

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasmepsin II (Plasmodium falciparum) | BDBM50072543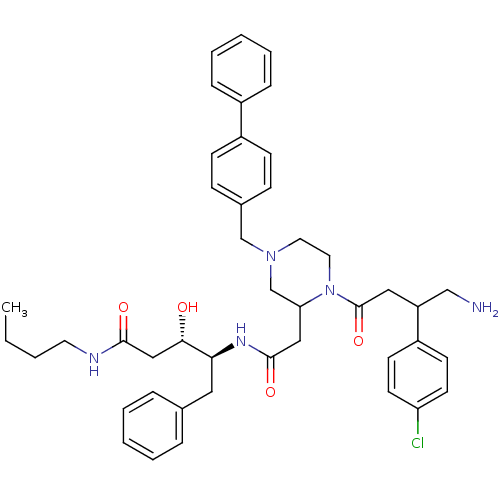 ((3S,4S)-4-(2-{1-[4-Amino-3-(4-chloro-phenyl)-butyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against plasmepsin II | Bioorg Med Chem Lett 8: 3203-6 (1999) BindingDB Entry DOI: 10.7270/Q2D21WSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50072545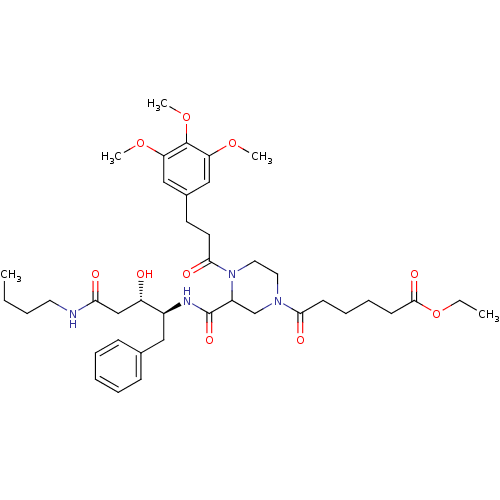 (6-{3-((1S,2S)-1-Benzyl-3-butylcarbamoyl-2-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 3203-6 (1999) BindingDB Entry DOI: 10.7270/Q2D21WSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50072542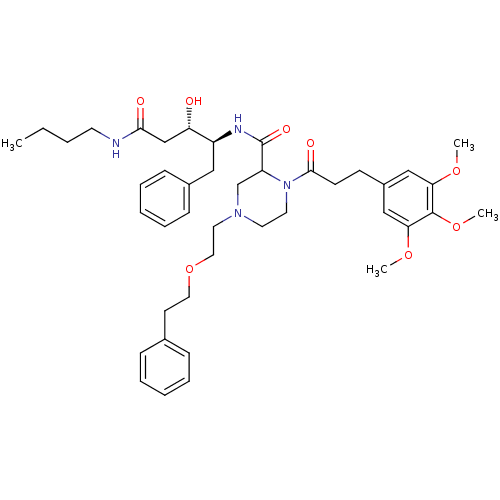 (4-(2-Phenethyloxy-ethyl)-1-[3-(3,4,5-trimethoxy-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 3203-6 (1999) BindingDB Entry DOI: 10.7270/Q2D21WSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50072544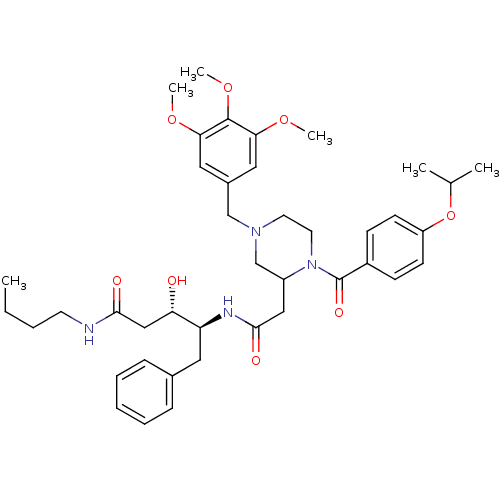 ((3S,4S)-3-Hydroxy-4-{2-[1-(4-isopropoxy-benzoyl)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 3203-6 (1999) BindingDB Entry DOI: 10.7270/Q2D21WSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM315477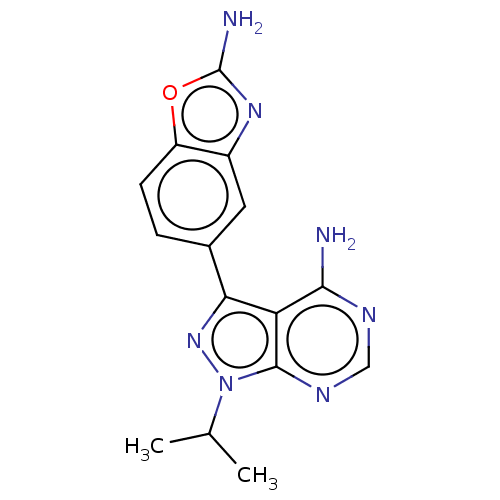 (US10172858, Table 1.1 | US10172858, Table 1.22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in the presence of ATP incubated for 30 mins by Lance ultra assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50579370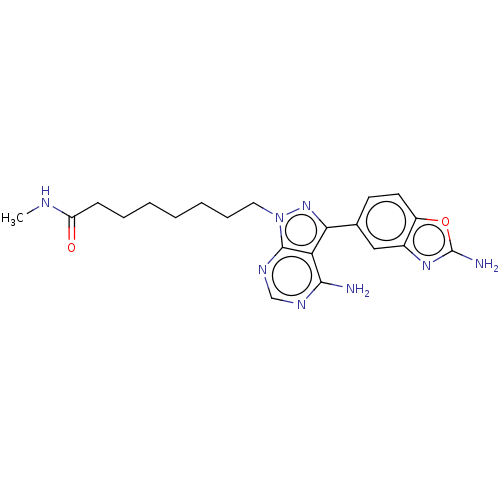 (CHEMBL4870348) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50566959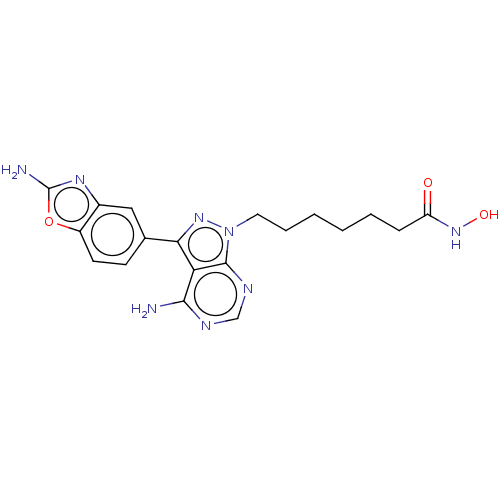 (CHEMBL4846560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50566959 (CHEMBL4846560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50579370 (CHEMBL4870348) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50579370 (CHEMBL4870348) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in the presence of ATP incubated for 30 mins by Lance ultra assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50566959 (CHEMBL4846560) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC3 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50579370 (CHEMBL4870348) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC6 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50579370 (CHEMBL4870348) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC3 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50566959 (CHEMBL4846560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in the presence of ATP incubated for 30 mins by Lance ultra assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50579371 (CHEMBL4846776) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC6 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50566959 (CHEMBL4846560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC6 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50313645 (1-cyclopentyl-3-(1H-pyrrolo[2,3-b]pyridin-5-yl)-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in the presence of ATP incubated for 30 mins by Lance ultra assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50579371 (CHEMBL4846776) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC6 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50579371 (CHEMBL4846776) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224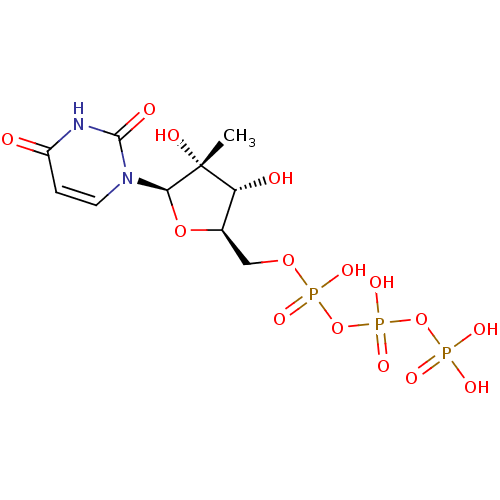 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 1a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 1a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50579371 (CHEMBL4846776) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC3 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 3a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 6a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 3a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 6a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC3 (unknown origin) using Ac-peptide as a substrate pretreated for 15 mins followed by substrate addition | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 1b infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 1b infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 4a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 4a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50579371 (CHEMBL4846776) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mTOR (unknown origin) using U-Light-4E-BP1 peptide as a substrate in the presence of ATP incubated for 30 mins by Lance ultra assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113824 BindingDB Entry DOI: 10.7270/Q2NP287M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM259897 (US9505780, JQ-1) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114153 BindingDB Entry DOI: 10.7270/Q2ZC86Z5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM259897 (US9505780, JQ-1) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114153 