 Found 149 hits with Last Name = 'soares' and Initial = 'l'
Found 149 hits with Last Name = 'soares' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50587056

(CHEMBL5080685)Show SMILES COC(=O)c1c(CCCCCCCCNc2c3CCCCc3nc3ccccc23)cccc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0352 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587059

(CHEMBL5092094)Show SMILES COC(=O)c1c(O)cccc1CCCCCCCCNc1c2CCCCc2nc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.177 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587057
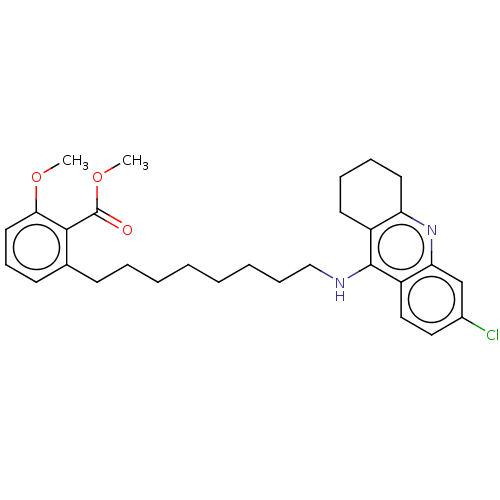
(CHEMBL5078555)Show SMILES COC(=O)c1c(CCCCCCCCNc2c3CCCCc3nc3cc(Cl)ccc23)cccc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.265 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587057

(CHEMBL5078555)Show SMILES COC(=O)c1c(CCCCCCCCNc2c3CCCCc3nc3cc(Cl)ccc23)cccc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587064
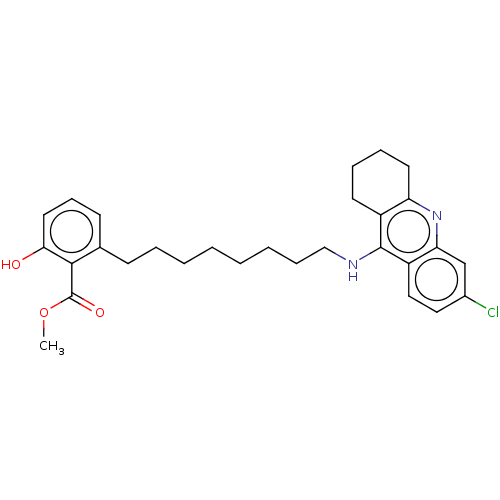
(CHEMBL5082773)Show SMILES COC(=O)c1c(O)cccc1CCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587062
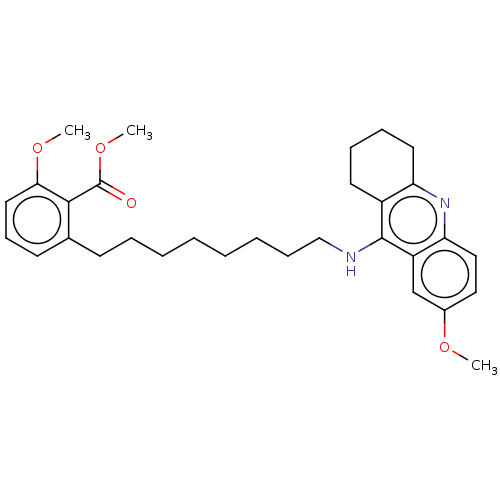
(CHEMBL5084379)Show SMILES COC(=O)c1c(CCCCCCCCNc2c3CCCCc3nc3ccc(OC)cc23)cccc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587066

