepsin D. | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50035828 (Ac-Trp-D-3-MPt(Pr)-Asp-Phe-NH2 | CHEMBL37900) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Cholecystokinin type A receptor by measuring its ability to displace [125I]-BH-CCK-8 from rat pancre... | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50035825 (Ac-Trp-L-3-MPt(Pr)-Asp-Phe-NH2 | CHEMBL34554) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor by displacement of [125I]-BH-CCK-8 from rat pancreatic acini | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM17941 ((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration on Bovine Cathepsin D | J Med Chem 34: 1935-43 (1991) BindingDB Entry DOI: 10.7270/Q2NG4R73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035831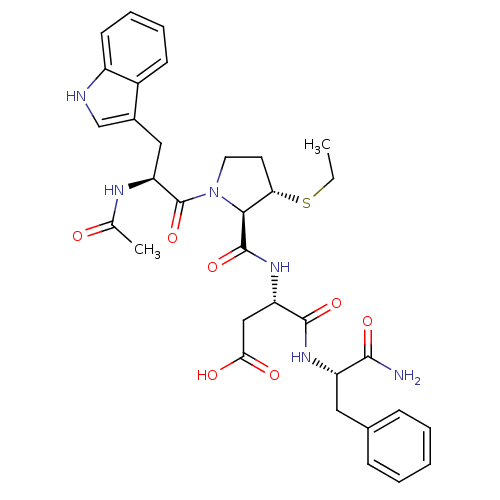 (Ac-Trp-D-3-MPt(Et)-Asp-Phe-NH2 | CHEMBL290444) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM50225752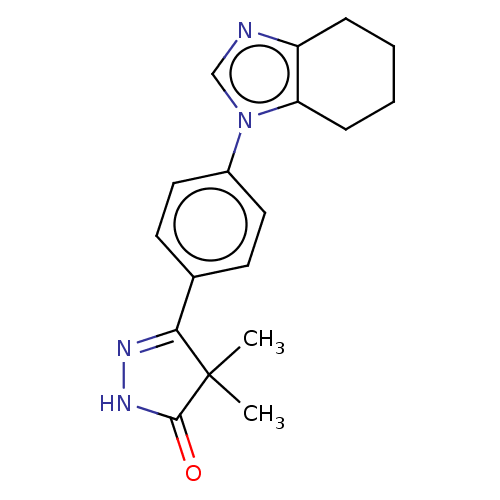 (CHEMBL46012) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description Inhibition of Guinea Pig cAMP specific phosphodiesterase (PDE III) | J Med Chem 30: 1724-8 (1987) BindingDB Entry DOI: 10.7270/Q20R9RN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035836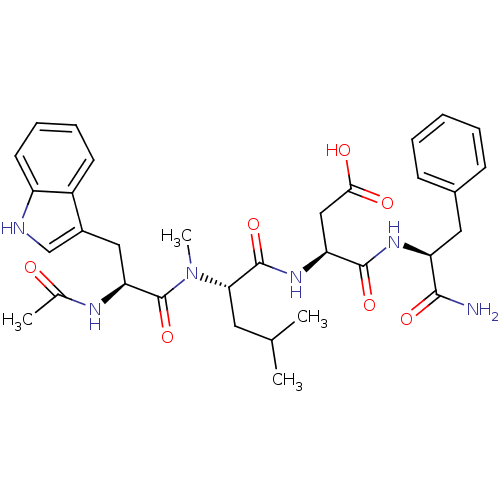 ((S)-3-((S)-2-{[(S)-2-Acetylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50035826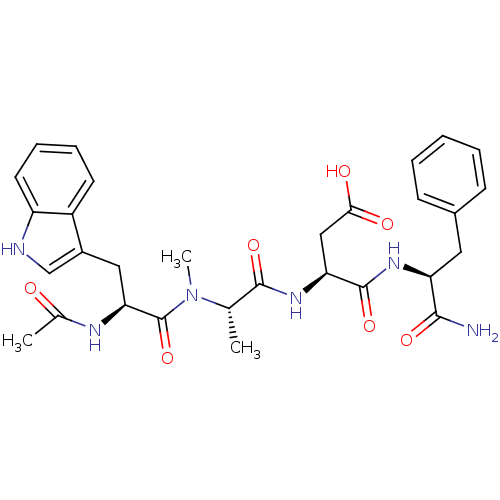 ((S)-3-((S)-2-{[(S)-2-Acetylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells | J Med Chem 38: 137-49 (1995) BindingDB Entry DOI: 10.7270/Q2QN65TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 82 total ) | Next | Last >> |