BindingDB Entry DOI: 10.7270/Q2ZC86Z5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50333129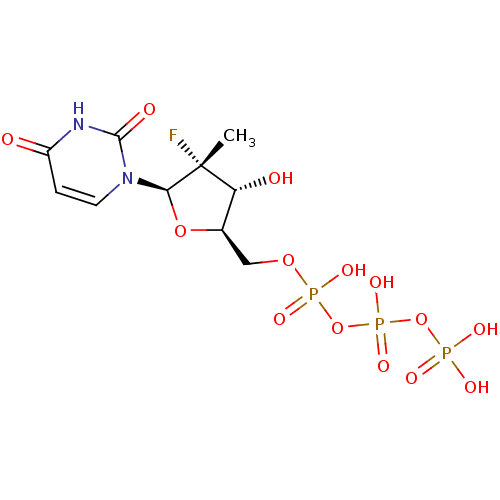 (((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 1a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50333129 (((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 1a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged PPAR-alpha LBD expressed in Escherichia coli BL21 (DE3) PlysS after 30 mins in presence of fluorescein ligand FL... | ACS Med Chem Lett 7: 590-4 (2016) Article DOI: 10.1021/acsmedchemlett.6b00033 BindingDB Entry DOI: 10.7270/Q2CR5W99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 5a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 5a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50603594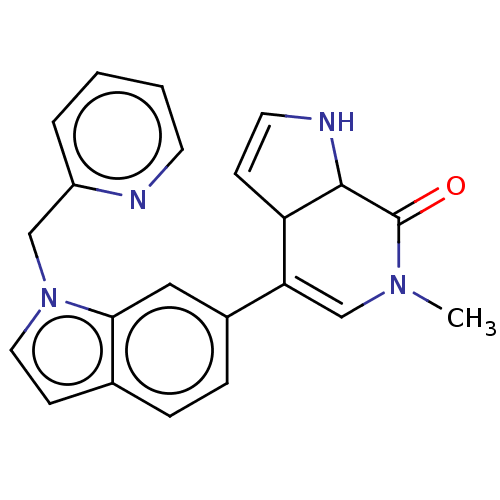 (CHEMBL5205457) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114153 BindingDB Entry DOI: 10.7270/Q2ZC86Z5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50603594 (CHEMBL5205457) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114153 BindingDB Entry DOI: 10.7270/Q2ZC86Z5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50585812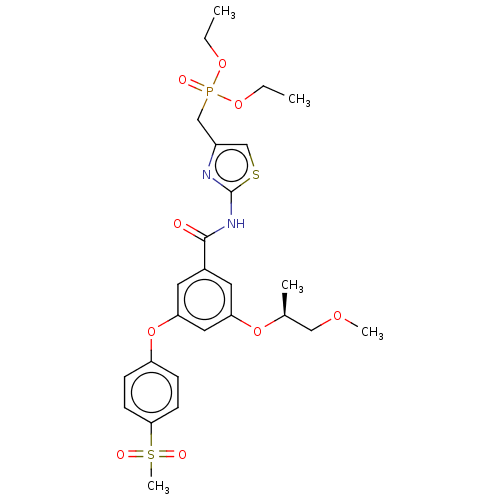 (CHEMBL5091943) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent labeled derivative from recombinant human hepatic glucokinase incubated for 30 mins in presence of 12 mM glucose by fluor... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02110 BindingDB Entry DOI: 10.7270/Q2057KV7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50585813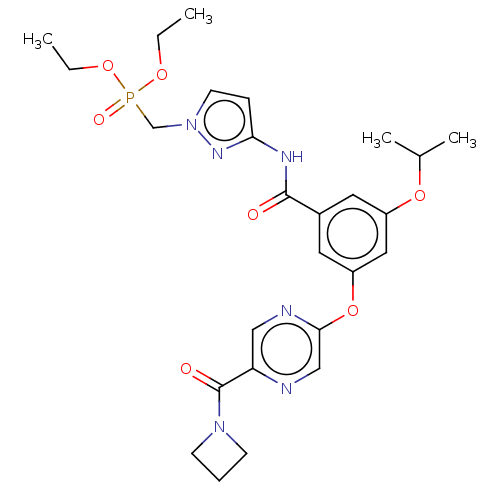 (CHEMBL5072532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent labeled derivative from recombinant human hepatic glucokinase incubated for 30 mins in presence of 12 mM glucose by fluor... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02110 BindingDB Entry DOI: 10.7270/Q2057KV7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50333129 (((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 6a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50333129 (((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 6a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314811 (2-((4-(2-(2-(4-chlorophenyl)-5-methyloxazol-4-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314813 (2-((3-(2-(2-(4-chlorophenyl)-5-methyloxazol-4-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 711 total ) | Next | Last >> |