(CHEMBL5073819) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587061
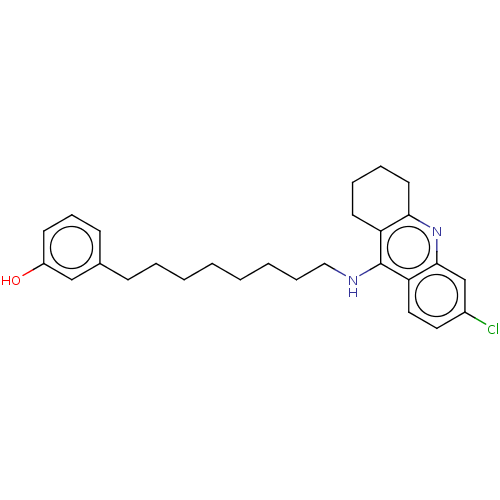
(CHEMBL5072428)Show SMILES Oc1cccc(CCCCCCCCNc2c3CCCCc3nc3cc(Cl)ccc23)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587064

(CHEMBL5082773)Show SMILES COC(=O)c1c(O)cccc1CCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587063
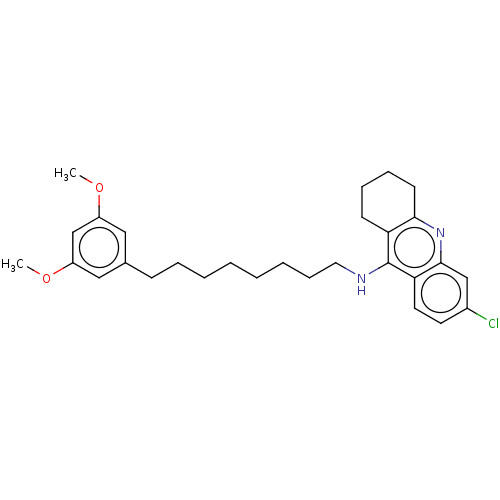
(CHEMBL5077234)Show SMILES COc1cc(CCCCCCCCNc2c3CCCCc3nc3cc(Cl)ccc23)cc(OC)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587058

(CHEMBL5088557)Show SMILES COc1cccc(CCCCCCCCNc2c3CCCCc3nc3cc(Cl)ccc23)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587055

(CHEMBL5091660) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621711
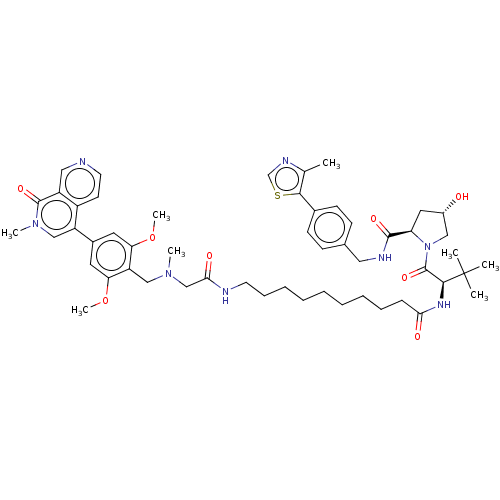
((2S,4R)-1-[(2S)-2-[10-[2-([[2,6-dimethoxy-4-(2-met...)Show SMILES COc1cc(cc(OC)c1CN(C)CC(=O)NCCCCCCCCCC(=O)N[C@@H](C(=O)N1C[C@@H](O)C[C@@H]1C(=O)NCc1ccc(cc1)-c1scnc1C)C(C)(C)C)-c1cn(C)c(=O)c2cnccc12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621671
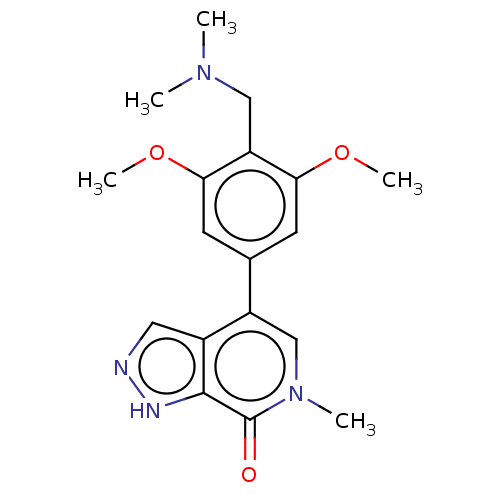
(US11773085, Compound B43 | methyl)-3,5-dimethoxyph...)Show SMILES COc1cc(cc(OC)c1CN(C)C)-c1cn(C)c(=O)c2[nH]ncc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621672

