

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H3N2 neuraminidase | Bioorg Med Chem 23: 7777-84 (2015) Article DOI: 10.1016/j.bmc.2015.11.028 BindingDB Entry DOI: 10.7270/Q2FB55X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H1N1 wild type neuraminidase | Bioorg Med Chem 23: 7777-84 (2015) Article DOI: 10.1016/j.bmc.2015.11.028 BindingDB Entry DOI: 10.7270/Q2FB55X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479825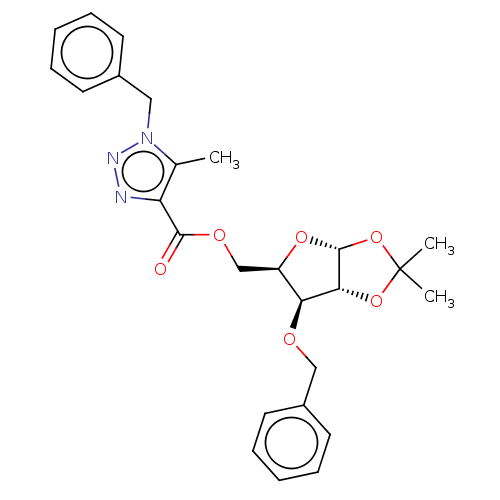 (CHEMBL515099) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense Curated by ChEMBL | Assay Description Inhibition of polymerase activity of HIV1 recombinant reverse transcriptase expressed in Escherichia coli | Eur J Med Chem 44: 373-83 (2009) Article DOI: 10.1016/j.ejmech.2008.02.047 BindingDB Entry DOI: 10.7270/Q2NP2776 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479824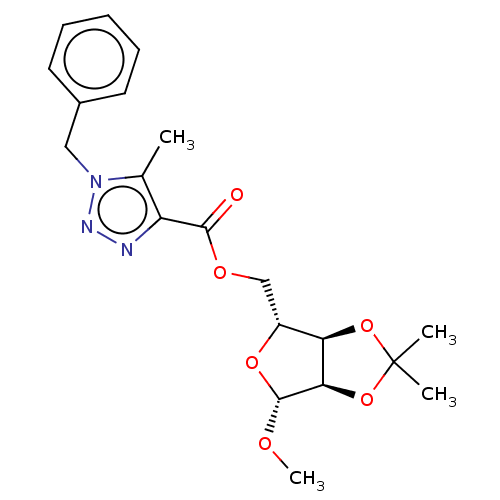 (CHEMBL474818) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense Curated by ChEMBL | Assay Description Inhibition of polymerase activity of HIV1 recombinant reverse transcriptase expressed in Escherichia coli | Eur J Med Chem 44: 373-83 (2009) Article DOI: 10.1016/j.ejmech.2008.02.047 BindingDB Entry DOI: 10.7270/Q2NP2776 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50500018 (CHEMBL3740548) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H3N2 neuraminidase E119V mutant | Bioorg Med Chem 23: 7777-84 (2015) Article DOI: 10.1016/j.bmc.2015.11.028 BindingDB Entry DOI: 10.7270/Q2FB55X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93122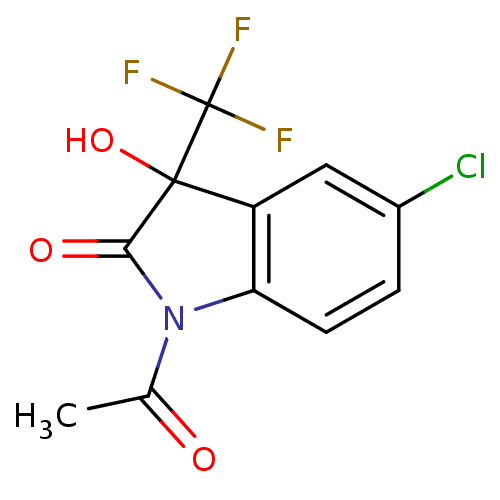 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 25) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50500018 (CHEMBL3740548) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H1N1 wild type neuraminidase | Bioorg Med Chem 23: 7777-84 (2015) Article DOI: 10.1016/j.bmc.2015.11.028 BindingDB Entry DOI: 10.7270/Q2FB55X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93121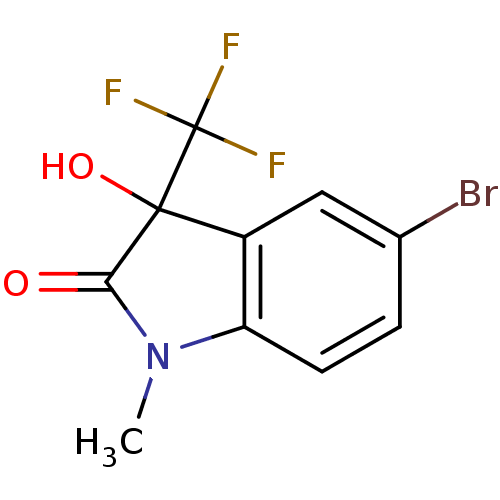 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 21) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | n/a | n/a | 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H3N2 neuraminidase E119V mutant | Bioorg Med Chem 23: 7777-84 (2015) Article DOI: 10.1016/j.bmc.2015.11.028 BindingDB Entry DOI: 10.7270/Q2FB55X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479826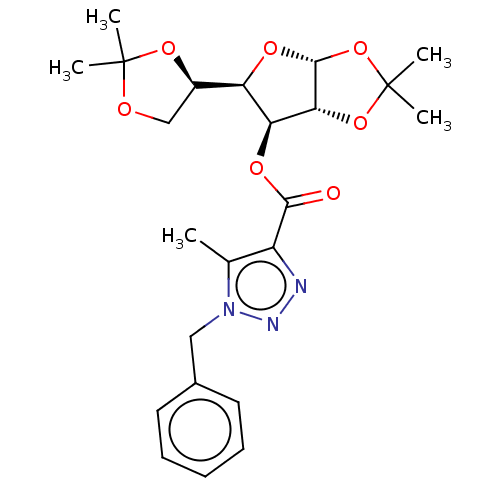 (CHEMBL473157) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense Curated by ChEMBL | Assay Description Inhibition of polymerase activity of HIV1 recombinant reverse transcriptase expressed in Escherichia coli | Eur J Med Chem 44: 373-83 (2009) Article DOI: 10.1016/j.ejmech.2008.02.047 BindingDB Entry DOI: 10.7270/Q2NP2776 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93119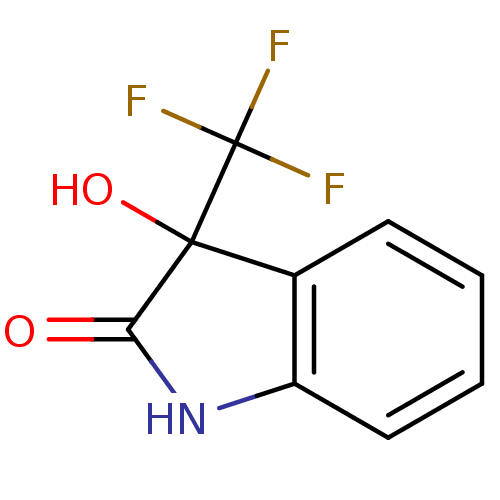 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 16) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 6.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93120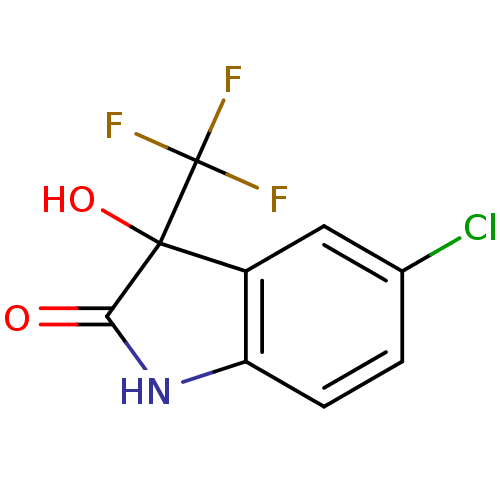 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 18) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93123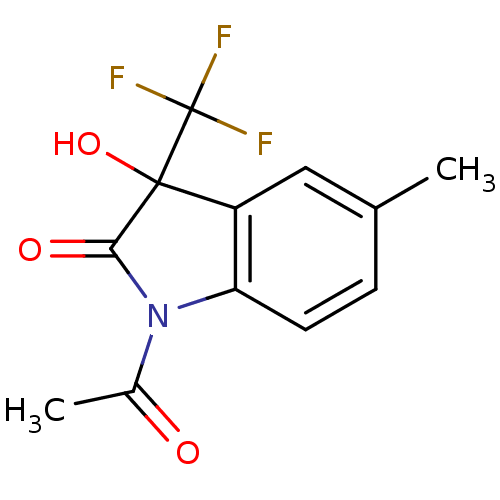 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 26) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 8.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50500018 (CHEMBL3740548) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H1N1 neuraminidase H275Y mutant | Bioorg Med Chem 23: 7777-84 (2015) Article DOI: 10.1016/j.bmc.2015.11.028 BindingDB Entry DOI: 10.7270/Q2FB55X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50500018 (CHEMBL3740548) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H3N2 neuraminidase | Bioorg Med Chem 23: 7777-84 (2015) Article DOI: 10.1016/j.bmc.2015.11.028 BindingDB Entry DOI: 10.7270/Q2FB55X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H1N1 neuraminidase H275Y mutant | Bioorg Med Chem 23: 7777-84 (2015) Article DOI: 10.1016/j.bmc.2015.11.028 BindingDB Entry DOI: 10.7270/Q2FB55X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||