

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver carboxylesterase (Sus scrofa) | BDBM50570559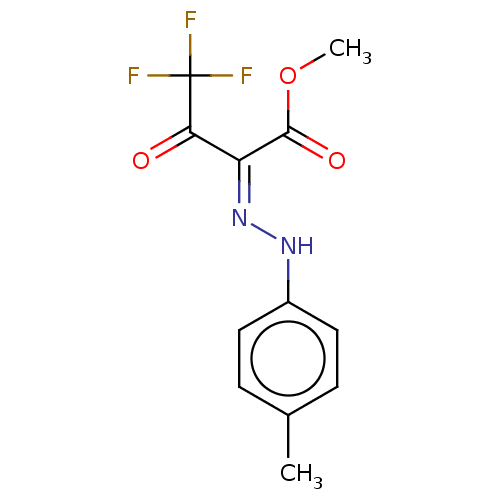 (CHEMBL4868687) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of porcine liver carboxylesterase by double reciprocal Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500766 (CHEMBL3754409) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500766 (CHEMBL3754409) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500752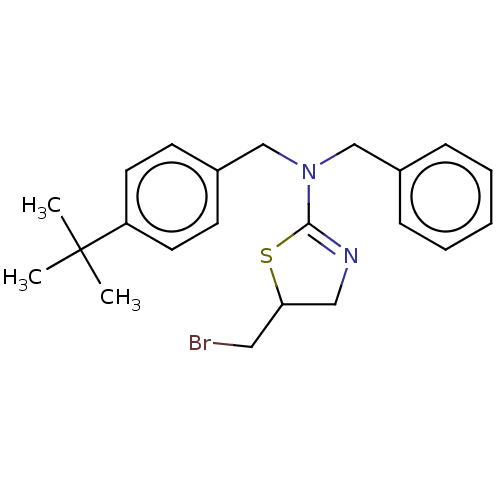 (CHEMBL3754327) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500752 (CHEMBL3754327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500760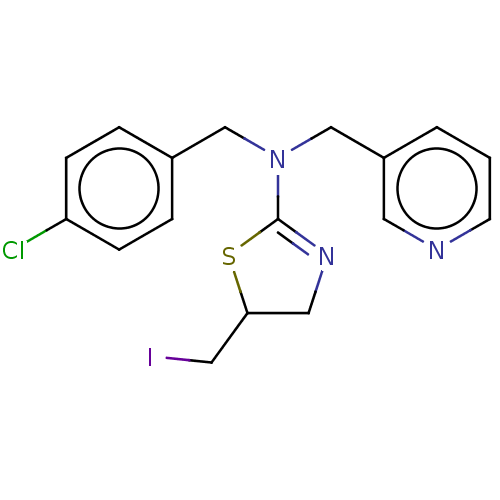 (CHEMBL3754622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500766 (CHEMBL3754409) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500755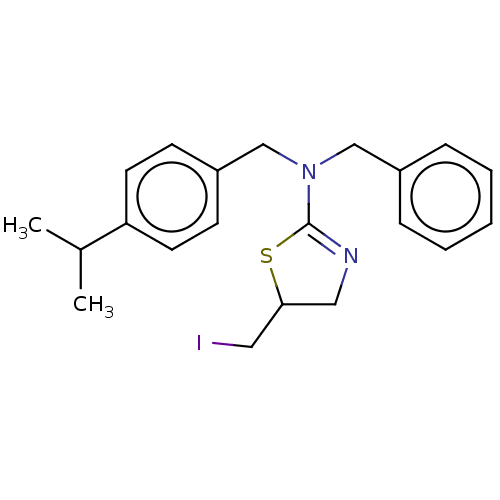 (CHEMBL3752466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500756 (CHEMBL3752908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500755 (CHEMBL3752466) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500757 (CHEMBL3752682) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500760 (CHEMBL3754622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500758 (CHEMBL3753216) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500755 (CHEMBL3752466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500748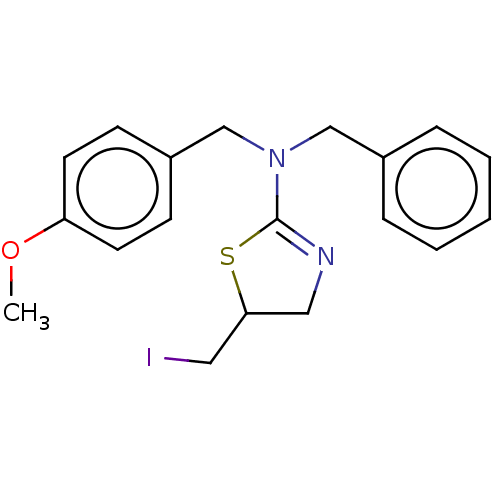 (CHEMBL3753156) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500746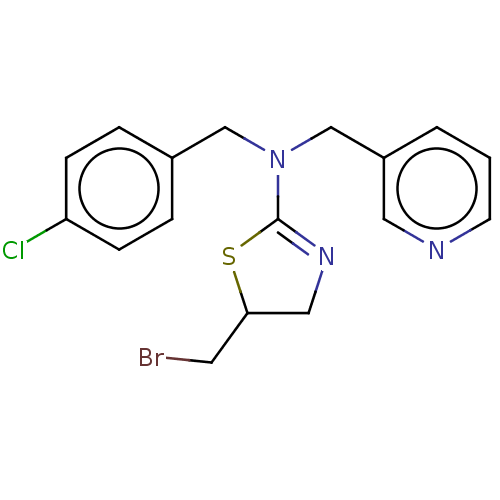 (CHEMBL3753531) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50570559 (CHEMBL4868687) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human erythrocyte