(US11773085, Compound B44 | methyl]-N-[8-(phenylami...)Show SMILES COc1cc(cc(OC)c1CN1CC(C1)C(=O)NCCCCCCCCNc1ccccc1)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621681
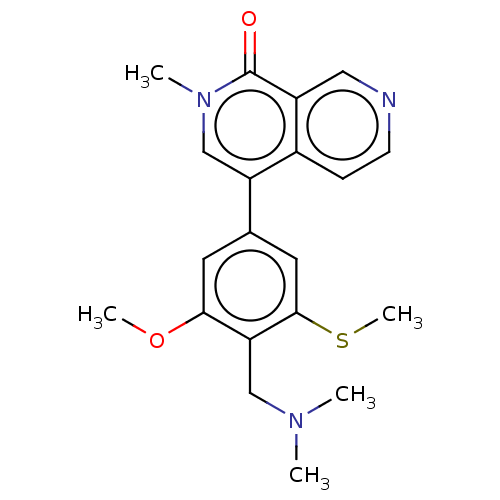
(4-[4-[(dimethylamino)methyl]-3-methoxy-5-(methylsu...)Show SMILES COc1cc(cc(SC)c1CN(C)C)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621698

(N-[[2,6-dimethoxy-4-(2-methyl-1-oxo-1,2-dihydro-2,...)Show SMILES COc1cc(cc(OC)c1CN(C)C(=O)CCCCCOc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621699
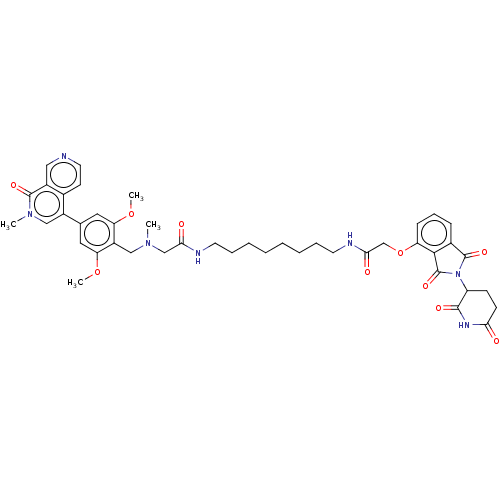
(N-[8-[2-([[2,6-dimethoxy-4-(2-methyl-1-oxo-1,2-dih...)Show SMILES COc1cc(cc(OC)c1CN(C)CC(=O)NCCCCCCCCNC(=O)COc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621705

(N-[[2,6-dimethoxy-4-(2-methyl-1-oxo-1,2-dihydro-2,...)Show SMILES COc1cc(cc(OC)c1CN(C)C(=O)CCCOc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621667
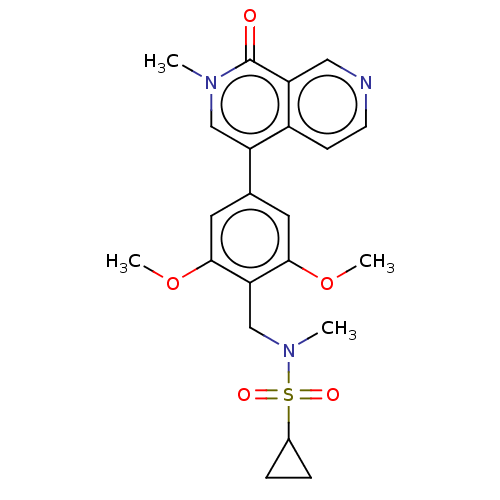
(US11773085, Compound B39 | cyclopropanesulfonamide...)Show SMILES COc1cc(cc(OC)c1CN(C)S(=O)(=O)C1CC1)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621708

(N-[[2,6-dimethoxy-4-(2-methyl-1-oxo-1,2-dihydro-2,...)Show SMILES COc1cc(cc(OC)c1CN(C)C(=O)CCOc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621706

(N-[[2,6-dimethoxy-4-(2-methyl-1-oxo-1,2-dihydro-2,...)Show SMILES COc1cc(cc(OC)c1CN(C)C(=O)CCCCOc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621666
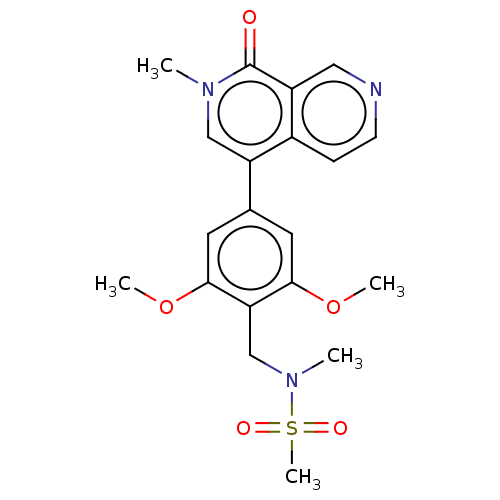
(N-[[2,6-dimethoxy-4-(2-methyl-1-oxo-2,7-naphthyrid...)Show SMILES COc1cc(cc(OC)c1CN(C)S(C)(=O)=O)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621663
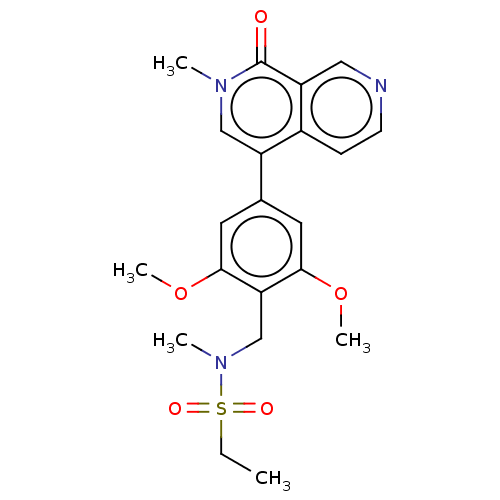
(N-[[2,6-dimethoxy-4-(2-methyl-1-oxo-1,2-dihydro-2,...)Show SMILES CCS(=O)(=O)N(C)Cc1c(OC)cc(cc1OC)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621668
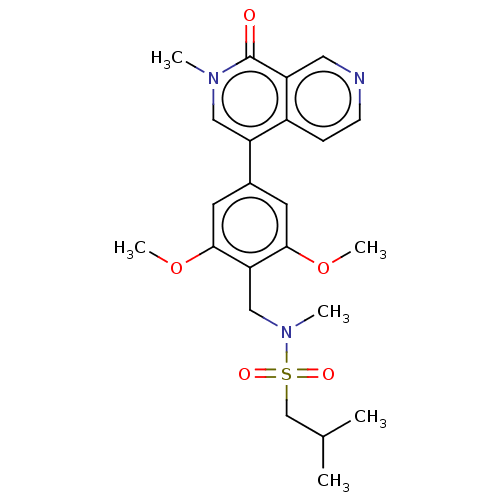