AChE by double reciprocal Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500746 (CHEMBL3753531) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50570559 (CHEMBL4868687) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of equine serum BuChE by double reciprocal Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570550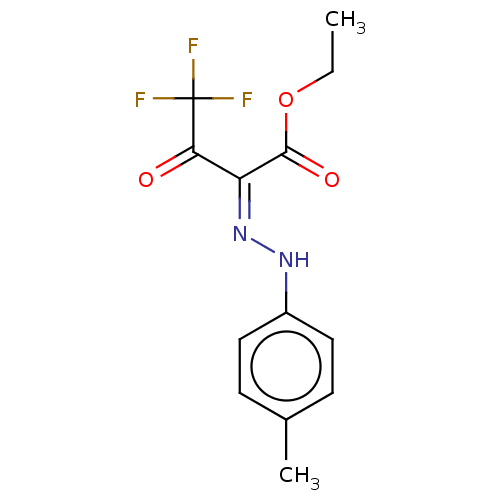 (CHEMBL4849361) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50570553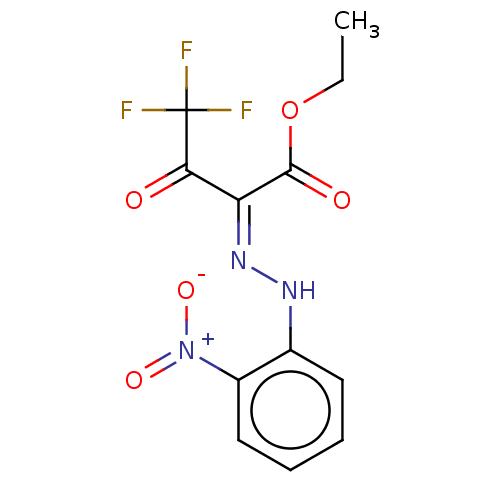 (CHEMBL4854298) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES2 expressed in mouse NSO cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570552 (CHEMBL4855755) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50570549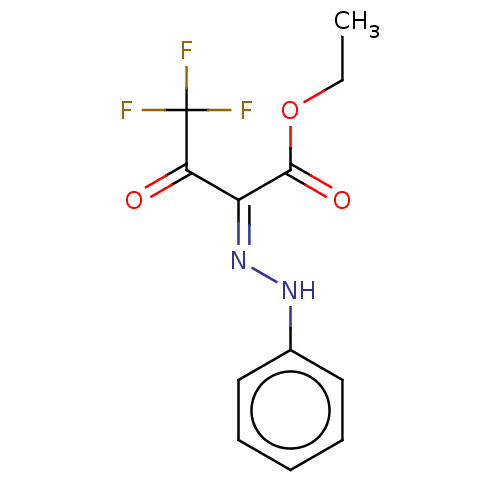 (CHEMBL4853742) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES2 expressed in mouse NSO cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570553 (CHEMBL4854298) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570555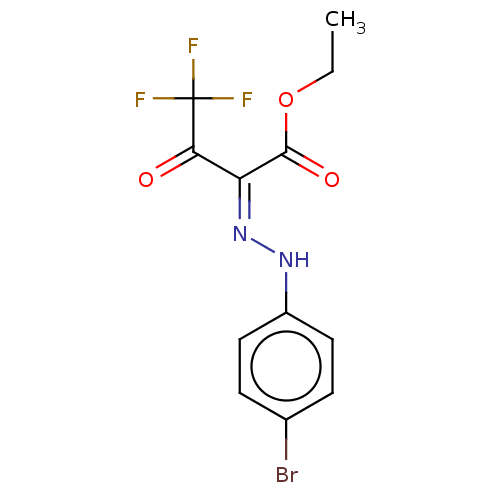 (CHEMBL4868014) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570551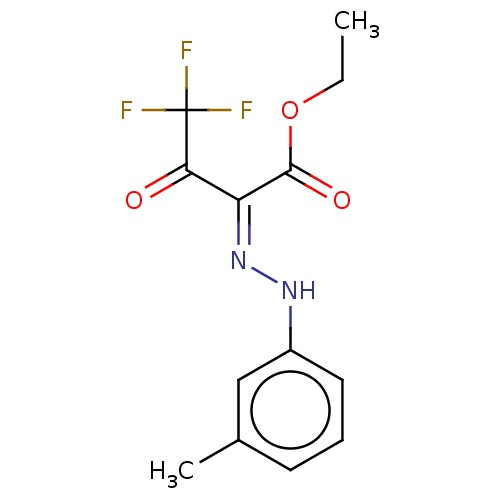 (CHEMBL4853637) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570549 (CHEMBL4853742) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570549 (CHEMBL4853742) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570554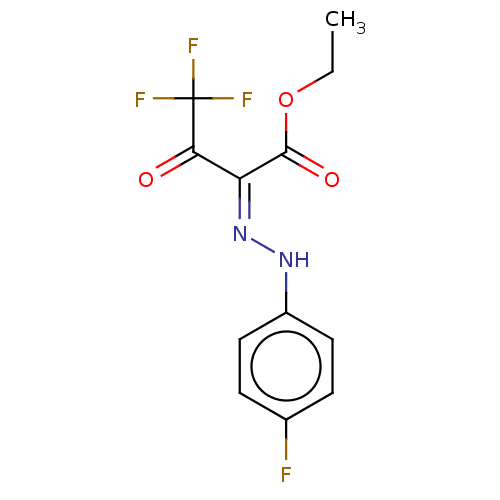 (CHEMBL4858186) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570551 (CHEMBL4853637) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570550 (CHEMBL4849361) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570561 (CHEMBL4855838) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50570554 (CHEMBL4858186) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES2 expressed in mouse NSO cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50570555 (CHEMBL4868014) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES2 expressed in mouse NSO cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570558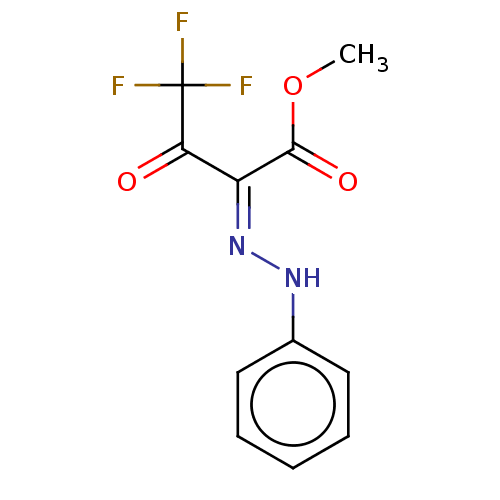 (CHEMBL4849436) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570552 (CHEMBL4855755) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570555 (CHEMBL4868014) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570559 (CHEMBL4868687) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50570561 (CHEMBL4855838) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES2 expressed in mouse NSO cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570560 (CHEMBL4872772) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570554 (CHEMBL4858186) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50570551 (CHEMBL4853637) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES2 expressed in mouse NSO cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570553 (CHEMBL4854298) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570559 (CHEMBL4868687) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated followed by substrate addition by Ellman's method | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570556 (CHEMBL4847987) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570564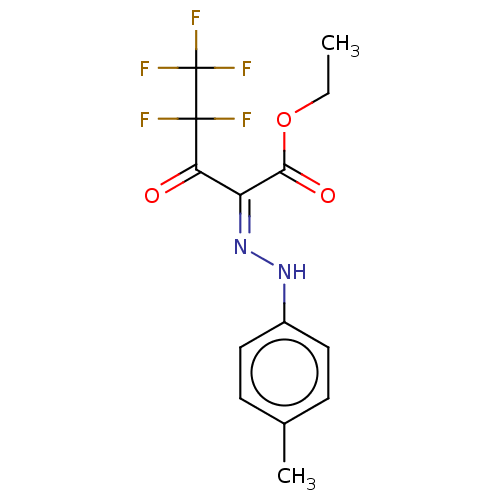 (CHEMBL4853013) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570560 (CHEMBL4872772) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570557 (CHEMBL4856140) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 161 total ) | Next | Last >> |