(US11773085, Compound B40 | ethylpropane-1-sulfonam...)Show SMILES COc1cc(cc(OC)c1CN(C)S(=O)(=O)CC(C)C)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621648
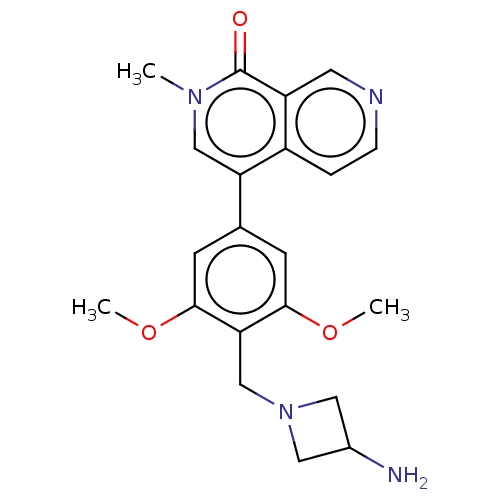
(4-[4-[(3-aminoazetidin-1-yl)methyl]-3,5-dimethoxyp...)Show SMILES COc1cc(cc(OC)c1CN1CC(N)C1)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621662
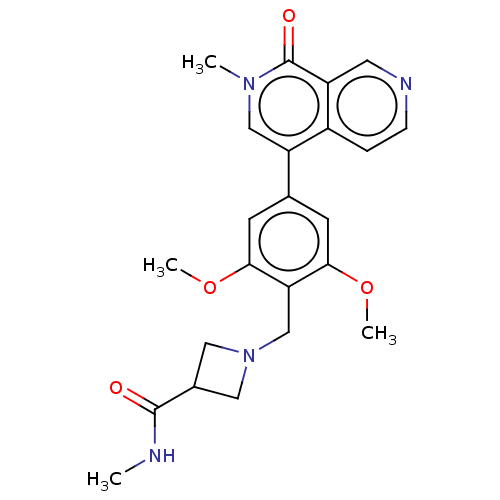
(US11773085, Compound B34)Show SMILES CNC(=O)C1CN(Cc2c(OC)cc(cc2OC)-c2cn(C)c(=O)c3cnccc23)C1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587065
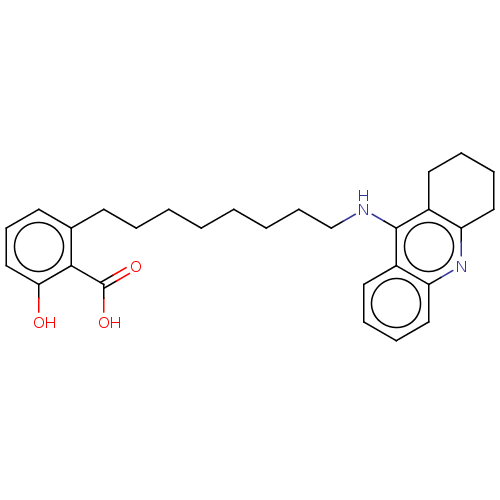
(CHEMBL5087646)Show SMILES OC(=O)c1c(O)cccc1CCCCCCCCNc1c2CCCCc2nc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587060
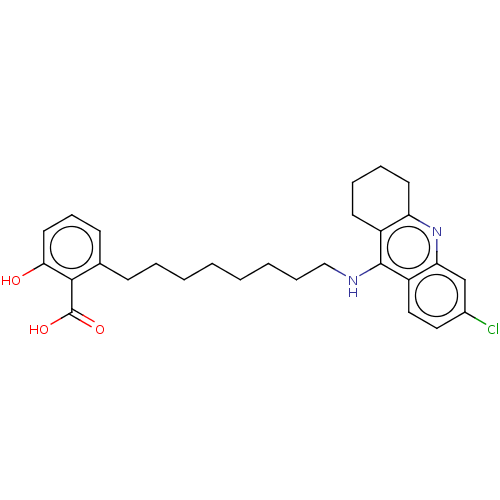
(CHEMBL5078914)Show SMILES OC(=O)c1c(O)cccc1CCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987

(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587059

(CHEMBL5092094)Show SMILES COC(=O)c1c(O)cccc1CCCCCCCCNc1c2CCCCc2nc2ccccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587061

(CHEMBL5072428)Show SMILES Oc1cccc(CCCCCCCCNc2c3CCCCc3nc3cc(Cl)ccc23)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587066

(CHEMBL5073819) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587056

(CHEMBL5080685)Show SMILES COC(=O)c1c(CCCCCCCCNc2c3CCCCc3nc3ccccc23)cccc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587058

(CHEMBL5088557)Show SMILES COc1cccc(CCCCCCCCNc2c3CCCCc3nc3cc(Cl)ccc23)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587054
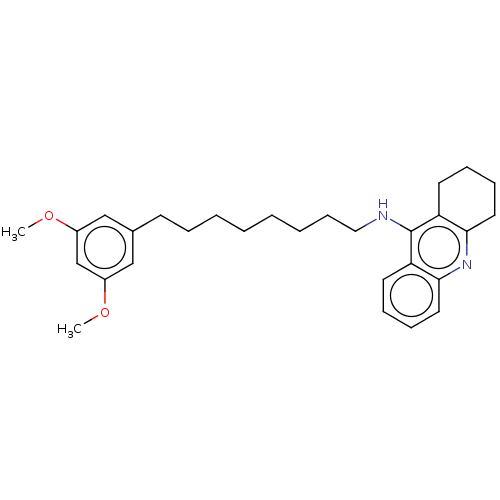
(CHEMBL5088541)Show SMILES COc1cc(CCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(OC)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587055

(CHEMBL5091660) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using ZMAL (Z-(Ac)Lys-AMC fluorogenic substrate incubated for 90 mins by fluorescence method |
ACS Med Chem Lett 10: 671-676 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00071
BindingDB Entry DOI: 10.7270/Q2BP068P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587054

(CHEMBL5088541)Show SMILES COc1cc(CCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(OC)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621546
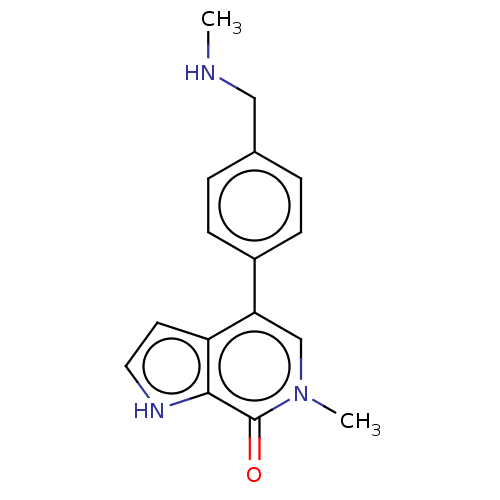
(US11773085, Compound B14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621554
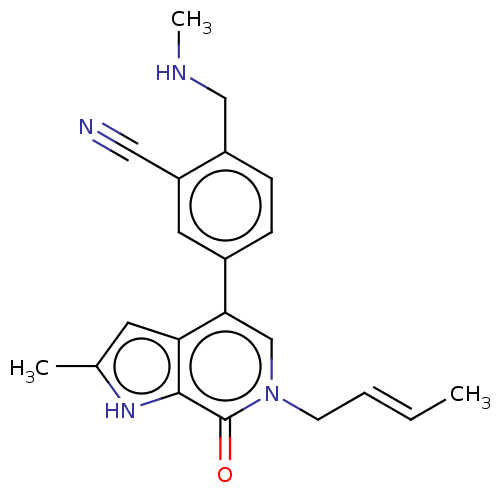
(US11773085, Compound B15)Show SMILES CNCc1ccc(cc1C#N)-c1cn(C\C=C\C)c(=O)c2[nH]c(C)cc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621588
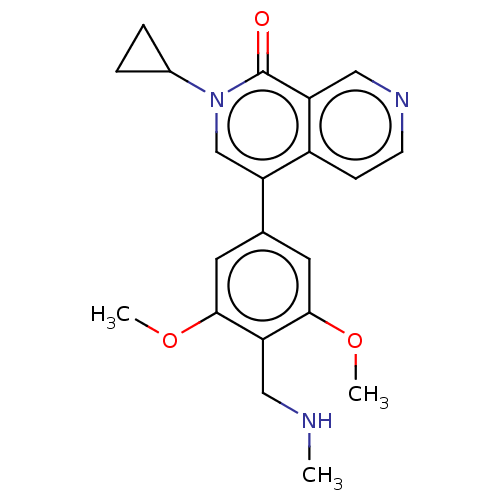
(2-cyclopropyl-4-[3,5-dimethoxy-4-[(methylamino)met...)Show SMILES CNCc1c(OC)cc(cc1OC)-c1cn(C2CC2)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621649
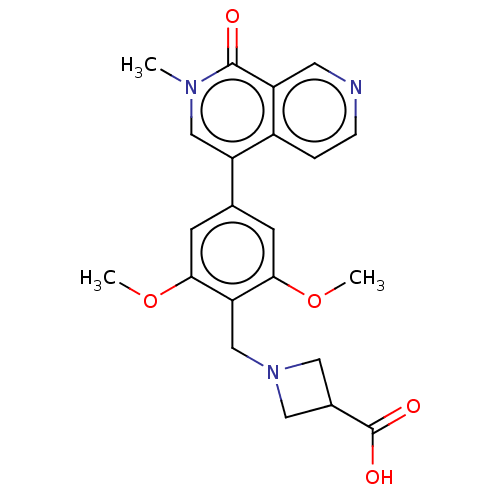
(US11773085, Compound B21 | acid )Show SMILES COc1cc(cc(OC)c1CN1CC(C1)C(O)=O)-c1cn(C)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621650
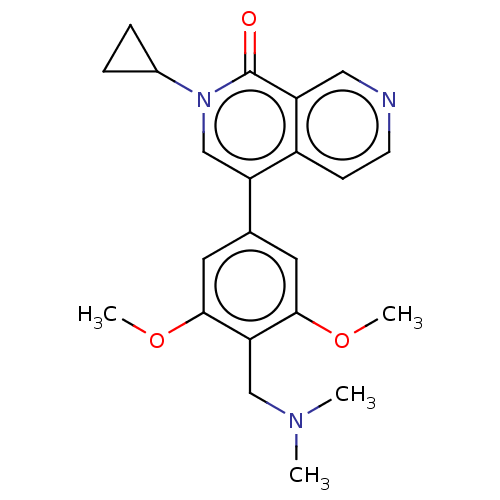
(2-cyclopropyl-4-[4-[(dimethylamino)methyl]-3,5-dim...)Show SMILES COc1cc(cc(OC)c1CN(C)C)-c1cn(C2CC2)c(=O)c2cnccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM50183449
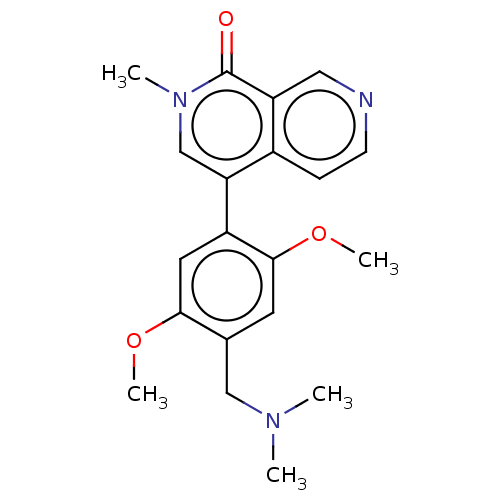
(CHEMBL3823101 | US11773085, Compound B23)Show SMILES COc1cc(c(OC)cc1CN(C)C)-c1cn(C)c(=O)c2cnccc12 Show InChI InChI=1S/C20H23N3O3/c1-22(2)11-13-8-19(26-5)15(9-18(13)25-4)17-12-23(3)20(24)16-10-21-7-6-14(16)17/h6-10,12H,11H2,1-5H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM50183448
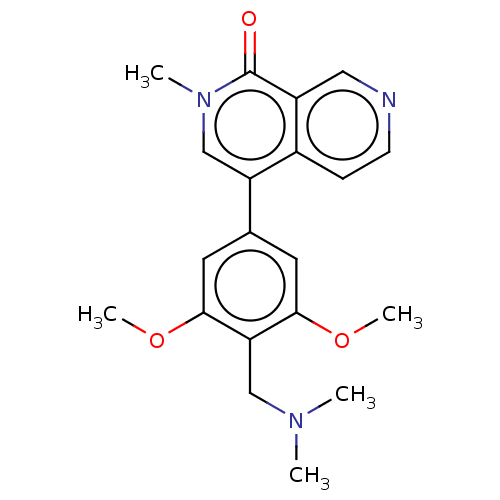
(CHEMBL3823478 | US11773085, Compound B2)Show SMILES COc1cc(cc(OC)c1CN(C)C)-c1cn(C)c(=O)c2cnccc12 Show InChI InChI=1S/C20H23N3O3/c1-22(2)11-17-18(25-4)8-13(9-19(17)26-5)16-12-23(3)20(24)15-10-21-7-6-14(15)16/h6-10,12H,11H2,1-5H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621656
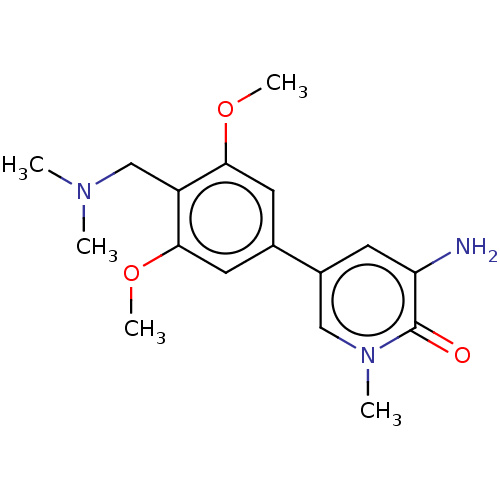
(3-amino-5-(4-((dimethylamino)methyl)-3,5-dimethoxy...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621660
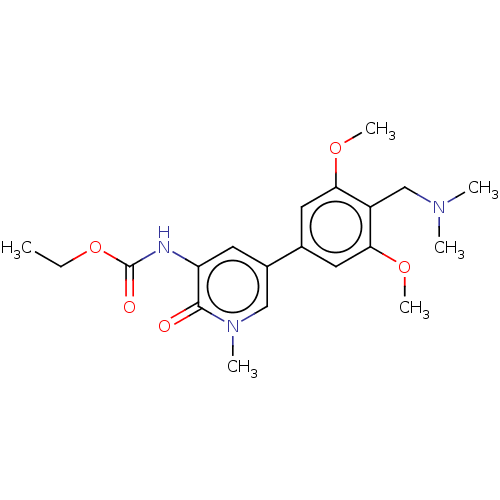
(N-(5-[4-[(dimethylamino)methyl]-3,5-dimethoxypheny...)Show SMILES CCOC(=O)Nc1cc(cn(C)c1=O)-c1cc(OC)c(CN(C)C)c(OC)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9 [133-239]
(Homo sapiens (Human)) | BDBM621664
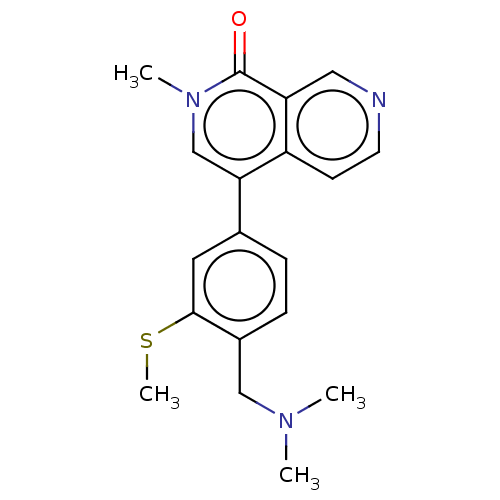
(4-[4-[(dimethylamino)methyl]-3-(methylsulfanyl)phe...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data